Abstract
Aim:
Platelet function is intricately linked to the pathophysiology of critical Illness, and some studies have shown that antiplatelet therapy (APT) may decrease mortality and incidence of acute respiratory distress syndrome (ARDS) in these patients. Our objective was to understand the efficacy of APT by conducting a meta-analysis.
Materials and Methods:
We conducted a meta-analysis using PubMed, Central, Embase, The Cochrane Central Register, the ClinicalTrials.gov Website, and Google Scholar. Studies were included if they investigated critically ill patients receiving antiplatelet therapy and mentioned the outcomes being studied (mortality, duration of hospitalization, ARDS, and need for mechanical ventilation).
Results:
We found that there was a significant reduction in all-cause mortality in patients on APT compared to control (odds ratio [OR]: 0.83; 95% confidence interval [CI]: 0.70–0.97). Both the incidence of acute lung injury/ARDS (OR: 0.67; 95% CI: 0.57–0.78) and need for mechanical ventilation (OR: 0.74; 95% CI: 0.60–0.91) were lower in the antiplatelet group. No significant difference in duration of hospitalization was observed between the two groups (standardized mean difference: −0.02; 95% CI: −0.11–0.07).
Conclusion:
Our meta-analysis suggests that critically ill patients who are on APT have an improved survival, decreased incidence of ARDS, and decreased need for mechanical ventilation.
Keywords: Acute respiratory distress syndrome, Antiplatelet therapy, Critical illness, Meta-analysis, Sepsis
INTRODUCTION
Despite recent advances in the treatment, the burden of sepsis and septic shock remains high, with an incidence between 11 and 240 per 100,000 population, hospital costs of more than $20 billion annually in the United States, and a mortality as high as 80%.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] Among patients admitted to the Intensive Care Unit (ICU), acute respiratory distress syndrome (ARDS) is a leading cause of increased mortality and long-term reduction in quality of life.[16,17,18] Despite the great burden of sepsis and ARDS, very few effective strategies are available for treatment.[16,17,18,19]
Platelets are a vital component of normal hemostasis as well as pathological thrombosis. Antiplatelet therapy (APT) works by interfering with one of the several steps of platelet activation including adhesion, release, and/or aggregation.[20] While the benefit of APT is well established for primary and secondary prevention of cardiovascular and cerebrovascular diseases,[21] recent studies have revealed that these agents may also benefit patients with serious infections, sepsis, and those who are admitted to the ICU.[22,23,24,25,26,27,28,29,30,31,32,33,34,35]
Platelets play an important role in the inflammatory cascade that results in the development of ARDS.[36,37,38] Observational studies suggest that prehospital use of APT may be protective against the development of ARDS. In addition, data also suggests that these agents may be of benefit in patients with existing ARDS.[22]
In light of this data, potential use of APT in reducing length of stay and mortality in these patients carries significant weight and has global health and economic ramifications. Given the possible benefit of antiplatelet agents as described in observational data, we aimed to perform a systematic review of literature and meta-analysis to further study the efficacy of these agents in critically ill patients.
MATERIALS AND METHODS
Search strategy
A computerized literature search of all publications in the PubMed, Central, Embase, the Cochrane database, ClinicalTrials.gov website, and Google Scholar databases was performed. We also utilized manual searches for article reference lists and conference proceedings. This was last assessed as up-to-date on March 1, 2016.
Search terms included varying combinations of the following: “ICU,” “critical illness,” “sepsis,” “septic shock,” “ARDS,” “pneumonia,” “infection,” “mechanical ventilation,” “antiplatelet drugs,” “aspirin,” “clopidogrel,” “prasugrel,” “ticlopidine,” “cilostazol,” “dipyridamole,” “tirofiban,” eptifibatide,” “abciximab,” “anagrelide,” “ticagrelor,” “vorapaxar,” “atopaxar,” and “Pentoxifylline.”
Inclusion criteria
The PRISMA statement of reporting systematic reviews and meta-analysis was applied to the methods for this study [Supplementary Table 1].[40]
Supplementary Table 1.
PRISMA 2009 checklist
| Section/topic | Number | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including as applicable: Background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to PICOS | 4 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., web address), and, if available, provide registration information including registration number | - |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 4,5 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 4 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 4 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 4,5 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 4,5 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumption and simplifications made | 4,5 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 6 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 6 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 6 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 6 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were prespecified | 6 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 7 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | 7 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (Item 12) | 7 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | Figures 1–4 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | 7,8 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (Item 15) | 7,8 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [Item 16]) | 7,8 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policymakers) | 9 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 12 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 12 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 13 |
PICOS: Participants, interventions, comparisons, outcomes, and study design
The following inclusion criteria were used:
Studies on critically ill patients including: Studies with adult patients admitted to the ICU or postoperative patients or patients admitted to the hospital with serious infections/sepsis/systemic inflammatory response syndrome (SIRS)/septic shock
Studies where one or more antiplatelet agents were used: Irreversible COX inhibitor (aspirin); adenosine diphosphate inhibitors (clopidogrel, prasugrel, ticlopidine, and ticagrelor); phosphodiesterase inhibitors (cilostazol, anagrelide, Pentoxifylline); adenosine reuptake inhibitors (dipyridamole); glycoprotein IIb/IIIa inhibitors (tirofiban, eptifibatide, and abciximab); and protease activated receptor-1 antagonist (atopaxar, vorapaxar).
