Abstract
Background and Objectives:
Adequate nutritional supplementation in infants with cardiac malformations after surgical repair is a challenge. Critically ill infants in the early postoperative period are in a catabolic stress. The mismatch between estimated energy requirement (EER) and the intake in the postoperative period is multifactorial, predisposing them to complications such as immune deficiency, more infection, and growth failure. This study aimed to assess the feasibility and efficacy of enriched breast milk feed on postoperative recovery and growth of infants after open heart surgery.
Methodology:
Fifty infants <6 months of age were prospectively randomized in the trial for enteral nutrition (EN) postoperatively from day 1 to 10, after obtaining the Institute Ethics Committee's approval. They were equally divided into two groups on the basis of the feed they received: Control group was fed with expressed breast milk (EBM; 0.65 kcal/ml) and intervention group was fed with EBM + energy supplementation/fortification with human milk fortifier (7.5 kcal/2 g)/Simyl medium-chain triglyceride oil (7.8 kcal/ml). Energy need for each infant was calculated as per EER at 90 kcal/kg/day, as the target requirement. The intra- and post-operative variables such as cardiopulmonary bypass and aortic cross-clamp times, ventilation duration, Intensive Care Unit (ICU), and hospital length of stay and mortality were recorded. Anthropometric and hematological parameters and infection control data were recorded in a predesigned pro forma. Data were analyzed using Stata 14.1 software.
Results:
The duration of mechanical ventilation, length of ICU stay (LOIS), length of hospital stay (LOHS), infection rate, and mortality rate were lower in the intervention group compared to the control group although none of the differences were statistically significant. Infants in control group needed mechanical ventilation for about a day more (i.e., 153.6 ± 149.0 h vs. 123.2 ± 107.0 h; P = 0.20) than those in the intervention group. Similarly, infants in control group stayed for longer duration in the ICU (13.2 ± 8.9 days) and hospital (16.5 ± 9.8 days) as compared to the intervention group (11.0 ± 6.1 days; 14.1 ± 7.0 days) (P = 0.14 and 0.17, respectively). The LOIS and LOHS were decreased by 2.2 and 2.4 days, respectively, in the intervention group compared to control group. The infection rate (3/25; 5/25) and mortality rate (1/25; 2/25) were lower in the intervention group than those in the control group. The energy intake in the intervention group was 40 kcal more (i.e., 127.2 ± 56.1 kcal vs. 87.1 ± 38.3 kcal) than the control group on the 10th postoperative day.
Conclusions:
Early enteral/oral feeding after cardiac surgery is feasible and recommended. In addition, enriching the EBM is helpful in achieving the maximum possible calorie intake in the postoperative period. EN therapy might help in providing adequate nutrition, and it decreases ventilation duration, infection rate, LOIS, LOHS, and mortality.
Keywords: Congenital heart disease, Enriched expressed breast milk, Enteral feeds, Infants, Open heart surgery, Postoperative period
INTRODUCTION
Caloric imbalance is common in the perioperative period among infants with congenital heart disease (CHD). Critically ill infants are at high risk of developing nutritional deficiencies due to imbalance between their energy expenditure and caloric intake.[1] This imbalance contributes significantly to morbidity[1] and mortality. The nutritional deficiency is associated with compromised immune defense, more infection, growth failure, longer hospital stay, and increased costs. Most infants with CHD have a normal weight for gestational age at birth but develop malnutrition in early infancy.[2,3] A high metabolic rate and limited endogenous substrate reserves predispose children to develop acute energy deficiencies in any stressful situation as in the case of post open heart surgery.[4] Infants with cyanotic heart diseases (total anomalous pulmonary venous connection (TAPVC), tetralogy of Fallot (TOF), transposition of the great arteries (TGA) frequently have decreased weight and height compared with healthy infants.[5,6] Infants with acyanotic heart diseases such as a large left-right shunt, atrial septal defect (ASD), and ventricular septal defect (VSD) also have reduced weight gain but growth may be maintained during infancy in some of these infants.[7,8] Calorie intake is often reduced in infants with CHD and is related to poor weight gain.[2,8,9] Others have reported cumulative energy and protein deficit and excess in the first 8 days of Pediatric Intensive Care Unit (PICU) stay in critically ill pediatric patients.[9]
Malnutrition is relatively common preoperatively in children with CHD.[2] Postoperative acute caloric deficiency after cardiac surgery with cardiopulmonary bypass (CPB)[4] adversely affects recovery and outcomes such as increased mechanical ventilation, infection rate, ICU and hospital stay, and ultimately the growth and development of these infants.[10,11,12] The present study was conducted to evaluate the feasibility and efficacy of early enteral feeding with caloric fortification of expressed breast milk (EBM) in the convalescence of these infants postoperatively.
