Introduction
Forty years ago, a group of physicians gathered in Nice, France, with a shared optimism for the promise of systemic therapies to treat cancer. This conference became the inaugural meeting of what is now the European Society for Medical Oncology (ESMO), which today convenes nearly 20,000 individuals from around the world. At that time, in 1975, it was becoming clear that contemporary scientific discoveries related to the properties of chemotherapeutic agents were spring-loading the oncologist's ability to address malignancies. Clinicians were able to employ adjuvant chemotherapy for the treatment of early-stage breast cancer, and combination chemotherapy for patients with testicular cancer, both of which would soon lead to marked improvements in overall survival. Medicines such as cyclophosphamide, cisplatin, bleomycin and others—which we often take for granted in our practices today—were then new instruments in the clinician's anticancer toolkit.
The number of systemic therapies and treatment approaches we currently have to address the needs of our patients with cancer is vast. With advanced techniques in radiology, pathology, radiation oncology, surgical oncology and other subspecialties, the benefit of medicines can be further augmented for certain types of cancer. A woman diagnosed with early-stage breast cancer in Europe today has an 80% chance of long-term survival. Similarly, a child diagnosed in Europe with acute lymphoblastic leukaemia has a nearly 90% chance to live a long and relatively healthy life after treatment. Overall, the notion that cancer is a universal death sentence has shifted from reality to fallacy in wealthy parts of the world.
Yet, as these tremendous gains in advanced economies have resulted in improvements in life expectancy, and undoubtedly economic gains from productivity life-years (not to mention the social and moral imperatives implicit in cancer care), the conception of cancer as a death sentence is not a fallacy in many impoverished regions of the world: it is a reality.
The authors of this editorial have been sobered time and again by the sight of patients with cancer in wards of hospitals where treatment options readily available in developed countries have not extended their reach. Even after a century of discovery in therapeutics, our society's ability to deliver medicines to places where they are needed has been an imperfect—if not an exiguous—performance.
Current advances rely on availability of certain interventions, including medicines considered essential for the success of therapy in certain cancers. The WHO recognised the importance of these medications to improve the outcome of patients worldwide, and, since 1977, proposed and subsequently maintained, the WHO Model List of Essential Medicines.1 Containing only a few anticancer medicines at the beginning, the list has reached its 19th version, and has been recently updated, prompting us to reflect on the worldwide implications of this project.
ESMO has endeavoured to reduce these inequities as part of its societal mission, and has been among the global leaders of several crucial policy changes that are designed to dramatically improve access to cancer treatment worldwide. Our partnership with the Union for International Cancer Control (UICC), in particular, alongside many other collaborators around the world, is highlighted in the present editorial.
Global need
More than 8 million people died of cancer in 2013, the majority of whom were living in the developing world. This massive discrepancy in mortality derives largely (if not entirely) from disparities in access to cancer prevention, diagnosis and treatment, rather than any difference in pathology across geographies or ethnicities (although genetic variations remain an area of critical research). Many organisations, including the UICC, ESMO and the American Society for Clinical Oncology (ASCO), are responding to the WHO's Global Action Plan (GAP), which was outlined following the United Nations High-level Meeting on Noncommunicable Diseases (NCDs) in September 2011. One of the targets of the WHO GAP is to reach 80% coverage of essential medicines and technologies for NCDs by 2025, including those medicines and technologies for cancer.
In line with this goal, UICC coordinated with the WHO Essential Medicines List (EML) Secretariat at the WHO Essential Medicines Department to convene a group of institutions and individuals to review and propose modifications to the cancer medicines on its Model List of Essential Medicines for Adults and Children (WHO EML). The WHO EML is updated every 2 years in a process where additions or changes to a medicine can be proposed. A period is set aside for public comment, and the WHO Expert Committee on the Selection and Use of Essential Medicines reviews the public health relevance—available evidence for both, benefits and harms—as the main selection criteria. In January 2014, the UICC and the Dana-Farber Cancer Institute (DFCI) in Boston, USA, launched a 12-month review alongside partners from ESMO, ASCO and other cancer institutions from around the world. A core group of individuals (including the authors) proposed a disease-based approach (and not a medicine-by-medicine approach) and elaborated a priority list of 29 types of cancers.
Historically, medicines proposed to the Lists had been recommended on an individual agent basis. Our core group determined, however, that inclusion needed to be disease- and regimen-based. The diseases we would select for analysis were those demonstrating a high epidemiological burden, and those with high response rates to systemic therapies (over no treatment, or over surgery). An exhaustive review period led to a decision to consider treatment regimens for 29 types of cancer, both paediatric and adult.