The following exclusion criteria were applied to the search:
Studies with nonhuman participants
Studies which did not have a direct comparison between antiplatelet users and nonantiplatelet users
Studies which did not report one or more of the end-points for this meta-analysis (mortality, ARDS, length of hospital stay, and need for mechanical ventilation)
Studies where the drug being studied was not an antiplatelet agent; for example, studies on only nonsteroidal anti-inflammatory drugs (NSAID), antithrombotic agents, and statins. Two reviewers (DM and JS) independently extracted the data from identified studies.
Disagreements were resolved by consensus, or if necessary, by a third party (MA). An attempt was made to obtain data from authors of all ongoing studies which met the search criteria.
Study end-points
The primary outcome of this analysis was all-cause mortality. Secondary outcomes included incidence of acute lung injury (ALI) or ARDS, length of hospital stay, and need for mechanical ventilation. Individual study definitions for ALI, ARDS, and sepsis were used for this meta-analysis [Table 1].
Table 1.
Description of included studies
| Author(s) | Title | Year of publication | Type of study | Number of patients | Inclusion criteria | Primary outcome | Type of antiplatelet drug used | Severity scoring | Timing of APT | Duration of maximum follow-up | Definition of ALI/ARDS | Definition of sepsis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Winning et al.[35] | Antiplatelet drugs and outcome in severe infection: Clinical impact and underlying mechanisms | 2009 | Observational | 224 | Patients who were admitted to the hospital for CAP | Need for treatment in ICU Length of hospital stay | Aspirin, clopidogrel | SOFA 2.95±2.03 (control) versus 2.74±1.18 (APT) | Patients on APT for at least 6 months before admission | 28 days | ||
| Winning et al.[34] | Antiplatelet drugs and outcome in mixed admissions to an ICU | 2010 | Observational | 615 | Patients admitted to the ICU within 24 h after arrival to the hospital | Death during ICU treatment or discharge from ICU | Aspirin, clopidogrel | APACHE II: 19 (13-19) (control) versus 25 (19-32) (APT) | Patients on APT before admission | Duration of ICU stay | ||
| Erlich et al.[25] | Prehospitalization APT is associated with a reduced incidence of ALI | 2011 | Observational | 161 | Age>18 years+at least one risk factor for ALI (high-risk trauma, aspiration, sepsis, shock, pneumonia, and pancreatitis) | Development of ALI or ARDS during hospitalization | Aspirin, clopidogrel, ticlopidine, cilostazol, dipyridamole, anagrelide, persantine | APACHE III: 39 (27-54) (control) versus 46 (34-57) (APT) | Patients on APT before admission | American- European Consensus Conference criteria[40] | ||
| Lösche et al.[50] | Do aspirin and other antiplatelet drugs reduce the mortality in critically ill patients? | 2012 | Observational | 224 | Patients admitted to the hospital with CAP | Length of hospital stay Admission to ICU | Aspirin, ticlopidine, clopidogrel | SOFA odds ratio 0.19 (0.04-0.87) | Patients on APT for at least 6 months before admission | Duration of hospital stay | ||
| Lösche et al.[50] | Do Aspirin and other antiplatelet drugs reduce the mortality in critically ill patients? | 2012 | Observational | 834 | ICU admission for severe sepsis or septic shock | ICU mortality | Aspirin | APACHE II: 22.6±9.2 (control) versus 24.1±8.3 (APT) | Duration of ICU stay | |||
| Eisen et al.[24] | Acetylsalicylic acid usage and mortality in critically ill patients with the SIRS and sepsis | 2012 | Observational | 2890 | Patients admitted to the ICU with SIRS | Hospital mortality | Aspirin | APACHE II: 17.78 (control) versus 17.47 (APT) | Patients on APT in the 24 h period at time of detection of SIRS | America College of Chest Physicians/Society of Critical Care Medicine Consensus Conference criteria[48] | ||
| Losche et al.[29] | Association of prehospitalization aspirin therapy and ALI: results of a multicenter international observational study of at-risk patients | 2012 | Observational | 3855 | Admission to the hospital with the presence of at least one major risk factor for ALI and age>18 years.(aspiration, pneumonia, sepsis, shock, pancreatitis, high-risk trauma, or high-risk surgery) | Development of ALI/ARDS during hospitalization | Aspirin | APACHE II: 9 (5-14) (control) versus 12 (8-16) (APT) | Documentation of use or administration of APT at time of hospital admission | Duration of hospital stay | American- European Consensus Conference criteria[40] | |
| Gross et al.[27] | Clopidogrel treatment and the incidence and severity of community acquired pneumonia in a cohort study and meta-analysis of APT in pneumonia and critical illness | 2013 | Observational | 23,882 | Patients who received at least 6 consecutive prescriptions of clopidogrel | Incidence and severity of pneumonia | clopidogrel | NA | ≥6 prescription claims of APT | Duration of hospital stay | ||
| Harr et al.[28] | APT is associated with decreased transfusion- associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients | 2013 | Observational | 839 | Blunt trauma mechanism, ED arrival within 6 h of injury, ED base deficit>6 mEq/L or ED systolic blood pressure<90 mm Hg, and a blood product transfusion within the first 12 h of ED arrival | Lung dysfunction defined by Denver lung dysfunction score of 2 or 3 Denver MOF score (>3) Mortality | Aspirin, ticlopidine, clopidogrel | NA | Patients on APT before trauma | 28 days | Denver lung dysfunction score Grades 2 or 3, which corresponds to PaO2:FiO2 ratio<200 | |
