Abstract
Background and Objectives:
Percutaneous portal vein (PV) stent placement is used to manage PV occlusion or stenosis caused by malignancy. The use of endoscopic ultrasonography (EUS) has expanded to include vascular interventions. The aim of this study was to examine the technical feasibility and safety of EUS-guided transhepatic PV stent placement in a live porcine model.
Materials and Methods:
EUS-guided transhepatic PV stent placement was performed in six male miniature pigs under general anesthesia using forward-viewing echoendoscope. Under EUS guidance, the left intrahepatic PV was punctured with a 19-gauge fine-needle aspiration (FNA) needle and a 0.025 inch guidewire inserted through the needle and into the main PV. The FNA needle was then withdrawn and a needle-knife inserted to dilate the tract. Under EUS and fluoroscopic guidance, a noncovered metal stent was inserted over the guidewire and released into the main PV.
Results:
A PV stent was placed successfully in all six pigs with no technical problems or complications. The patency of the stent in the main PV was confirmed using color Doppler EUS and transhepatic portal venography. Necropsy of the first three animals revealed no evidence of bleeding and damage to intra-abdominal organs or vessels. No complications occurred in the remaining three animals during the 8 weeks observation period.
Conclusions:
EUS-guided transhepatic PV stent placement can be both technically feasible and safe in a live animal model.
Keywords: Endoscopic ultrasonography, feasibility, portal vein, safety, stent
INTRODUCTION
Endoscopic ultrasonography (EUS) and EUS-guided interventions are often used to diagnose and manage intra-abdominal diseases.[1,2,3,4] More recently, EUS has been used to diagnose and treat vascular conditions.[5,6,7,8] EUS-guided coil embolization or cyanoacrylate injections have been used to manage gastric varices.[9,10] The feasibility and safety of EUS-guided portal vein (PV) puncture and catheterization in a live porcine model have been documented.[11,12,13] The utility of contrast-enhanced harmonic EUS (CEH-EUS) for diagnosing celiac artery dissection and superior mesenteric artery (SMA) dissection was reported in our study group.[14]
Percutaneous PV stent placement is occasionally used to treat patients with malignant PV obstruction caused by hepatocellular carcinoma or pancreatic and biliary cancer although the indications are not well established.[15,16] PV stent relieves portal venous pressure and can improve gastroesophageal varices, refractory ascites, superior mesenteric vein congestion, bowel edema, and malabsorption caused by portal hypertension. Palliative PV stent placement in patients with malignant PV obstruction may prolong survival and improve their general condition and quality of life.[15,16,17] Furthermore, PV stent placement may be useful for relieving benign PV stenosis or obstruction caused by necrotizing pancreatitis, suppurative appendicitis, or liver transplantation surgery.[18,19]
To date, PV stent placement has been performed by interventional radiologists via the percutaneous access route. However, EUS-guided transhepatic PV stent placement may have several advantages over the traditional percutaneous approach. For example, EUS provides a detailed image of the PV and adjacent vascular structures, thereby allowing selection of the most preferable port of entry into the PV.[6] Color Doppler and pulse-wave Doppler can delineate vascular structures and real-time blood flow characteristics clearly, help detect abnormal vascular flow, and assess the degree of stenosis and flow velocity.[14,20] Furthermore, the use of color Doppler can reduce both the dose of contrast, the risk of nephrotoxicity, and exposure to radiation. The aim of the present study was to examine the technical feasibility and safety of EUS-guided transhepatic PV stent placement in a live animal model.
MATERIALS AND METHODS
The animal study was approved by the Research Animal Care Committee of Asan Medical Center. EUS-guided transhepatic PV stent placement was performed consecutively in six miniature pigs.
Animal preparation
Male miniature pigs (Sus scrofa domesticus) (8–10 months old; 25 kg) were used. Food was withheld for 48 h before the procedure. Pigs received only clear sugar-sweetened water for gastric preparation. Preanesthesia medication comprised intramuscular injection of atropine sulfate (0.05 mg/kg), tiletamine hydrochloric acid (HCl) plus zolazepam (7.5 mg/kg; Zoletil®; Virbac animal health, Fort Worth, Texas, United States), and xylazine HCl (1–2 mg/kg; Rompun®; Bayer Health Care, Lerverkusen, Germen). Procedures were performed under general anesthesia (1.5%–2.5% isoflurane). Blood pressure, heart rate, respiration rate, and arterial oxygen saturation were monitored during anesthesia.
