Abstract
Background
We developed a prediction tool for recurrence and survival in patients with stage IV colorectal cancer (CRC) following surgically curative resection.
Patients and methods
From January 1983 to December 2012, 113 patients with CRC and synchronous liver and/or lung metastatic CRC were investigated at the Osaka Medical Center for Cancer and Cardiovascular Diseases. All patients underwent curative resection of primary and metastatic lesions. In the group of patients who underwent surgery from 1983 to 2008, a Cox regression model was used to develop prediction models for 1-year, 3-year and 5-year cancer-specific survival (CSS) and relapse-free survival (RFS). In the other group of patients who underwent surgery from 2009 to 2012, the developed prediction model was validated.
Results
Univariate analysis of clinicopathological factors showed that the following factors were significantly correlated with CSS and RFS: preoperative serum carcinoembryonic antigen level, tumour location, pathologically defined tumour invasion and lymph node metastasis, and synchronous metastatic lesions. Using these variables, novel prediction models predicting CSS and RFS were constructed using the Cox regression model with concordance indexes of 0.802 for CSS and 0.631 for RFS. The prediction models were validated by external data sets in an independent patient group.
Conclusions
We developed novel and reliable personalised prognostic models, integrating tumour, node, metastasis (TNM) factors as well as the preoperative serum carcinoembryonic antigen level, tumour location and metastatic lesions, to predict patients' prognosis following surgically curative resection. This individualised prediction model may help clinicians in the treatment of postoperative stage IV CRC following surgically curative resection.
Keywords: prediction tool, colorectal cancer, distant metastasis, overall survival, relapse-free survival
Key questions.
What is already known about this subject?
Many cases of metastatic colorectal cancer (CRC) are difficult to cure. Some previous studies have found that a subset of liver-isolated or lung-isolated metastatic CRC is potentially curable with surgery. Although we would like to know the provability of the relapse and cancer death, there is no reliable prediction tool.
What does this study add?
We developed novel reliable prediction models for stage IV CRC after surgically curative resection of primary and metastatic lesions. It was constructed with concordance indexes of 0.802 and 0.631 for cancer-specific survival and relapse-free survival after curative surgical resection, respectively. This is the first report representing the prediction models for stage IV CRC after concurrent curative surgical resection.
How might this impact on clinical practice?
The models we have generated will provide a valuable tool to help physicians manage patients with stage IV CRC after curative surgical resection. In addition to the recent advances in chemotherapy treatment, this model will contribute to selecting high-risk patients with stage IV CRC who will require postoperative treatments following curative surgical resection, resulting in better outcomes.
Introduction
In developed countries with increasingly ageing populations, cancer is one of the most prominent illnesses in terms of public welfare and health measures. Colorectal cancer (CRC) is a frequent malignancy and one of the leading causes of cancer-related death.1 Approximately one in five patients with CRC has a distant metastatic disease at the time of presentation. Metastatic dissemination of the primary tumour is directly related to patient survival, and distant metastases are a major cause of death in patients with CRC. Systemic chemotherapy is the standard approach to treat metastatic CRC, and the past decade has shown remarkable progress in therapies for CRC. Many new drugs are currently in use for metastatic CRC, and the average median survival duration has increased in recent years largely because of the availability of new active agents such as irinotecan, oxaliplatin, cetuximab and bevacizumab.2–4 Although many cases of metastatic CRC are difficult to cure, previous studies have found that a subset of liver-isolated or lung-isolated metastatic CRC is potentially curable with surgery.5 6 The role of synchronous curative resection for CRC with lung and/or liver metastases has been restricted. Previous studies on this treatment were retrospective and had small sample sizes with short-term follow-up periods.6 7 We also reported clinicopathological characteristics of stage IV CRC.8 However, it is difficult to determine the benefits of concurrent curative resection for primary and metastatic sites, and predict the prognosis.