| Valerio-Rojas et al.[33] | Outcomes of severe sepsis and septic shock patients on chronic antiplatelet treatment: A historical cohort study | 2013 | Observational | 651 | ≥18 years, diagnosis of severe sepsis or septic shock at the time of ICU admission and use of APT before admission | Hospital mortality | Aspirin, clopidogrel, ticlopidine, dipyridamole | APACHE III: 55 (42-68) (control) versus 57.5 (46-74.8) (APT) | Patients on APT at time of ICU admission | Duration of hospital stay | American- European Consensus Conference criteria[40] | America College of Chest Physicians/Society of Critical Care Medicine Consensus Conference criteria[48] |
| Otto et al.[31] | Effects of low-dose acetylsalicylic acid and atherosclerotic vascular diseases on the outcome in patients with severe sepsis or septic shock | 2013 | Observational | 886 | Only patients with severe sepsis/septic shock and minimum ICU stay of 48 h | ICU mortality Discharge from ICU Hospital mortality | Aspirin, clopidogrel | NA | Patient on APT for at least 2 days during ICU stay | Duration of hospital stay | ||
| Faverio et al.[26] | Antiplatelets improve survival among critically ill mechanically ventilated patients | 2014 | Observational | 150 | Critically ill mechanically ventilated patients for 1 day or more managed at a tertiary medical ICU | Mortality during ICU and hospital stay | Aspirin, clopidogrel | APACHE II>25 | Patient on prehospital or in-hospital APT | Duration of hospital stay | ||
| Chen et al.[23] | Prehospital aspirin use is associated with reduced risk of ARDS in critically ill patients: A propensity- adjusted analysis | 2015 | Observational | 1149 | Patient who are 18 years old or older admitted to the medical, surgical, cardiovascular, and trauma ICUs who remained in the ICU for at least 2 days | ARDS in first 4 days of ICU stay | Aspirin | NA | Patients on APT before admission | Duration of hospital stay | American- European Consensus Conference criteria[40] | America College of Chest Physicians/Society of Critical Care Medicine Consensus Conference criteria[48] |
| Tsai et al.[32] | Association of prior antiplatelet agents with mortality in sepsis patients: A nationwide population-based cohort study | 2015 | Observational | 683,421 | All patients with a first time discharge diagnosis of sepsis | In-hospital mortality from sepsis | Aspirin, clopidogrel, ticlopidine | NA | Patients on APT currently or within 30 days before admission | ICD 9 code for sepsis | ||
| Mazzeffi et al.[30] | Preoperative aspirin use and lung injury after aortic valve replacement surgery: A retrospective cohort study | 2015 | Observational | 375 | All adult patients having aortic valve replacement surgery with cardiopulmonary bypass | Occurrence of ARDS | Aspirin | NA | Patients receiving preoperative APT | Berlin definition[67] | ||
| Boyle et al.[22] | Aspirin therapy in patients with ARDS is associated with reduced ICU mortality: A prospective analysis | 2015 | Observational | 202 | All adult patients (>16 years-old) requiring invasive mechanical ventilation | ICU mortality | Aspirin | APACHE II: 18 (13-24) in (control) versus 21 (17-24) (APT) | Patients on prehospital APT, in ICU APT or both | Duration of hospital stay | American- European Consensus Conference criteria[40] | |
| Al Harbi et al.[48] | Association between aspirin therapy and the outcome in critically ill patients: A nested cohort study | 2016 | Observational | 763 | ≥18-year-old with expected ICU length of stay of >48 h. Patients who were pregnant, had do-not-resuscitate status within 24 h of admission, were terminally ill or admitted to the ICU after cardiac arrest, seizures, liver transplantation, or burn injury were excluded | All cause ICU mortality and in-hospital mortality | Aspirin | APACHE II: 22.9±8.2 (control) versus 26.5±7.2 (APT) | Patients who had either continuation of a pre-ICU prescription or a newly prescribed APT in the ICU | Duration of ICU stay |
CAP: Community-acquired pneumonia, SIRS: Systemic inflammatory response syndrome, APACHE: Acute Physiology and Chronic Health Evaluation, SOFA: Sequential Organ Failure Assessment, APT: Antiplatelet therapy, NA: Not available, ICU: Intensive Care Unit, ALI: Acute lung injury, ARDS: Acute respiratory distress syndrome, ED: Emergency department
In studies where multiple follow-up periods were available, the longest follow-up was included in the analysis.
Study analysis
Data were summarized across treatment arms using the Mantel-Haenszel odds ratio (OR) or standardized mean difference (SMD). We evaluated heterogeneity of effects using the Higgins’ I2 statistic. Fixed effects model was used except in cases where heterogeneity was significant (defined as I2 >40%). In these cases, random effects models were used.[42]
To address publication bias, we used four methods: Funnel plots,[43] Begg and Mazumdar test,[44] Egger test,[45] and Duval and Tweedie's test.[46] Sensitivity analyses were performed using the one-study out method, addressing the influence of each study by testing whether deleting each individually would significantly change the pooled results of the meta-analysis on the final effect and its precision. We also carried out a sensitivity analysis by comparing studies on aspirin with studies where patients were on APT other than aspirin, either alone or in combination with aspirin. Finally, chronological cumulative analyses were used to test if the effect size and precision shifts based on technical advancement in critical care medicine. The statistical analysis was performed by the Comprehensive Meta-Analysis version 2.0 software (Biostat, Inc., New Jersey, USA).