METHODOLOGY
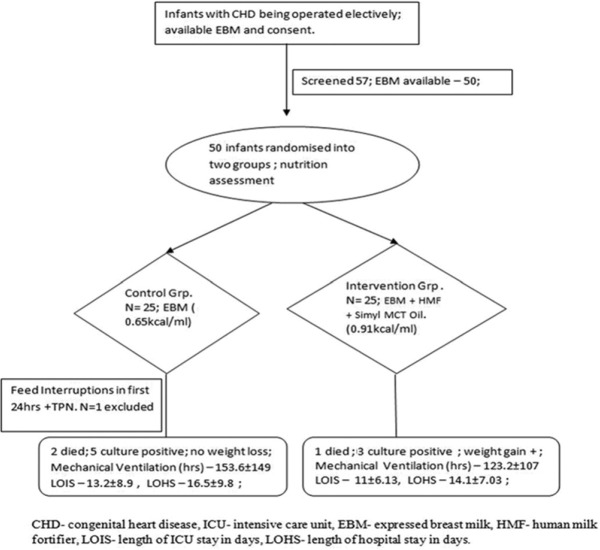
Fifty-seven infants below 6 months of age with CHD were recruited for this pilot trial during the 6-month period. Seven infants were excluded from the study because of nonavailability of mother's milk. Remaining fifty infants undergoing elective open heart surgery from May 2015 to December 2015 were enrolled in the study. The Institutional Research and Ethics Committee approval and written informed consent from the parents were obtained. Those included for the study were screened as per the inclusion criteria for age <6 months, availability of mother's milk, elective surgeries, and exclusion criteria such as prematurity, proven infection, comorbidities other than cardiac malformations, noncardiac congenital anomalies, emergency operations, and closed heart operations. They were randomized into two groups in the preoperative period using a computer-generated random list. Envelopes (brown) marked control and intervention groups according to the random list were kept under the supervision of the ICU nursing staff-in-charge and were opened for the enrolled babies postoperatively in the ICU. These patients were assigned either control group (n = 25), to receive EBM, and intervention group (n = 25), to receive EBM fortified with human milk fortifier (HMF®, 1 sachet per 40 ml) and Simyl medium-chain triglyceride (MCT) oil (0.1/20 ml). The randomization process was blinded to the intensivists and the dietician conducting the study. The flow diagram depicting the enrollment of infants with CHD into the pilot study as per the CONSORT guidelines is shown in Figure 1. A predesigned pro forma with pre-, intra-, and post-operative parameters was attached to the ICU chart of individual babies. Demographic details including final diagnosis, socioeconomic status, feeding practices before surgery (breast-fed or bottle-fed or both), gestational age and weight of the baby, and noncardiac congenital anomalies were collected preoperatively. Intraoperative details of CPB time, aortic cross-clamp (AOXCl) time were recorded. We chose these time factors because prolonged AOXCl time increases gut hypoperfusion and increases risk of feed intolerance postoperatively. Postoperatively (from day 1) clinical measurements such as length in centimeters (cm), weight in kilograms (kg), and hematological parameters such as hemoglobin, total leukocyte count, differential blood count, urea, creatinine, phosphate (PO4), bilirubin, aspartate aminotransferase, and alanine aminotransferase were measured on alternate days. C-reactive protein (CRP) and serum albumin were measured at less frequent intervals. After the babies were assigned to control and intervention groups (as per the random number list), they were fed as per the protocol by the bedside staff. The feeding regimen was driven by the dietician. Each patient was assessed by the dietician daily for requirement of total calories per day prospectively, presence or absence of feeding, method of feeding (oral/nasogastric tube feeding), duration of feeding, frequency of stools, and reason for discontinuation of feedings. The nutrient consumption of each baby was calculated based on the actual feed volume given to the baby in the previous 24 h period. Total feed volume was estimated after considering the fluid restriction depending on the cardiac status of the patient. The infants in control group were fed with 0.65 kcal/ml while those in the intervention group received 0.91 kcal/ml. All the babies were fed enterally through Ryle's tube from day 1 of surgery even if on ventilator. Once the patients were self-ventilating, the calculated amount of target calorie was given orally gradually. Enteral nutrition (EN) varied