Development of a prognostic prediction model is important to determine the necessity of intensive follow-up and adjuvant therapy. By predicting recurrence and metastasis, such a model could lead to adequate treatment of CRC after curative surgical resection.9 10 The tumour, node, metastasis (TNM) staging system of the International Union Against Cancer (Union for International Cancer Control; UICC) is a reliable prognostic system for patients with CRC of all stages.11 However, even TNM staging does not consolidate demographic features, tumour characteristics and other histopathological features to predict recurrence and survival. Therefore, development of a model for the prediction of individual outcomes would be a useful tool in this age of personalised medicine.
To develop such a model for the prediction of cancer relapse and survival, we constructed a prediction tool for patients with stage IV CRC after concurrent surgically curative resection of primary and metastatic lesions. Development of the tool is based on a statistically calculated formula constructed from potential prognostic factors and thus provides a prediction probability for individual outcomes that will benefit patients in selecting treatment choices after having undergone surgically curative resection.
Patients and methods
One hundred and thirteen patients were identified as having a diagnosis of stage IV CRC with liver and/or lung metastases at Osaka Medical Center for Cancer and Cardiovascular Diseases from 1983 to 2012. All patients had histologically confirmed CRC with distant metastasis and underwent curative resection for primary and metastatic lesions. This study was approved by the Institutional Review Board of Osaka Medical Center for Cancer and Cardiovascular Diseases. The patient records were anonymised prior to the analysis.
Surgical specimens were fixed in formalin, processed through a graded ethanol series, and embedded in paraffin. The sections were stained with H&E and Elastica van Gieson stain, and the histological grade, degree of lymphatic invasion and degree of venous invasion were examined. Data on age, sex, the serum level of the tumour marker carcinoembryonic antigen (CEA), primary tumour site (rectum or colon), pathological finding (histological grade, tumour invasiveness, lymph node metastases, lymphatic invasion and venous invasion) and perioperative chemotherapy were retrieved from the patients' medical records for evaluation. Preoperative determination of the extent of tumour spread was performed using X-ray, CT, MRI and/or positron emission tomography. Intraoperative findings contributed to the determination of metastatic tumour involvement. After surgery, all patients underwent follow-up blood examinations to assess the serum levels of the tumour markers CEA and carbohydrate antigen 19–9, and further imaging with abdominal ultrasonography, CT, chest X-ray and/or positron emission tomography every 3–6 months following the Japanese guidelines.12 Postoperatively, patients received chemotherapy (oxaliplatin, irinotecan and 5-fluorouracil-based drugs) following informed consent. Clinicopathological factors were assessed according to the TNM classification of the UICC.11 The primary and secondary end points of the study were the cancer-specific survival (CSS) time and relapse-free survival (RFS).
Kaplan-Meier survival curves were plotted and compared with the generalised log-rank test. Univariate analysis was performed using a proportional hazards model for RFS and CSS after primary curative resection to identify independent factors. Two-sided p values of <0.1 were considered statistically significant.
In 83 patients who underwent concurrent curative operations (R0) from January 1983 to December 2008, a Cox proportional hazards regression model for primary and metastatic sites was used as a learning set to develop the prediction model for CSS and RFS. As a validation set, an independent group of 30 patients who underwent curative operations for CRC from January 2009 to December 2012 was used to validate the prediction model. Each prediction model was validated by the following two procedures: internal validation using the study patients from which the model was developed, and external validation using these independent validation patients. Harrell et al's13 concordance index was calculated for each prediction model as a nomogram. All statistical analyses were performed using R 3.1.2.
Results
The characteristics of all 113 patients are listed in table 1. The patients' ages ranged from 16 to 81 years, and 69 patients (61.1%) were male. Primary tumours were located in the rectum (40 patients, 35.4%) or colon (73 patients, 64.6%). The most common site of metastases at presentation was the liver (97 patients, 85.8%), followed by the lung (11 patients, 9.7%). The median number of liver or lung metastatic sites was 2 (range 1–7) and 1 (range 1–3), respectively.
Table 1.