Individual study quality appraisal
Two authors (DM, JS) independently assessed the risk of bias of included studies using the standardized Newcastle-Ottawa scale.[47] This validated instrument for appraising observational studies measures the risk of bias in eight categories: Representativeness of the exposed cohort (S1); selection of the nonexposed cohort (S2); ascertainment of exposure (S3); demonstration that the outcome of interest was not present at the start of the study (S4); comparability (C1 and C2); assessment of outcome (E1); was follow-up long enough for outcomes to occur (E2); and adequacy of follow-up of cohorts (E3) [Supplementary Table 2]. Discrepancies were resolved by discussion or adjudication by a third author (MA).
Supplementary Table 2.
Newcastle-Ottawa scale for observational studies included in our meta-analysis
| Author(s) | Selection | Comparability | Exposure | Total stars | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | C2 | E1 | E2 | E3 | ||
| Winning et al.[35] | x | x | x | x | x | x | x | x | 8 | |
| Winning et al.[35] | x | x | x | x | x | x | 6 | |||
| Erlich et al.[25] | x | x | x | x | x | x | X | 7 | ||
| Losche et al.(Study 1)[50] | x | x | x | x | x | x | 6 | |||
| Losche et al.(Study 2)[30] | x | x | x | x | x | x | 6 | |||
| Eisen et al.[24] | x | x | x | x | x | x | x | x | 8 | |
| Kor et al.[29] | x | x | x | x | x | x | x | x | 8 | |
| Gross et al.[27] | x | x | x | x | x | x | 6 | |||
| Harr et al.[28] | x | x | x | x | x | x | 6 | |||
| Valerio-Rojas et al.[33] | x | x | x | x | x | x | x | x | 8 | |
| Otto et al.[31] | x | x | x | x | x | x | 6 | |||
| Faverio et al.[26] | X | X | X | X | X | X | 6 | |||
| Chen et al.[23] | X | X | X | X | X | X | 8 | |||
| Tsai et al.[32] | X | X | X | X | X | 5 | ||||
| Mazzeffi et al.[30] | X | X | X | X | X | X | X | X | 8 | |
| Boyle et al.[22] | X | X | X | X | X | X | X | 7 | ||
| Al Harbi et al.[48] | X | X | X | X | X | X | 6 | |||
RESULTS
Our search yielded 1862 articles which were narrowed down to 15 individual full-text articles and 1 conference abstract,[22,23,24,25,26,27,28,29,30,31,32,33,34,35,48] and included three different studies, out of which two were included in our analysis. This process gave us 17 individual studies with a total of 721,763 patients to include in our analysis [Supplementary Figure 1 (317.6KB, tif) ].[22,23,24,25,26,27,28,29,30,31,32,33,34,35,48]
Flowchart of search strategy for systematic review
All the studies reported event rate, except for 3[22,23,31] that reported the overall effect using confidence interval (CI) overall effect rather than event rate. This effect was incorporated in the analysis. Among the 17 studies, 16 used aspirin,[22,23,24,25,26,28,29,30,31,32,33,34,35,48] 10 used clopidogrel,[23,25,26,27,29,31,32,33,34,35] 5 used ticlopidine,[25,28,29,32,33] 2 used dipyridamole,[25,33] and only 1 used anagrelide, cilostazol, and persantine [Table 1].[25] Most of our studies included patients on prehospitalization APT except the study by Boyle et al.[22] and Al Harbi et al.[48] which included some patients with de novo initiation of APT during hospitalization.
In an effort to stratify or compare patients on APT, 7 studies used the Acute Physiology and Chronic Health Evaluation (APACHE) II score,[22,24,25,26,29,34,48] 2 studies used the APACHE III score,[25,33] 2 studies used the Sequential Organ Failure Assessment Score[29,35] while the rest did not use any of these risk scores [Table 1].
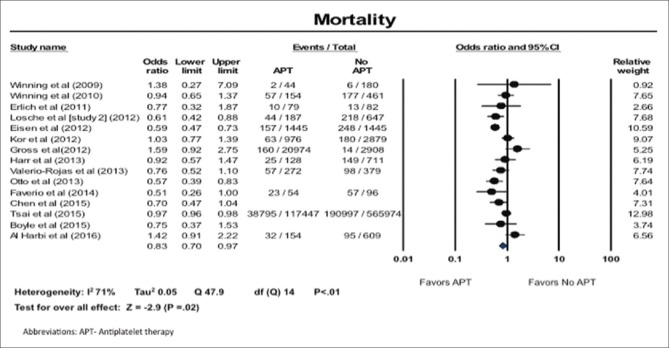
All-cause mortality
We found that all-cause mortality was significantly lower in patients on APT (OR: 0.83; 95% CI: 0.70–0.97). There was high heterogeneity in the results; I2 of 71% [Figure 1].
Figure 1.
All-cause mortality
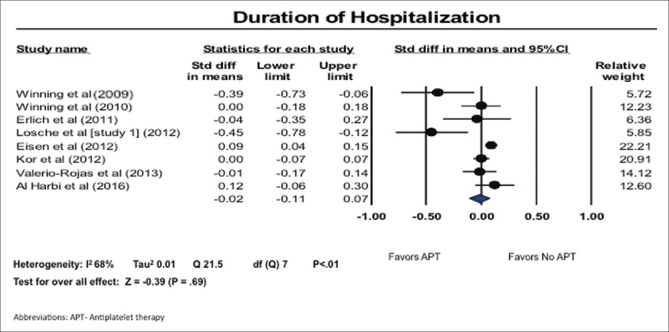
Duration of hospitalization
We also found that while the length of hospital stay was shorter in patients on APT, this effect did not reach statistical significance (SMD, −0.02; 95% CI: −0.11–0.07). There was high heterogeneity in the results; I2 of 68% [Figure 2].