Endoscopic ultrasonography-guided portal vein stenting procedure
A forward-viewing curvilinear array echoendoscope (XGIF-UCT160J-AL5; Olympus Medical Systems, Japan) with an EUS processor (EU-ME1; Olympus Medical Systems, Japan) was advanced into the stomach and the portosplenic junction identified by tracing the intrahepatic PV branch of the left liver lobe at a frequency of 7.5 MHz. Under EUS guidance, the left intrahepatic PV was punctured with a 19-gauge fine-needle aspiration (FNA) needle (Expect™ 19ga Flex Needle; Boston Scientific Co., Natick, MA, United States), the stylet withdrawn, and proper positioning of the needle confirmed by aspiration of blood. Portal venography was performed by injecting 10 mL of iodinated contrast dye (VISIPAQUE™ iodixanol; GE healthcare, Piscataway, NJ, United States) through the needle under fluoroscopic and EUS guidance. Then, a 0.025 inch guidewire (VisiGlide, G-240-2545A; Olympus Medical Systems, Japan) was inserted through the needle and placed in the main PV. The guidewire was maintained in the main PV while the needle was withdrawn from the echoendoscope channel. The initial puncture site was then cauterized and dilated using a needle-knife (5.5Fr distal tip; 7Fr outer diameter; Micro-knife XL®, Boston Scientific Co., Natick, MA, United States). After creating a transhepatic tract, a 7Fr stent delivery system mounted with a noncovered metal stent (diameter, 8 mm; length, 6 cm; BONASTENT® Biliary, DU-BB-060418; Standard Sci-Tech Inc., Seoul, South Korea) was inserted over the guidewire. The stent was then released in the main PV under EUS and fluoroscopic guidance. After deploying the PV stent, the blood flow through the stent was confirmed using color Doppler EUS. Next, a 4Fr endoscopic retrograde cholangiopancreatography catheter (Glo-Tip®, GT-1-UT; Cook Endoscopy, Winston-Salem, NC, United States) was inserted over the guidewire and the guidewire was withdrawn. Portal venography was again performed through the catheter, and stent patency in the main PV was confirmed. The catheter was then removed from the PV. Finally, the echoendoscope was removed and the animal was monitored closely for 2 h to check sudden or fatal complications. The first three animals were then observed for a further 1 week. After this time, stent patency in the main PV was evaluated again using color Doppler EUS and transhepatic portogram. The animals were then euthanized using intravenous potassium chloride under general anesthesia and necropsied. The other three animals were observed for 8 weeks to monitor survival and euthanized.
RESULTS
EUS-guided puncture of the left intrahepatic PV with a 19-gauge FNA needle was successful in all six animals [Figure 1]. The guidewire was placed in the main PV through the needle without any difficulty. After creating a transhepatic tract using a needle-knife, the stent delivery system was easily advanced to the main PV. In all cases, the stent was released in the main PV without any problems. After successful deployment of the stent, a portogram was obtained and flow of the contrast through the deployed stent was confirmed [Figure 2]. There was no fluctuation in heart rate, blood pressure, respiration rate, or arterial oxygen saturation during the procedure. No complications were observed during the first 2 h postprocedure.
Figure 1.
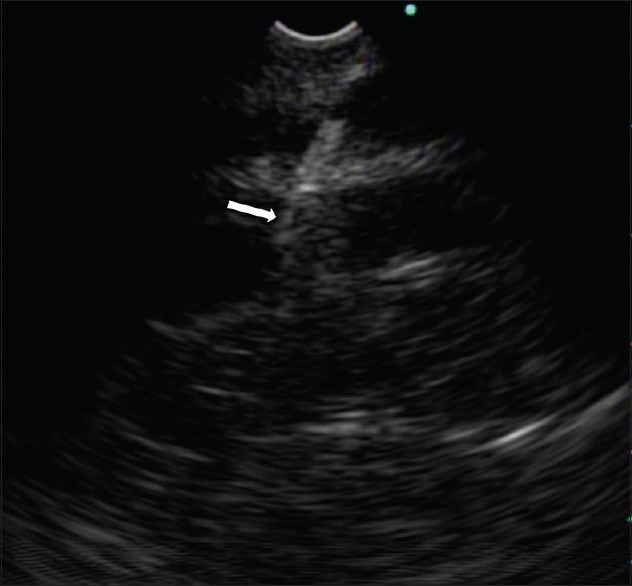
Endoscopic ultrasonographic view. The 19-gauge fine-needle aspiration needle (arrow) in the left intrahepatic portal vein
Figure 2.
