Abstract
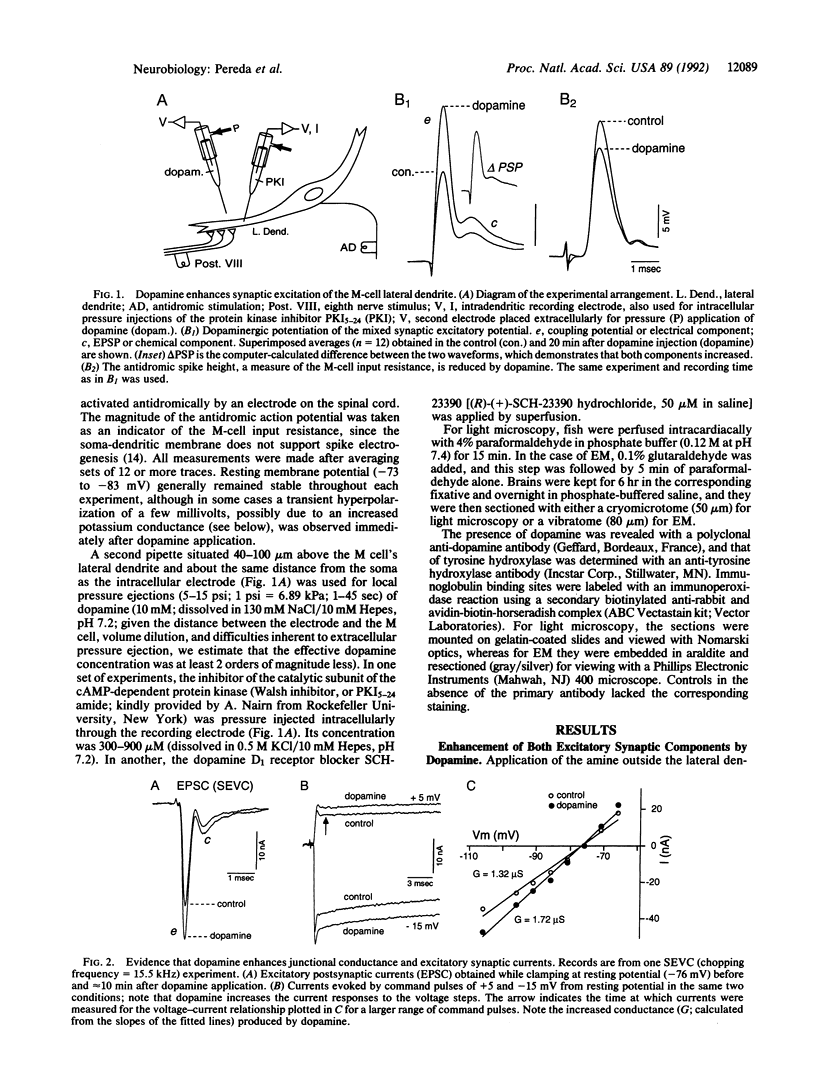
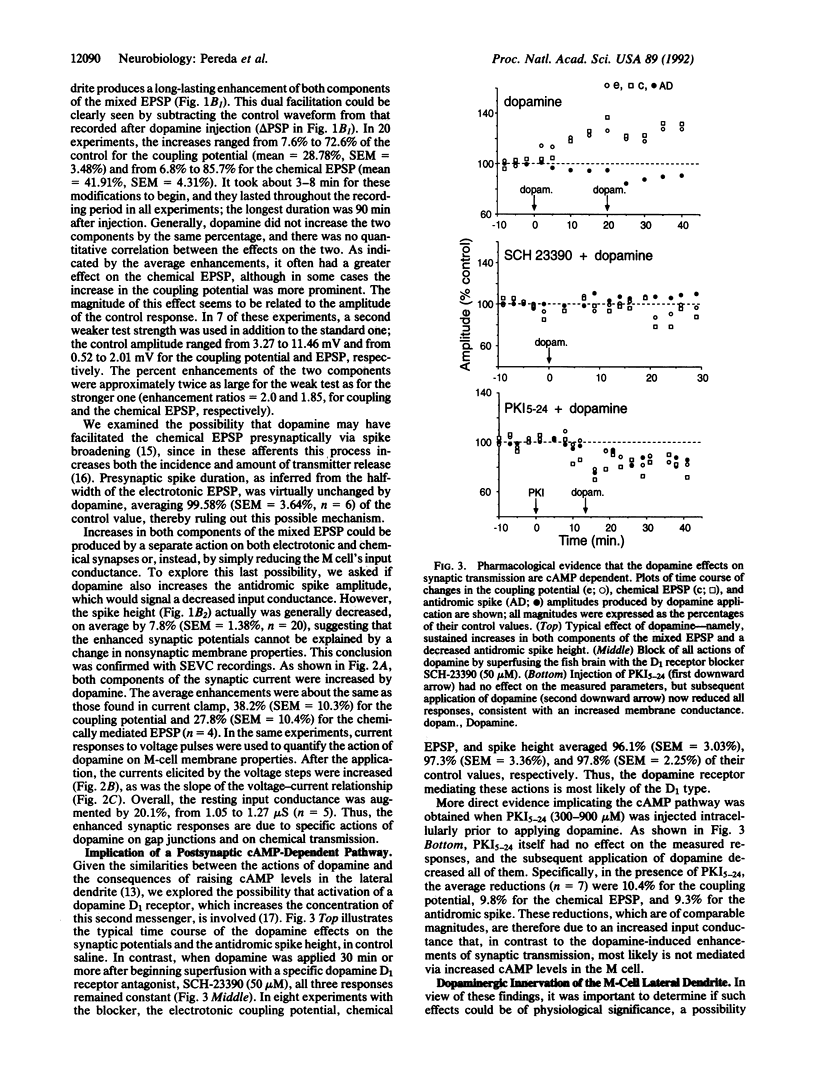
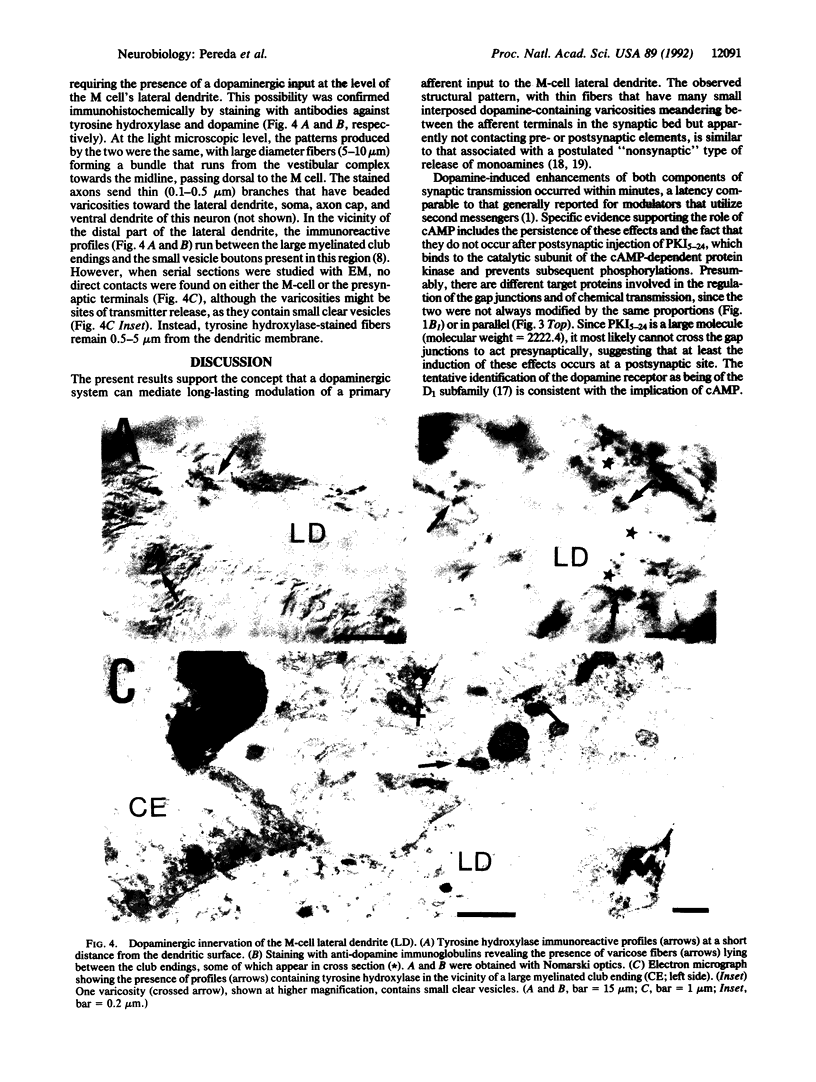
The transmitter dopamine reduces electrotonic coupling between retinal horizontal cells and increases their sensitivity to glutamate. Since in other systems single afferents establish mixed electrotonic and chemical excitatory synapses with their targets, dopamine might be expected there to depress one component of excitation while enhancing the other. This hypothesis was tested by applying dopamine locally in the vicinity of the lateral dendrite of the goldfish Mauthner cell (M cell) and monitoring the composite electrotonic and chemical excitatory postsynaptic potentials and currents evoked by ipsilateral eighth nerve stimulation. Dopamine produces persistent enhancements of both components of the postsynaptic response while it also increases input conductance. All these dopamine actions are prevented by superfusing the brain with saline containing the dopamine D1 receptor antagonist SCH-23390. Postsynaptic injections of the cAMP-dependent protein kinase inhibitor (Walsh inhibitor, or PKI5-24) block the dopamine-induced changes in synaptic transmission, implicating a cAMP-dependent mechanism. Furthermore, there is a dopaminergic innervation of the M cell, as demonstrated immunohistochemically with antibodies against dopamine and the rate-limiting enzyme in its synthetic pathway, tyrosine hydroxylase. Varicose immunoreactive fibers lie in the vicinity of the distal part of the lateral dendrite between the large myelinated club endings that establish the mixed synapses. As determined with electron microscopy, the dopaminergic fibers contain small vesicles, and they do not have synaptic contacts with either the afferents or the M cell, remaining instead in the synaptic bed. Taken together, these results suggest that dopamine released at a distance from these terminals increases the gain of this primary sensory input to the M cell, most likely through a phosphorylation mechanism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Burt J. M., Spray D. C. Inotropic agents modulate gap junctional conductance between cardiac myocytes. Am J Physiol. 1988 Jun;254(6 Pt 2):H1206–H1210. doi: 10.1152/ajpheart.1988.254.6.H1206. [DOI] [PubMed] [Google Scholar]