Figure 2.
Duration of hospitalization
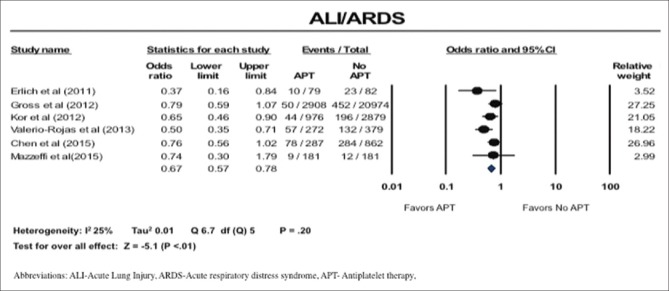
Incidence of acute lung injury/acute respiratory distress
The incidence of ALI and ARDS was reduced in patients on APT (OR: 0.67; 95% CI: 0.57–0.78) [Figure 3]. There was low heterogeneity in these studies; I2 of 25% [Figure 3].
Figure 3.
Incidence of acute lung injury/acute respiratory distress
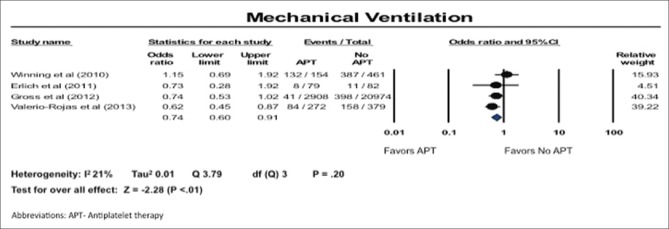
Need for mechanical ventilation
The need for mechanical ventilation was less in patients on APT (OR: 0.74; 95% CI: 0.60–0.91). There was low heterogeneity in these studies; I2 of 21% [Figure 4].
Figure 4.
Need for mechanical ventilation
Sensitivity analysis and cumulative analysis
Sensitivity analysis whereby each study was removed individually did not demonstrate significant difference or change in the overall outcomes, except in the analyses of need for mechanical ventilation. When the study by Valerio-Rojas et al.[33] was removed for the outcome, the effect becomes nonsignificant (OR: 0.83; 95% CI: 0.64–1.08). We also carried out a sensitivity analysis by comparing studies on aspirin with studies where patients were on APT other than aspirin, either alone or in combination with aspirin. Comparison of these two groups showed consistent results across all outcomes. Chronological cumulative analysis for each outcome did not find any significant change in the final effect outcomes [Supplementary Figures 2 (1.6MB, tif) and 3 (906KB, tif) ].[33]
Cumulative meta-analysis: All-cause mortality; duration of hospitalization; acute lung injury/acute respiratory distress syndrome; need for mechanical ventilation (APT: Antiplatelet therapy, ALI: Acute lung injury, ARDS: Acute respiratory distress syndrome)
Sensitivity analysis for acute lung injury/acute respiratory distress syndrome which reveals that there is a nonsignificant trend toward decreased need for mechanical ventilation after removal of a study by Valerio-Rojas et al.[33] (APT: Antiplatelet therapy)
Publication bias
Funnel plot analysis did not show bias for all outcomes. Similar results were observed after quantifying with others’ methods (Begg and Mazumdar, Egger, and Duval and Tweedie's tests)[43,44,45,46] [Supplementary Figures 4 (1.1MB, tif) and 5 (825.5KB, tif) ]. The individual study quality appraisal and the risk of bias are summarized in Supplementary Table 2.
Funnel plots: All-cause mortality (a), duration of hospitalization (b), acute lung injury/acute respiratory distress syndrome (c), need for mechanical ventilation (d)
Tests for publication bias: All-cause mortality (a); duration of hospitalization (b); acute lung injury/acute respiratory distress syndrome (c); need for mechanical ventilation (d)
DISCUSSION
Our meta-analysis of 17 observational cohort studies with over 720,000 patients revealed that critically ill patients on APT have improved survival when compared to those who do not receive APT. To the best of our knowledge, this is the largest meta-analysis on this topic. A recent meta-analysis by Wang et al. aimed to summarize similar evidence but includes only 9 studies as compared to the 17 in this meta-analysis.[39] This is partly due to a different search strategy as well as our inclusion of conference abstracts and literature published after their cutoff date of November 2015. In our study, the use of APT was also found to be associated with a reduced incidence of ALI/ARDS. Sensitivity analysis revealed that this beneficial effect is not limited to aspirin but rather is consistent across the different antiplatelet drugs used in the included studies. Of note, while bleeding risk or need for transfusion was not assessed using meta-analysis techniques given the small number of studies, an increased risk of these outcomes was not seen in studies that did report these results.[24,33,34] In fact, a large single-center study reported a decreased risk of bleeding[24] while another study reported a decreased need for transfusion in patients on APT.[33]
Previously performed animal studies have shown results similar to our meta-analysis. A study on the effects of clopidogrel on experimentally-induced endotoxemia in mice revealed a trend toward improved survival beyond 48 h.[35] A study of clopidogrel in polymicrobial sepsis in mice reported decreased thrombocytopenia and end-organ damage.[50] Blockade of the glycoprotein IIb/IIIa receptor has shown to decrease mortality in rabbits with Escherichia coli endotoxin-induced shock.[51] Another study investigating E. coli sepsis in baboon models revealed decreased incidence of microangiopathic hemolysis and renal insufficiency.[52]
Platelet function is intricately linked to the pathophysiology of sepsis and its complications. Sepsis decreases the hemostatic function of platelets while the capabilities of platelets for molecular expression and cytokine production remain unimpaired and growth factor production is upregulated.[53] The antimicrobial peptides produced by platelets (known as defensins) are bactericidal and essential to the host immune response; however, the resultant inflammatory response may contribute significantly to the microvascular dysfunction characteristic of sepsis.[54] In addition, during sepsis, there is an increase in phagocytic neutrophil-platelet complexes. These complexes, while aiding in pathogen elimination, also lead to an overwhelming inflammatory response that damages the host. In fact, a study focusing on platelet function in patients with sepsis revealed that while platelet-leukocyte adhesion increased in sepsis, there was a decrease in circulating platelet-neutrophil complexes in patients who died and also in those who had multi-organ dysfunction.[55] This suggests that there may be peripheral sequestration of these complexes in sepsis, which, in turn, may lead to end-organ damage. Platelet activation also results in hypercoagulation and disseminated intravascular coagulation.[56]