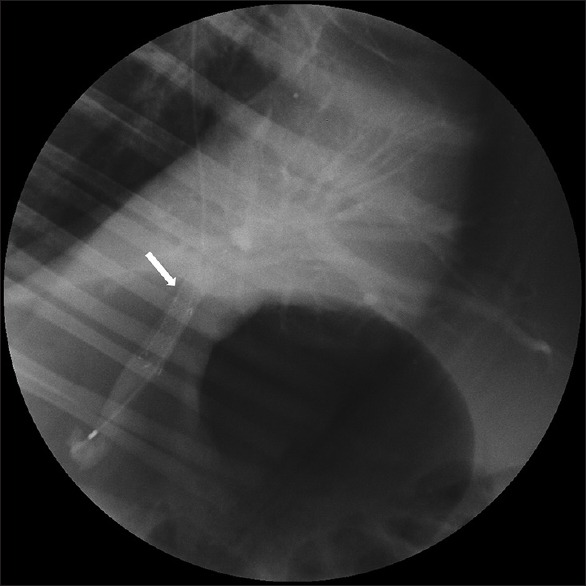
Fluoroscopic view. The patency of the portal vein stent (arrow) was confirmed by monitoring the flow of contrast injected into the portal vein
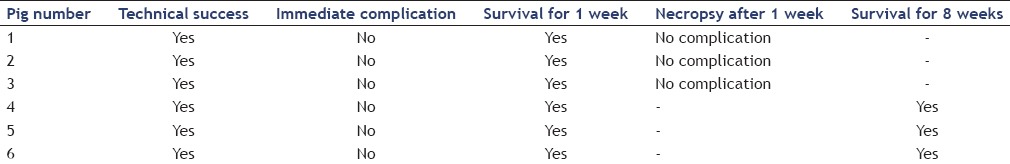
None of the first three animals showed signs of distress or infection, bleeding, peritonitis, or other complications during the 1 week observation period. After 1 week, blood flow through the PV stent was confirmed by color Doppler EUS. The flow of contrast through the stent in the PV was confirmed by portal venography. Necropsy revealed no evidence of significant bleeding and damage to intra-abdominal organs or vessels [Figure 3]. Observation of the remaining three animals for 8 weeks revealed no signs of distress or complications. The results of EUS-guided transhepatic PV stent placement in the six pigs are summarized in Table 1.
Figure 3.
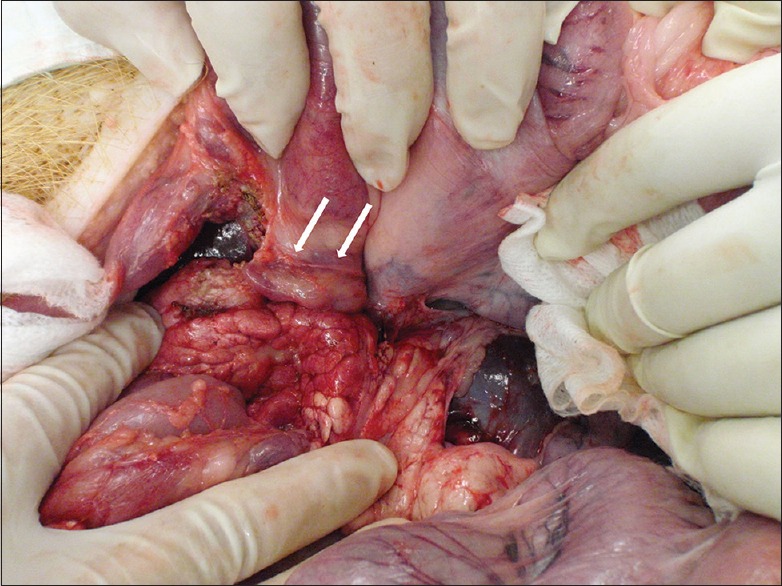
Necropsy revealed no evidence of damage to the portal vein, liver, or adjacent intra-abdominal organs and vessels. The stent in the main portal vein (arrow) is shown
Table 1.