Clinicopathological factors in 113 patients with stage IV colorectal cancer
| Factors | N=113 |
|---|---|
| Age (years) | 62 (16–81) |
| Sex | |
| Male | 69 (61.1) |
| Female | 44 (38.9) |
| Primary colorectal tumour | |
| Rectum group | 40 (35.4) |
| Rectosigmoid | 11 |
| Upper rectum | 17 |
| Lower rectum | 11 |
| Anal canal | 1 |
| Colon group | 73 (64.6) |
| Caecum | 8 |
| Ascending | 18 |
| Transverse | 10 |
| Descending | 4 |
| Sigmoid | 33 |
| Histological grade | |
| Well | 26 (23.0) |
| Moderate | 84 (74.3) |
| Others | 3 (2.7) |
| Tumour invasion | |
| T3 | 75 (66.4) |
| T4a | 33 (29.2) |
| T4b | 5 (4.4) |
| Lymph node metastasis | |
| N0 | 35 (31.0) |
| N1 | 34 (30.1) |
| N2a | 30 (26.5) |
| N2b | 14 (12.4) |
| Lymphatic invasion | |
| Absent | 5 (6.2) |
| Present | 106 (93.8) |
| NA | 2 (0.0) |
| Venous invasion | |
| Absent | 13 (11.5) |
| Present | 98 (86.7) |
| NA | 2 (1.8) |
| Synchronous liver metastases | 97 |
| Solitary | 47 |
| ≥2 | 50 |
| Synchronous lung metastases | 11 |
| Solitary | 6 |
| ≥2 | 5 |
| Synchronous liver and lung metastases | 7 |
Data are presented as mean±SD, n (%), or n.
Well: well-differentiated adenocarcinoma; moderate: moderately differentiated adenocarcinoma; others: poorly differentiated, mucinous adenocarcinoma, or squamous cell carcinoma.
NA, not available.
The cohort of 83 patients in the learning set was examined to develop prediction models for CSS and RFS. The median CSS and RFS were 4.27 and 1.21 years, respectively (figures 1 and 2). The median follow-up time was 3.34 years (range 0.39–21.73 years). After concurrent surgically curative resection of both primary and metastatic lesions, 29 patients (25.7%) had no recurrence (median follow-up time, 5.53 years; range 2.17–21.73 years).
Figure 1.
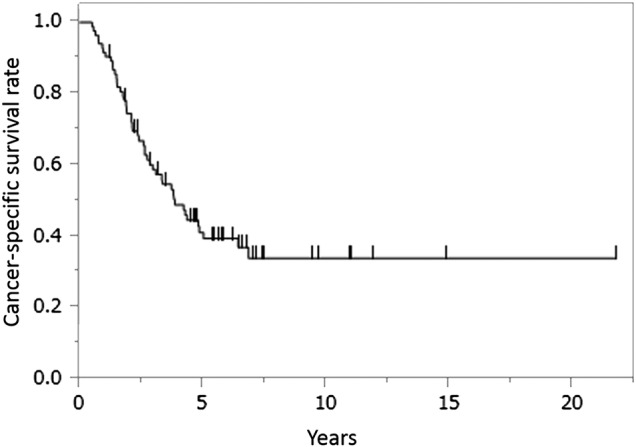
Cancer-specific survival curve after surgically curative resection for stage IV colorectal cancer (CRC). Kaplan-Meier plots show the cancer-specific survival curve after curative surgical resection in 113 patients with stage IV CRC.
Figure 2.

Relapse-free survival curve after surgically curative resection for stage IV colorectal cancer (CRC). Kaplan-Meier plots show the relapse-free survival curve after curative surgical resection in 113 patients with stage IV CRC.
Table 2 provides the results of the univariate analysis of factors related to CSS. The preoperative serum CEA level (>40 ng/mL, HR=2.750, p=0.002), tumour location (upper and lower rectum and anal canal, HR=2.524, p=0.003), pathologically defined tumour invasion (T4, HR=2.390, p=0.004) and lymph node metastasis (N2, HR=2.089, p=0.013), and synchronous metastasis (liver and lung, HR=3.315, p=0.031) were significantly correlated with CSS.