ARDS is a devastating complication in critically ill patients. The pathophysiology of ARDS is characterized by damage to the alveolar-capillary barrier resulting in increased vascular permeability and influx of protein-rich fluid into interstitial and alveolar membranes.[57] Platelet activation plays a critical role in this process. Bronchoalveolar lavage fluid from patients with ARDS has excessive quantities of platelet-specific alpha granules, thereby demonstrating the increased platelet activity in these patients.[58] Activation of platelets leads to adhesion of platelets to the endothelium and release of inflammatory and thrombotic agents along with leukocyte recruitment, edema, and production of neutrophil extracellular traps (NET).[59] In ARDS, a high concentration of proinflammatory factors in the alveoli can lead to overproduction of NET, which causes direct-tissue injury. They also further activate platelets to promote fibrin deposition and perpetuate the ongoing inflammatory cascade.[60,61] Our meta-analysis demonstrates a decreased incidence of ALI/ARDS in patients on antiplatelet medications. This is in line with animal studies done previously. Treatment with aspirin reduced transfusion-associated lung injury in mice.[37] Another study revealed that in rabbit lungs with ALI, blockade of thromboxane A2 (a mediator inhibited by aspirin) eliminated pulmonary hypertension and improved oxygenation.[62]
There are currently several randomized controlled trials in progress that aim to evaluate the role of APT in sepsis and ARDS. One phase II study aims to randomly assign patients with sepsis/septic shock to aspirin use versus placebo. The primary outcome for this study is a reduction of organ dysfunction. Another study aims to study the effect of aspirin in reducing the severity of ARDS as determined by the oxygenation index. In a similar phase II study, researchers are studying the efficacy of aspirin in preventing ARDS in patients who are at increased risk for ALI.[63,64,65]
Limitations
There are several limitations to our meta-analysis. First, all the included studies were observational (reflecting the paucity of randomized trials on this topic) and, therefore, prone to bias. Second, this is a meta-analysis performed on study-level data. Third, the definitions and reporting of adverse outcomes and risk of enrolled patients differed across studies. Fourth, most of our studies included patients with prehospital antiplatelet use and as such inferences cannot be extended to new initiation of APT in patients admitted with critical illness. Furthermore, one of the included studies had coexisting NSAID use in both the aspirin group and the control,[24] which could possibly have influenced the effect on mortality and duration of hospitalization. Previous data are controversial on the use of NSAIDs in sepsis and we cannot be sure of how the inclusion of this study would change the effect size.[66] Another limitation of our analysis is that the new definition of sepsis (2016) and ARDS (2012) could not be taken into account as it would lead to the removal of a large number of studies still using the older definitions.[41,49,67] These limitations may explain some of the heterogeneity seen in this meta-analysis for various outcomes. On the other hand, despite these limitations, the consistency of the magnitude, direction of the overall effect, and stability of the results after the sensitivity analyses, in conjunction with the large number of patients studied (the largest patient population studied to-date for a meta-analysis on this topic), support the strength of the conclusions.
CONCLUSION
Our meta-analysis shows that critically ill patients receiving APT have a moderately improved survival, decreased incidence of ARDS, and decreased need for mechanical ventilation. These data need to be validated by large randomized controlled trials, which are lacking in this area.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 3.Braun L, Riedel AA, Cooper LM. Severe sepsis in managed care: Analysis of incidence, one-year mortality, and associated costs of care. J Manag Care Pharm. 2004;10:521–30. doi: 10.18553/jmcp.2004.10.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elhag KM, Mustafa AK, Sethi SK. Septicaemia in a teaching hospital in Kuwait-I: Incidence and aetiology. J Infect. 1985;10:17–24. doi: 10.1016/s0163-4453(85)80004-9. [DOI] [PubMed] [Google Scholar]
- 5.Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, et al. Epidemiology of sepsis in Germany: Results from a national prospective multicenter study. Intensive Care Med. 2007;33:606–18. doi: 10.1007/s00134-006-0517-7. [DOI] [PubMed] [Google Scholar]
- 6.Finfer S, Bellomo R, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand Intensive Care Units. Intensive Care Med. 2004;30:589–96. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 7.Flaatten H. Epidemiology of sepsis in Norway in 1999. Crit Care. 2004;8:R180–4. doi: 10.1186/cc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: Secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care. 2006;10:R42. doi: 10.1186/cc4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoa NT, Diep TS, Wain J, Parry CM, Hien TT, Smith MD, et al. Community-acquired septicaemia in Southern Viet Nam: The importance of multidrug-resistant Salmonella typhi. Trans R Soc Trop Med Hyg. 1998;92:503–8. doi: 10.1016/s0035-9203(98)90891-4. [DOI] [PubMed] [Google Scholar]
- 10.Jawad I, Lukšic I, Rafnsson SB. Assessing available information on the burden of sepsis: Global estimates of incidence, prevalence and mortality. J Glob Health. 2012;2:010404. doi: 10.7189/jogh.02.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 12.Salvo I, de Cian W, Musicco M, Langer M, Piadena R, Wolfler A, et al. The Italian SEPSIS study: Preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Med. 1995;21(Suppl 2):S244–9. doi: 10.1007/BF01740762. [DOI] [PubMed] [Google Scholar]
- 13.Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, et al. Brazilian sepsis Epidemiological Study (BASES study) Crit Care. 2004;8:R251–60. doi: 10.1186/cc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 15.Torio CM, Andrews RM. National inpatient hospital costs: The most expensive conditions by payer, 2011. Statistical Brief #160 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs Vol 2016. 2011. [Last accessed on 2016 Jan 03]. Available from: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb160.jsp .