Endoscopic ultrasonography-guided transhepatic portal vein stent placement in the six miniature pigs
DISCUSSION
With the development of EUS technique, EUS can be used for diverse procedures including drainage for pancreaticobiliary disease, celiac plexus intervention, vascular intervention, and natural orifice transluminal endoscopic surgery.[2,3,4,5,6,21,22] Intra-abdominal vascular access and therapy are promising areas within the field of EUS-guided intervention; therefore, several clinical or experimental studies have examined EUS-guided vascular interventions.[5,6] Experimental studies show that EUS-guided PV catheterization and PV pressure measurement in a porcine model are technically feasible.[11,12,23] In a pilot study, investigators performed EUS-guided transduodenal puncture of the extrahepatic PV.[11] Necropsy revealed hematoma at the puncture site within the extrahepatic PV in all pigs. However, in another study, investigators found no evidence of bleeding after EUS-guided transhepatic puncture of the intrahepatic PV.[12] It was thought that the hepatic parenchyma tamponaded the puncture site in the intrahepatic PV, thereby preventing postprocedural bleeding. Another study showed that EUS-guided creation of an intrahepatic portosystemic shunt (an alternative to the traditional transjugular intrahepatic portosystemic shunt) was also both technically feasible and safe in a porcine model.[23] In a clinical study, the diameter, the flow velocity, and the flow volume of gastric varices were evaluated using EUS.[24] Our own group showed that a combination color Doppler EUS and CEH-EUS could be a useful complementary modality for assessing celiac artery dissection and SMA dissection without the need to expose the patient to radiation.[14]
Here, we demonstrated the technical feasibility and safety of PV stent placement using EUS-guided transhepatic approach in a live animal model. EUS-guided transhepatic PV stent was placed successfully in all six pigs without any technical difficulties or immediate complications. A needle-knife (distal tip, 5.5Fr; outer diameter, 7Fr) was used to create a transhepatic tract and a stent delivery system of the same size (outer diameter 7Fr) was inserted without the need for bougination or balloon dilation. We selected this approach to reduce the chances of complications, particularly significant bleeding at the PV puncture site. If bougination or balloon dilation was necessary to advance the stent delivery system (as in the case of pseudocyst drainage or biliary drainage), a significant amount of active bleeding might have occurred.
This study has several limitations. First, healthy animals (without portal hypertension and coagulopathy) were used; therefore, the risk of bleeding may have been underestimated. Second, most of the devices used in the present study were designed for biliary intervention. More research (and development) of devices dedicated to EUS-guided vascular intervention is necessary. Third, lack of sterility is a potential drawback of EUS-guided vascular intervention; this could result in bacteremia and affect long-term survival. Fourth, after dilation transhepatic tract using needle knife, it would expect some bile leak and bile peritonitis in the absence of a covered stent traversing the tract. We found no evidence of peritonitis during the 8 weeks observation period in the present study; however, peritonitis can become life-threatening in immunocompromised patients with liver cirrhosis or malignancy: The patients who are potential candidates for PV stent placement. The above mentioned factors are the main obstacles to the clinical application of EUS-guided transhepatic PV stent placement.
CONCLUSIONS
EUS-guided transhepatic PV stent placement can be both technically feasible and safe in a live animal model. Further studies with large numbers of animals are needed to assess the safety of this technically challenging intervention before it can be considered suitable for wider clinical application.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was supported by the grant (2014-201) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
REFERENCES
- 1.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 2.Giovannini M, Pesenti C, Rolland AL, et al. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473–7. doi: 10.1055/s-2001-14967. [DOI] [PubMed] [Google Scholar]
- 3.Bhutani MS. Endoscopic ultrasound comes of age: Mature, established, creative and here to stay! Endosc Ultrasound. 2014;3:143–51. doi: 10.4103/2303-9027.138782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbonari AP, Bonfim J, Colaiacovo R, et al. Pancreatic cancer with portal vein invasion diagnosed by endoscopic ultrasound. Endosc Ultrasound. 2015;4:158–9. doi: 10.4103/2303-9027.156758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxena P, Lakhtakia S. Endoscopic ultrasound guided vascular access and therapy (with videos) Endosc Ultrasound. 2015;4:168–75. doi: 10.4103/2303-9027.162994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weilert F, Binmoeller KF. EUS-guided vascular access and therapy. Gastrointest Endosc Clin N Am. 2012;22:303–14. doi: 10.1016/j.giec.2012.04.019. x. [DOI] [PubMed] [Google Scholar]