Table 2.
Univariate analysis for cancer-specific survival (Cox proportional hazards regression model)
| Factors | HR | 95% CI | p Value |
|---|---|---|---|
| Age (<61/≥62) | 1.106 | 0.621 to 1.952 | 0.728 |
| Sex (male/female) | 1.230 | 0.689 to 2.273 | 0.489 |
| Primary colorectal tumour (rectum/others) | 2.524 | 1.373 to 4.554 | 0.003 |
| CEA level (>40/≤40) | 2.750 | 1.457 to 5.001 | 0.002 |
| Tumour invasion (T4a–b/T3) | 2.390 | 1.338 to 4.251 | 0.004 |
| Lymph node metastasis (N2/N0–1) | 2.089 | 1.172 to 3.692 | 0.013 |
| Lymphatic invasion (present/absent) | 0.879 | 0.419 to 2.149 | 0.758 |
| Venous invasion (present/absent) | 1.290 | 0.522 to 4.290 | 0.614 |
| Synchronous liver and lung metastases (both/alternative) | 3.315 | 1.133 to 7.778 | 0.031 |
Underlined values indicate p values of <0.1.
Rectum includes upper and lower rectum and anal canal.
CEA, carcinoembryonic antigen.
Table 3 provides the results of the univariate analysis of clinicopathological factors related to RFS. In the univariate analysis, preoperative serum CEA level (>40 ng/mL, HR=1.824, p=0.032), pathologically defined lymph node metastasis according to the TNM classification (N2, HR=1.753, p=0.032) and synchronous metastasis (both liver and lung/lung, HR=4.332, p=0.020; liver/lung, HR=2.010, p=0.034) were significantly correlated with RFS.
Table 3.
Univariate analysis for relapse-free survival (Cox proportional hazards regression model)
| Factors | HR | 95% CI | p Value |
|---|---|---|---|
| Age (<61/≥62 years) | 1.125 | 0.677 to 1.854 | 0.645 |
| Sex (male/female) | 1.073 | 0.641 to 1.772 | 0.786 |
| Primary colorectal tumour (rectum/others) | 1.237 | 0.704 to 2.094 | 0.448 |
| CEA level (≥40/≤40) | 1.824 | 1.054 to 3.042 | 0.032 |
| Tumour invasion (T4a–b/T3) | 1.229 | 0.728 to 2.035 | 0.433 |
| Lymph node metastasis (N2/N0–1) | 1.753 | 1.051 to 2.892 | 0.032 |
| Lymphatic invasion (present/absent) | 0.718 | 0.371 to 1.564 | 0.380 |
| Venous invasion (present/absent) | 1.025 | 0.452 to 2.943 | 0.958 |
| Synchronous metastasis (both/lung) (liver/lung) |
4.332 2.010 |
1.284 to 13.148 1.049 to 4.353 |
0.020 0.034 |
Underlined values indicate p values of <0.1.
Rectum includes upper and lower rectum and anal canal.
Both: synchronous liver and lung metastases.
CEA, carcinoembryonic antigen.
Of the 113 patients, 88 (77.9%) received adjuvant chemotherapy (oxaliplatin-based, irinotecan-based and/or 5-fluorouracil-based drugs) after curative resection. In this study, the chemotherapy choice was not significantly related to patient outcome (table 4).
Table 4.
Univariate analysis for cancer-specific survival regarding chemotherapy after concurrent surgical resection (Cox proportional hazards regression model)
| Chemotherapy | HR | 95% CI | p Value |
|---|---|---|---|
| Adjuvant chemotherapy (any/none) | 1.093 | 0.585 to 2.229 | 0.790 |
Underlined values indicate statistical significance.
Any chemotherapies include oxaliplatin, irinotecan and 5-fluorouracil based drugs.