Disease-based briefings for each cancer were then developed individually or by small groups of oncologists (and included treatment regimens, requirements for diagnosis and treatment, toxicity panels, epidemiology and systematic reviews), which were then peer-reviewed by at least two additional oncologists. A central committee aggregated the recommendations, sometimes seeking guidance from additional specialists, to come to consensus.
Over the course of the year, a group of 100 cancer experts—who contributed as volunteers—from five continents, demonstrated the oncology community's great interest and commitment to availing cancer medicines to those in need.
Out of these briefings, 52 different anticancer agents were recommended to the WHO. The full proposal—including all disease briefings and ancillary supportive documentation on regulatory and pricing information—was reviewed and deliberated on at the 19th Meeting of the WHO Expert Committee on the Selection and Use of Essential Medicines in Geneva in April, 2015.
Ultimately, 16 additions were made by the WHO Expert Committee (16 new for the Adult List, 7 new to the Children's List), which collectively impact the treatment options for 26 different types of cancer, many of which could have not been treated at all without the newly-added medicines. Further, the 2015 EML now states the disease for which it has efficacy next to the medical name, instead of all medicines being listed under one categorical heading for cancer. Six medicines were rejected on the basis that they currently did not have sufficient evidence presented for the proposed indication.
Effectuating real change
The driving purpose of the effort was to develop a package that could be used as a decision-making tool by policymakers around the world. The WHO EML can now be used to cross-reference the types of cancer being treated in a given country with the list of medicines for each disease, and thus formulate or strengthen national medicine formularies and procurement processes accordingly.
We believe this addition of 16 medicines and the reaffirmation of the 30 cancer medicines already on the WHO EML, as well as the new format of the cancer medicines that is linked to both the diseases for, and regimens with which they are used, will advance the world towards reaching the GAP target of 80% availability of medicines for NCDs, and will also help to lay the foundation on which strong cancer treatment systems can be established in low-income and middle-income settings. Currently, there are wide discrepancies between the numbers of cancer medicines listed on national EMLs (some countries having only a handful listed), as has been recently shown by the ESMO-led European2 and Global Opioid Policy Initiative3 studies, and the ESMO European and International Antineoplastic Medicines Surveys,4 5 which, despite being ‘perception surveys at a snapshot in time’, represent a real first attempt to identify issues and shortages related to the availability to patients of cancer therapies and pain medicines. A vital next step is the formal review by national health sectors that can employ the new WHO EML as a reference and alignment with national cancer control plans. The UICC and its partners are eager to engage with countries that wish to apply this disease-based framework and welcomed outreach.
Second, we believe the new WHO EML, published in 2015, will demystify misconceptions about the availability of cancer medicines. For example, 42/46 cancer medicines have multiple manufacturers and globally available pricing information, suggesting availability is high. Similarly, 43/46 of these highly-effective cancer medicines are no longer under patent protection and the remainder will see this protection expire in the coming 2–3 years. We hope this new list will empower oncologists and cancer advocates around the world to demand routine availability of the cancer medicines needed to save lives. A brief cost assessment was published with the applications and is available in reference 6. It showed the medicines required for a full course of treatment for several highly treatable cancers can be <US$500.
We also believe the WHO EML can help set the tone for important dialogue related to regulatory mechanisms—nationally, regionally and globally—as well as pharmaceutical industry partnerships, supply chain mechanisms and overall care delivery by skilled personnel.
It is the aim of ESMO, the UICC, DFCI, ASCO and more than 90 oncologists who contributed their expertise to this work, that the foundation laid by creating a new model for selecting essential medicines for cancer care—and developing a new list itself—will, over time, reduce the disparities that persist in survival outcomes today. With the ever-increasing set of medicines we have in our anticancer toolkit, it is long overdue that we bring cancer care to the developing world. Having celebrated its 40th Anniversary in 2015, ESMO invites you to join us in these efforts.
For more information about ESMO's European and Global Public Policy initiatives, please visit our Public Policy website section7 or send us an email (feedback@esmo.org) if you would like to join a particular project and support our efforts.
For more information about UICC public policy initiatives, please visit: http://www.UICC.org/essentialmeds.
An infographic regarding the review process of the 2015 WHO Model List of Essential Medicines is represented here with permission from UICC (figure 1).
Figure 1.