- Descarries L., Beaudet A., Watkins K. C. Serotonin nerve terminals in adult rat neocortex. Brain Res. 1975 Dec 26;100(3):563–588. doi: 10.1016/0006-8993(75)90158-4. [DOI] [PubMed] [Google Scholar]
- Frey U., Schroeder H., Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990 Jul 2;522(1):69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Greengard P., Jen J., Nairn A. C., Stevens C. F. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991 Sep 6;253(5024):1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- Jensen R. J. Effects of the dopamine antagonist (+)-SCH 23390 on intracellularly recorded responses of ganglion cells in the rabbit retina. Vis Neurosci. 1992 May;8(5):463–467. doi: 10.1017/s095252380000496x. [DOI] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp A. G., Dowling J. E. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. 1987 Jan 29-Feb 4Nature. 325(6103):437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Knapp A. G., Schmidt K. F., Dowling J. E. Dopamine modulates the kinetics of ion channels gated by excitatory amino acids in retinal horizontal cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):767–771. doi: 10.1073/pnas.87.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E. R., Knapp A. G., Dowling J. E. Enhancement of kainate-gated currents in retinal horizontal cells by cyclic AMP-dependent protein kinase. Brain Res. 1989 Mar 6;481(2):399–402. doi: 10.1016/0006-8993(89)90822-6. [DOI] [PubMed] [Google Scholar]
- Lin J. W., Faber D. S. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. I. Characteristics of electrotonic and chemical postsynaptic potentials. J Neurosci. 1988 Apr;8(4):1302–1312. doi: 10.1523/JNEUROSCI.08-04-01302.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. W., Faber D. S. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. II. Plasticity of excitatory postsynaptic potentials. J Neurosci. 