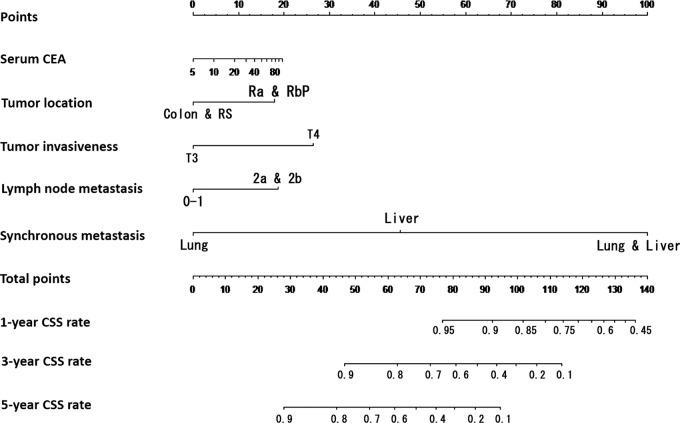
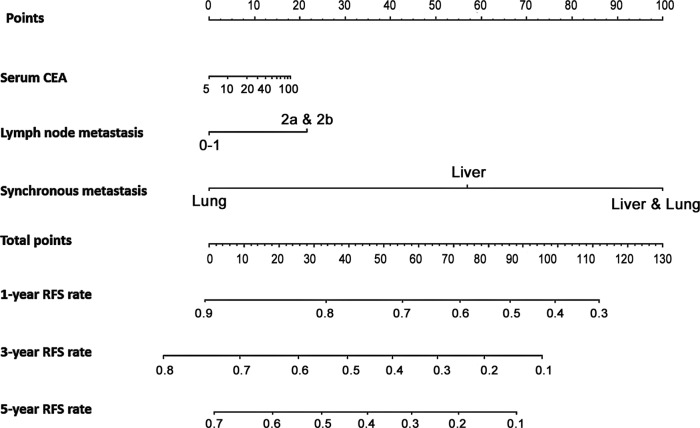
These statistically significant clinicopathological factors were used to develop prediction models for 1-year, 3-year and 5-year CSS and RFS after concurrent surgical resection of both primary and metastatic lesions. Nomograms of 1-year, 3-year and 5-year CSS and RFS were developed (figures 3 and 4). The events in the prediction models depended on identification of cancer-related death and recurrence, respectively.
Figure 3.
Nomogram to predict cancer-specific survival (CSS) after curative surgical resection for stage IV colorectal cancer. The prediction model of 1-year, 3-year and 5-year CSS after concurrent surgical resection of both primary and metastatic lesions was constructed using the Cox regression model. Clinicopathological factors used were the preoperative serum carcinoembryonic antigen (CEA) level, tumour location, pathologically defined tumour invasion and lymph node metastasis, and synchronous metastatic lesions.
Figure 4.
Nomogram to predict relapse-free survival (RFS) after curative surgical resection for stage IV colorectal cancer. The prediction model of 1-year, 3-year and 5-year RFS after concurrent surgical resection of both primary and metastatic lesions was constructed using the Cox regression model. Clinicopathological factors used were the preoperative serum carcinoembryonic antigen (CEA) level, pathologically defined lymph node metastasis and synchronous metastatic lesions.
Factors associated with prediction of CSS included preoperative serum CEA level, tumour location, pathologically defined tumour invasion and lymph node metastasis, and synchronous metastatic lesions. Factors associated with prediction of RFS included preoperative serum CEA level, pathologically defined lymph node metastasis and synchronous metastatic lesions. The predictive performance was evaluated by measuring the calibration comparing the prediction probability with the CSS and RFS after curative surgical resection. The prediction models were validated using the external data set as an independent patient group (n=30). The concordance indexes (C-indexes) for the CSS and RFS after concurrent surgical resection were 0.802 and 0.631, respectively. The C-indexes in the external validation were 0.702 and 0.599.