- 16.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 17.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 18.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 19.Boyle AJ, Mac Sweeney R, McAuley DF. Pharmacological treatments in ARDS; a state-of-the-art update. BMC Med. 2013;11:166. doi: 10.1186/1741-7015-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrono C, Bachmann F, Baigent C, Bode C, De Caterina R, Charbonnier B, et al. Expert consensus document on the use of antiplatelet agents. The task force on the use of antiplatelet agents in patients with atherosclerotic cardiovascular disease of the European society of cardiology. Eur Heart J. 2004;25:166–81. doi: 10.1016/j.ehj.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle AJ, Di Gangi S, Hamid UI, Mottram LJ, McNamee L, White G, et al. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced Intensive Care Unit mortality: A prospective analysis. Crit Care. 2015;23(19):109. doi: 10.1186/s13054-015-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Janz DR, Bastarache JA, May AK, O’Neal HR, Jr, Bernard GR, et al. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: A propensity-adjusted analysis. Crit Care Med. 2015;43:801–7. doi: 10.1097/CCM.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen DP, Reid D, McBryde ES. Acetyl salicylic acid usage and mortality in critically ill patients with the systemic inflammatory response syndrome and sepsis. Crit Care Med. 2012;40:1761–7. doi: 10.1097/CCM.0b013e318246b9df. [DOI] [PubMed] [Google Scholar]
- 25.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: A population-based cohort study. Chest. 2011;139:289–95. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faverio P, Aliberti S, Reyes L, Sibila O, Orihuela C, Brown A, et al. Antiplatelets improve survival among critically ill mechanically ventilated patients. Chest. 2014;146:500A. [Google Scholar]
- 27.Gross AK, Dunn SP, Feola DJ, Martin CA, Charnigo R, Li Z, et al. Clopidogrel treatment and the incidence and severity of community acquired pneumonia in a cohort study and meta-analysis of antiplatelet therapy in pneumonia and critical illness. J Thromb Thrombolysis. 2013;35:147–54. doi: 10.1007/s11239-012-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harr JN, Moore EE, Johnson J, Chin TL, Wohlauer MV, Maier R, et al. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Crit Care Med. 2013;41:399–404. doi: 10.1097/CCM.0b013e31826ab38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Losche W, Boettel J, Kabisch B. Do aspirin and other antiplatelet drugs reduce the mortality in critically ill patients? Thrombosis 2012. 2012:720254. doi: 10.1155/2012/720254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzeffi M, Kassa W, Gammie J, Tanaka K, Roman P, Zhan M, et al. Preoperative aspirin use and lung injury after aortic valve replacement surgery: A retrospective cohort study. Anesth Analg. 2015;121:271–7. doi: 10.1213/ANE.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 31.Otto GP, Sossdorf M, Boettel J, Kabisch B, Breuel H, Winning J, et al. Effects of low-dose acetylsalicylic acid and atherosclerotic vascular diseases on the outcome in patients with severe sepsis or septic shock. Platelets. 2013;24:480–5. doi: 10.3109/09537104.2012.724482. [DOI] [PubMed] [Google Scholar]
- 32.Tsai MJ, Ou SM, Shih CJ, Chao PW, Wang LF, Shih YN, et al. Association of prior antiplatelet agents with mortality in sepsis patients: A nationwide population-based cohort study. Intensive Care Med. 2015;41:806–13. doi: 10.1007/s00134-015-3760-y. [DOI] [PubMed] [Google Scholar]
- 33.Valerio-Rojas JC, Jaffer IJ, Kor DJ, Gajic O, Cartin-Ceba R. Outcomes of severe sepsis and septic shock patients on chronic antiplatelet treatment: A historical cohort study. Crit Care Res Pract 2013. 2013:782573. doi: 10.1155/2013/782573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winning J, Neumann J, Kohl M, Claus RA, Reinhart K, Bauer M, et al. Antiplatelet drugs and outcome in mixed admissions to an Intensive Care Unit. Crit Care Med. 2010;38:32–7. doi: 10.1097/CCM.0b013e3181b4275c. [DOI] [PubMed] [Google Scholar]
- 35.Winning J, Reichel J, Eisenhut Y, Hamacher J, Kohl M, Deigner HP, et al. Anti-platelet drugs and outcome in severe infection: Clinical impact and underlying mechanisms. Platelets. 2009;20:50–7. doi: 10.1080/09537100802503368. [DOI] [PubMed] [Google Scholar]
- 36.Bates JJ, Watson RW, Glynn CM, O’Neill AJ, Fitzpatrick JM, Buggy DJ. Aspirin preserves neutrophil apoptosis after cardiopulmonary bypass. Shock. 2004;21:495–9. doi: 10.1097/01.shk.0000126146.94237.92. [DOI] [PubMed] [Google Scholar]