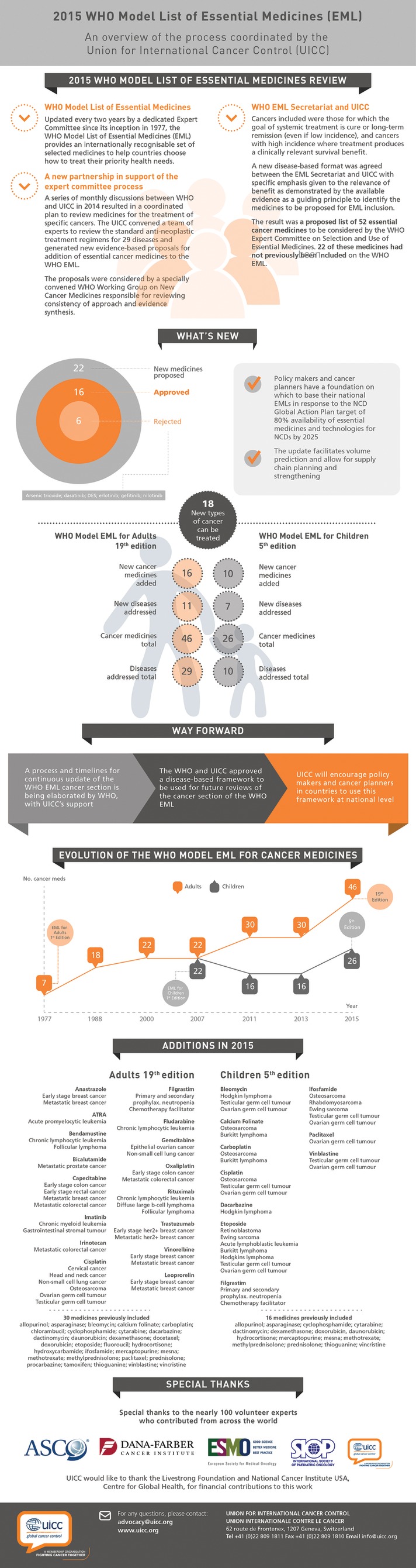
2015 WHO Model List of Essential Medicines (EML): An overview of the process coordinated by the Union for International Cancer Control (UICC)
Footnotes
Collaborators: Union for International Cancer Control EML Steering Committee Members: Tezer Kutluk—President-Elect, Union for International Cancer Control, Geneva, Switzerland. Ronald Barr—McMaster University, Hamilton, Ontario, Canada. Gilberto Lopes—Oncoclínicas Group, Johns Hopkins University, São Paulo, Brazil. Claire Wagner—Dana-Farber Cancer Institute, Boston, MA, USA. Lawrence N Shulman—Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, USA.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
Contributor Information
Collaborators: Tezer Kutluk, Ronald Barr, Gilberto Lopes, Claire Wagner, and Lawrence N Shulman
References
- 1.2015 WHO Model List of Essential Medicines. http://www.who.int/medicines/publications/essentialmedicines/en/
- 2.Cherny NI, Baselga J, de Conno F et al. . Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Europe: a report from the ESMO/EAPC Opioid Policy Initiative. Ann Oncol 2010;21:615–26. doi:10.1093/annonc/mdp581 [DOI] [PubMed] [Google Scholar]
- 3.Cherny NI, Cleary J, Scholten W et al. . The Global Opioid Policy Initiative (GOPI) project to evaluate the availability and accessibility of opioids for the management of cancer pain in Africa, Asia, Latin America and the Caribbean, and the Middle East: introduction and methodology. Ann Oncol 2013; 24(Suppl 11):xi7–13. doi:10.1093/annonc/mdt498 [DOI] [PubMed] [Google Scholar]
- 4.ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of anti-neoplastic medicines. Nathan I Cherny, Richard Sullivan, Julie Torode, Marika Saar, Alexandru Eniu. To be published in Annals of Oncology in 2016. http://www.esmo.org/Policy/Anti-Cancer-Medicines-Availability/European-Study [DOI] [PubMed]
- 5.ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of anti-neoplastic medicines. Nathan I Cherny, Richard Sullivan, Julie Torode, Marika Saar, Alexandru Eniu. To be published by ESMO in 2016. http://www.esmo.org/Policy/Anti-Cancer-Medicines-Availability/International-Study
- 6.http://www.who.int/selection_medicines/committees/expert/20/applications/UICC_EMLreview-cost_pricing-2014.pdf?ua=1
- 7.http://www.esmo.org/Policy