- 7.Choudhary NS, Puri R, Sud R. Paracholedochal varices causing biliopathy in a case of portal vein thrombosis. Endosc Ultrasound. 2015;4:76–7. doi: 10.4103/2303-9027.151372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takagi T, Irisawa A, Shibukawa G, et al. Intraductal ultrasonographic anatomy of biliary varices in patients with portal hypertension. Endosc Ultrasound. 2015;4:44–51. doi: 10.4103/2303-9027.151346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, et al. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: Results in 5 cases. Gastrointest Endosc. 2007;66:402–7. doi: 10.1016/j.gie.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Castro R, Pellicer-Bautista F, Giovannini M, et al. Endoscopic ultrasound (EUS)-guided coil embolization therapy in gastric varices. Endoscopy. 2010;42(Suppl 2):E35–6. doi: 10.1055/s-0029-1215261. [DOI] [PubMed] [Google Scholar]
- 11.Lai L, Poneros J, Santilli J, et al. EUS-guided portal vein catheterization and pressure measurement in an animal model: A pilot study of feasibility. Gastrointest Endosc. 2004;59:280–3. doi: 10.1016/s0016-5107(03)02544-6. [DOI] [PubMed] [Google Scholar]
- 12.Giday SA, Clarke JO, Buscaglia JM, et al. EUS-guided portal vein catheterization: A promising novel approach for portal angiography and portal vein pressure measurements. Gastrointest Endosc. 2008;67:338–42. doi: 10.1016/j.gie.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Magno P, Ko CW, Buscaglia JM, et al. EUS-guided angiography: A novel approach to diagnostic and therapeutic interventions in the vascular system. Gastrointest Endosc. 2007;66:587–91. doi: 10.1016/j.gie.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Paik WH, Choi JH, Seo DW, et al. Clinical usefulness with the combination of color Doppler and contrast-enhanced harmonic EUS for the assessment of visceral vascular diseases. J Clin Gastroenterol. 2014;48:845–50. doi: 10.1097/MCG.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 15.Yamakado K, Tanaka N, Nakatsuka A, et al. Clinical efficacy of portal vein stent placement in patients with hepatocellular carcinoma invading the main portal vein. J Hepatol. 1999;30:660–8. doi: 10.1016/s0168-8278(99)80197-4. [DOI] [PubMed] [Google Scholar]
- 16.Yamakado K, Nakatsuka A, Tanaka N, et al. Portal venous stent placement in patients with pancreatic and biliary neoplasms invading portal veins and causing portal hypertension: Initial experience. Radiology. 2001;220:150–6. doi: 10.1148/radiology.220.1.r01jl03150. [DOI] [PubMed] [Google Scholar]
- 17.Novellas S, Denys A, Bize P, et al. Palliative portal vein stent placement in malignant and symptomatic extrinsic portal vein stenosis or occlusion. Cardiovasc Intervent Radiol. 2009;32:462–70. doi: 10.1007/s00270-008-9455-9. [DOI] [PubMed] [Google Scholar]
- 18.Shan H, Xiao XS, Huang MS, et al. Portal venous stent placement for treatment of portal hypertension caused by benign main portal vein stenosis. World J Gastroenterol. 2005;11:3315–8. doi: 10.3748/wjg.v11.i21.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko GY, Sung KB, Yoon HK, et al. Early posttransplantation portal vein stenosis following living donor liver transplantation: Percutaneous transhepatic primary stent placement. Liver Transpl. 2007;13:530–6. doi: 10.1002/lt.21068. [DOI] [PubMed] [Google Scholar]
- 20.Brugge WR. EUS is an important new tool for accessing the portal vein. Gastrointest Endosc. 2008;67:343–4. doi: 10.1016/j.gie.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Sun S. Endoscopic ultrasound's vision: Probing our way to NOTES. Endosc Ultrasound. 2014;3:141–2. doi: 10.4103/2303-9027.138781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luz LP, Al-Haddad MA, DeWitt JA. EUS-guided celiac plexus interventions in pancreatic cancer pain: An update and controversies for the endosonographer. Endosc Ultrasound. 2014;3:213–20. doi: 10.4103/2303-9027.144515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buscaglia JM, Dray X, Shin EJ, et al. A new alternative for a transjugular intrahepatic portosystemic shunt: EUS-guided creation of an intrahepatic portosystemic shunt (with video) Gastrointest Endosc. 2009;69:941–7. doi: 10.1016/j.gie.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 24.Imamura H, Irisawa A, Shibukawa G, et al. Echo-endoscopic analysis of variceal hemodynamics in patient with isolated gastric varices. Endosc Ultrasound. 2014;3:238–44. doi: 10.4103/2303-9027.144542. [DOI] [PMC free article] [PubMed] [Google Scholar]