Discussion
CRC with distant metastases has a poor prognosis, although several recent chemotherapeutic advances have improved the overall outcomes of advanced metastatic CRC.14–17 Patients with localised metastases, such as liver or lung metastases, can achieve long-term survival through curative resection of the metastatic lesions.10 14 As such, models to predict the prognosis after curative surgical resection would be useful to determine the necessity of intensive follow-up to select adjuvant therapy. In the present study, clinicopathological analysis revealed that primary tumour location, preoperative serum CEA level, pathologically defined T and N factors according to the TNM classification of the UICC, and synchronous metastatic lesions were associated with a poor prognosis for CSS. In terms of location, tumours located in the lower rectum and anal canal showed a worse prognosis than those located in other locations.
Prevention of relapse is essential in CRC treatment following curative surgical resection. Although 5-fluorouracil was the only effective chemotherapeutic drug for treatment until the mid-1990s, active agents such as irinotecan, oxaliplatin, cetuximab and bevacizumab have been used for treatment of distant-metastatic CRC.3 4 18–20 A significant benefit of adjuvant chemotherapy has been demonstrated, and its administration has since become standard for stage III and high-risk stage II CRC.21–23 Although the benefit after curative surgical resection for stage IV CRC remains to be evaluated in several ongoing clinical trials, adjuvant chemotherapy would be promising for CRC that is highly suspicious for relapse after curative surgical resection.
One prognostic model reportedly integrated demographic and clinicopathological factors to provide for postoperative treatment in patients with rectal cancer.24 In this study, our novel and reliable personalised prognostic models integrated TNM factors, as well as the preoperative serum CEA level, tumour location and synchronous metastatic lesions to predict RFS and CSS of postoperative patients with stage IV CRC.
In this study, patients with stage IV CRC who underwent curative surgical resection of both primary and metastatic lesions were investigated to develop prediction models regarding CSS and RFS. Although there are limitations, such as the fact that perioperative treatments have changed over the past two decades and that a limited number of patients were included in a retrospective analysis, we believe that the models we have generated will provide a valuable tool to help physicians manage patients with stage IV CRC after curative surgical resection. In addition to the recent advances in chemotherapy treatment, this model will contribute to selecting high-risk patients with stage IV CRC who will require postoperative treatments following curative surgical resection, resulting in better outcomes.
In summary, surgical resection provides a potentially curative option even for selected patients with stage IV CRC with liver or lung metastasis. If the synchronous metastases are determined to be potentially resectable and the patient's performance status is good, surgical resection of the primary and metastatic lesions would be a good treatment option.
Conclusions
We have developed novel prognostic prediction models using multiple clinicopathological factors beyond TNM factors to provide individualised prognostic outcomes in patients with stage IV CRC after curative surgical resection. This model should help clinicians counsel patients on personalised treatments and follow-up.
Acknowledgments
The authors would like to thank Ms A. Ito for special technical assistance regarding sample preparation.
Footnotes
Contributors: NM and MO collected the data. NM, MO, MY, SN, TS, SM, HA, KG, KS, HT, JO, YF, MH and MY provided materials such as clinical information, database, software, surgical instruments, etc. NM and MO designed the study and analysed the data. NM and MO wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Funding: This work was supported in part by grants from the Uehara Foundation and Takeda Science.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Institutional Review Board of Osaka Medical Center for Cancer and Cardiovascular Diseases.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Siegel R, Ma J, Zou Z et al. . Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Sargent D, Goldberg RM et al. . Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209–14. 10.1200/JCO.2004.11.037 [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W et al. . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 4.Sobrero AF, Maurel J, Fehrenbacher L et al. . EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311–19. 10.1200/JCO.2007.13.1193 [DOI] [PubMed] [Google Scholar]