1988 Apr;8(4):1313–1325. doi: 10.1523/JNEUROSCI.08-04-01313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y. Fine structure of the synaptic endings on the Mauthner cell of the goldfish. J Comp Neurol. 1974 Aug 15;156(4):379–402. [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvey J. M., Burgard E. C., Decker G. Long-term potentiation: studies in the hippocampal slice. J Neurosci Methods. 1989 May;28(1-2):109–124. doi: 10.1016/0165-0270(89)90016-2. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Monsma F. J., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992 Feb;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983 Jan 20;301(5897):243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- Tuttle R., Masuko S., Nakajima Y. Freeze-fracture study of the large myelinated club ending synapse on the goldfish Mauthner cell: special reference to the quantitative analysis of gap junctions. J Comp Neurol. 1986 Apr 8;246(2):202–211. doi: 10.1002/cne.902460206. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Salter M. W., MacDonald J. F. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991 Sep 6;253(5024):1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Wolszon L. R., Faber D. S. The effects of postsynaptic levels of cyclic AMP on excitatory and inhibitory responses of an identified central neuron. J Neurosci. 1989 Mar;9(3):784–797. doi: 10.1523/JNEUROSCI.09-03-00784.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. O., Chang J. P., Peter R. E. Dopamine stimulates growth hormone release from the pituitary of goldfish, Carassius auratus, through the dopamine D1 receptors. Endocrinology. 1992 Mar;130(3):1201–1210. doi: 10.1210/endo.130.3.1347006. [DOI] [PubMed] [Google Scholar]
- Yang X. D., Faber D. S. Initial synaptic efficacy influences induction and expression of long-term changes in transmission. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4299–4303. doi: 10.1073/pnas.88.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. D., Korn H., Faber D. S. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990 Dec 6;348(6301):542–545. doi: 10.1038/348542a0. [DOI] [PubMed] [Google Scholar]