- 37.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–61. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–9. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Li H, Gu X, Wang Z, Liu S, Chen L. Effect of Antiplatelet therapy on acute respiratory distress syndrome and mortality in critically Ill patients: A meta-analysis. PLoS One. 2016;11:e0154754. doi: 10.1371/journal.pone.0154754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 42.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials. 2007;28:105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 44.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 45.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 47.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Vol. 2016. The Ottawa Hospital Research Institute; 2014. [Last accessed on 2016 Jun 02]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp . [Google Scholar]
- 48.Al Harbi SA, Tamim HM, Al-Dorzi HM, Sadat M, Arabi YM. Association between aspirin therapy and the outcome in critically ill patients: A nested cohort study. BMC Pharmacol Toxicol. 2016;17:5. doi: 10.1186/s40360-016-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 50.Seidel M, Winning J, Claus RA, Bauer M, Lösche W. Beneficial effect of clopidogrel in a mouse model of polymicrobial sepsis. J Thromb Haemost. 2009;7:1030–2. doi: 10.1111/j.1538-7836.2009.03352.x. [DOI] [PubMed] [Google Scholar]
- 51.Pu Q, Wiel E, Corseaux D, Bordet R, Azrin MA, Ezekowitz MD, et al. Beneficial effect of glycoprotein IIb/IIIa inhibitor (AZ-1) on endothelium in Escherichia coli endotoxin-induced shock. Crit Care Med. 2001;29:1181–8. doi: 10.1097/00003246-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 52.Taylor FB, Coller BS, Chang AC, Peer G, Jordan R, Engellener W, et al. 7E3 F(ab’)2, a monoclonal antibody to the platelet GPIIb/IIIa receptor, protects against microangiopathic hemolytic anemia and microvascular thrombotic renal failure in baboons treated with C4b binding protein and a sublethal infusion of Escherichia coli. Blood. 1997;89:4078–84. [PubMed] [Google Scholar]
- 53.Yaguchi A, Lobo FL, Vincent JL, Pradier O. Platelet function in sepsis. J Thromb Haemost. 2004;2:2096–102. doi: 10.1111/j.1538-7836.2004.01009.x. [DOI] [PubMed] [Google Scholar]
- 54.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–45. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 55.Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest. 1995;25:843–51. doi: 10.1111/j.1365-2362.1995.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 56.Levi M, de Jonge E, van der Poll T. Plasma and plasma components in the management of disseminated intravascular coagulation. Best Pract Res Clin Haematol. 2006;19:127–42. doi: 10.1016/j.beha.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 57.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 58.Idell S, Maunder R, Fein AM, Switalska HI, Tuszynski GP, McLarty J, et al. Platelet-specific alpha-granule proteins and thrombospondin in bronchoalveolar lavage in the adult respiratory distress syndrome. Chest. 1989;96:1125–32. doi: 10.1378/chest.96.5.1125. [DOI] [PubMed] [Google Scholar]
- 59.Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care. 2015;19:374. doi: 10.1186/s13054-015-1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs) – Formation and implications. Acta Biochim Pol. 2013;60:277–84. [PubMed] [Google Scholar]
- 62.Goff CD, Corbin RS, Theiss SD, Frierson HF, Jr, Cephas GA, Tribble CG, et al. Postinjury thromboxane receptor blockade ameliorates acute lung injury. Ann Thorac Surg. 1997;64:826–9. doi: 10.1016/s0003-4975(97)00490-6. [DOI] [PubMed] [Google Scholar]
- 63. [Last accessed on 2016 Jan 03];Aspirin for Treatment of Severe Sepsis. 2015 2016 Available from: https://www.clinicaltrials.gov/ct2/show/NCT01784159 . [Google Scholar]
- 64. [Last accessed 2016 Jan 03];LIPS-A: Lung Injury Prevention Study with Aspirin. 2014 2016 Available from: https://www.clinicaltrials.gov/ct2/show/NCT01504867 . [Google Scholar]
- 65. [Last accessed on 2016 Jan 03];Aspirin as a Treatment for ARDS (STAR): A Phase 2 Randomised Control Trial. 2014 2016 Available from: https://www.clinicaltrials.gov/ct2/show/NCT02326350 . [Google Scholar]
- 66.Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997;336:912–8. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 67.Jacob JA. New sepsis diagnostic guidelines shift focus to organ dysfunction. JAMA. 2016;315:739–40. doi: 10.1001/jama.2016.0736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of search strategy for systematic review
Cumulative meta-analysis: All-cause mortality; duration of hospitalization; acute lung injury/acute respiratory distress syndrome; need for mechanical ventilation (APT: Antiplatelet therapy, ALI: Acute lung injury, ARDS: Acute respiratory distress syndrome)
Sensitivity analysis for acute lung injury/acute respiratory distress syndrome which reveals that there is a nonsignificant trend toward decreased need for mechanical ventilation after removal of a study by Valerio-Rojas et al.[33] (APT: Antiplatelet therapy)
Funnel plots: All-cause mortality (a), duration of hospitalization (b), acute lung injury/acute respiratory distress syndrome (c), need for mechanical ventilation (d)
Tests for publication bias: All-cause mortality (a); duration of hospitalization (b); acute lung injury/acute respiratory distress syndrome (c); need for mechanical ventilation (d)