- 5.Headrick JR, Miller DL, Nagorney DM et al. . Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg 2001;71:975–9. 10.1016/S0003-4975(00)02522-4 [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Kim TY, Lee KH et al. . The beneficial effect of palliative resection in metastatic colorectal cancer. Br J Cancer 2013;108:1425–31. 10.1038/bjc.2013.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanzaki R, Higashiyama M, Oda K et al. . Outcome of surgical resection for recurrent pulmonary metastasis from colorectal carcinoma. Am J Surg 2011;202:419–26. 10.1016/j.amjsurg.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi N, Ohue M, Shingai T et al. . Clinicopathological characteristics and prognosis of stage IV colorectal cancer. Mol Clin Oncol 2015;3:1093–8. 10.3892/mco.2015.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology 2008;134:1296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornmann M, Formentini A, Ette C et al. . Prognostic factors influencing the survival of patients with colon cancer receiving adjuvant 5-FU treatment. Eur J Surg Oncol 2008;34:1316–21. 10.1016/j.ejso.2008.01.019 [DOI] [PubMed] [Google Scholar]
- 11.Sobin LH, Gospodarowicz MK. TNM classification of malignant tumors, seventh edition (2009). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Oxford: Wiley-Blackwell; 2009:100–9. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Itabashi M, Shimada Y et al. . Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012;17:1–29. 10.1007/s10147-011-0315-2 [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE Jr, Califf RM, Pryor DB et al. . Evaluating the yield of medical tests. JAMA 1982;247:2543–6. 10.1001/jama.1982.03320430047030 [DOI] [PubMed] [Google Scholar]
- 14.Bathe OF, Dowden S, Sutherland F et al. . Phase II study of neoadjuvant 5-FU+leucovorin+CPT-11 in patients with resectable liver metastases from colorectal adenocarcinoma. BMC Cancer 2004;4:32 10.1186/1471-2407-4-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koshariya M, Jagad RB, Kawamoto J et al. . An update and our experience with metastatic liver disease. Hepatogastroenterology 2007;54:2232–9. [PubMed] [Google Scholar]
- 16.Schwartzberg LS, Rivera F, Karthaus M et al. . PEAK: A Randomized, Multicenter Phase II Study of Panitumumab Plus Modified Fluorouracil, Leucovorin, and Oxaliplatin (mFOLFOX6) or Bevacizumab Plus mFOLFOX6 in Patients With Previously Untreated, Unresectable, Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer. J Clin Oncol 2014;32:2240–7. 10.1200/JCO.2013.53.2473 [DOI] [PubMed] [Google Scholar]
- 17.Price TJ, Peeters M, Kim TW et al. . Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014;15:569–79. [DOI] [PubMed] [Google Scholar]
- 18.Jin K, Lan H, Cao F et al. . Differential response to EGFR- and VEGF-targeted therapies in patient-derived tumor tissue xenograft models of colon carcinoma and related metastases. Int J Oncol 2012;41:583–8. 10.3892/ijo.2012.1469 [DOI] [PubMed] [Google Scholar]
- 19.McKay JA, Lloret C, Murray GI et al. . Application of the enrichment approach to identify putative markers of response to 5-fluorouracil therapy in advanced colorectal carcinomas. Int J Oncol 2000;17:153–8. [DOI] [PubMed] [Google Scholar]
- 20.Takatsuka S, Chung Y, Yamada N et al. . Characterization and purification of angiogenic factor derived from highly liver metastatic colon cancer cells. Int J Oncol 1997;11:1035–40. [DOI] [PubMed] [Google Scholar]
- 21.Moertel CG, Fleming TR, Macdonald JS et al. . Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352–8. 10.1056/NEJM199002083220602 [DOI] [PubMed] [Google Scholar]
- 22.Wolmark N, Rockette H, Mamounas E et al. . Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 1999;17:3553–9. [DOI] [PubMed] [Google Scholar]
- 23.Haller DG, Catalano PJ, Macdonald JS et al. . Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005;23:8671–8. 10.1200/JCO.2004.00.5686 [DOI] [PubMed] [Google Scholar]
- 24.Peng J, Ding Y, Tu S et al. . Prognostic nomograms for predicting survival and distant metastases in locally advanced rectal cancers. PLoS ONE 2014;9:e106344 10.1371/journal.pone.0106344 [DOI] [PMC free article] [PubMed] [Google Scholar]