in each baby as per the cardiovascular function, complexity of the surgery and the volume of fluid restriction in postoperative period (for example, depending on AOXCl time intraoperatively and low cardiac output status postoperatively or heart failure at any juncture). The target enteral feed volume was set at 100 ml/kg bw/day as recommended for normal infants of the same age group, but the given volume was varying in each infant depending on various postoperative factors as described above. The total daily calculated volume of feed was given enterally/orally in multiple divided doses as intermittent boluses at 2 hourly intervals by the bedside staff. EBM was collected from the individual mothers for their babies twice or thrice daily and was stored in refrigerator at 25° and was fortified with HMF/simyl MCT oil for the intervention group just before each feed. The enteral (through Ryle's tube)/oral feeding was started after 6 h on the 1st day of surgery for all the babies and was continued till the 10th postoperative day. EBM did not cost anything to the hospital and the other energy supplements such as HMF and simyl MCT oil were available as hospital supply and did not cost anything to the parents and also are of very less expense considering the small quantity required by each baby. Enteral feeding with mother's milk alone or with fortification was expected to produce side effects such as lactose intolerance, nausea, vomiting, and diarrhea in some infants, and for them the plan was in two parts. First-giving a period of gut rest (nil per oral) and maintenance intravenous fluid supplement for a temporary time period up to 24 h, prokinetics trial with domperidone syrup enterally and the second plan - in case of prolonged feed intolerance (more than 24 h at a stretch and up to 48 h), the calorie was supplemented by parenteral nutrition (PN) therapy, avoiding enteral feeds till the time gastro intestinal function and other cardiac functions become normal. The primary outcome was mortality and the secondary outcomes were duration of mechanical ventilation, ICU and hospital stay and incidence of microbiologically documented infection/sepsis status and any metabolic or humoral complication such as hypo/hyperglycemia.
Figure 1.
Consort diagram depicting - The flowchart of nutritional therapy for infants after repair of congenital cardiac malformation in Cardiac Surgical Intensive Care Unit
This prospective trial was conducted in the dedicated postoperative cardiac surgical ICU (CSICU) of cardiothoracic and the Vascular Surgery Department of the Institute. All the children with CHD after cardiac surgery were admitted to CSICU. The trend in the recent times with us in our high-volume setup is that increasing number of infants with congenital cardiac malformations are being diagnosed and operated, which motivated us to conduct this pilot study on early nutrition therapy to these critically high risk babies, and to see both the feasibility and efficacy of enteral calorie supplementation in the postoperative recovery in terms of ventilation duration, infection rate, length of ICU stay (LOIS), length of hospital stay (LOHS), and mortality. This study aimed to assess these variables prospectively which would help formulating our planned practice subsequently.
Feed (calculated amount of macronutrients) was given to both groups and it was constant for control group (0.65 kcal/ml) and intervention group (0.91 kcal/ml). As a unit policy, we prefer to use enteral route for feeding these babies, with mother's milk. Infants with CHD require more calorie than the others after major cardiac surgery. Although standards exist for caloric intake in healthy infants, such standards are lacking for babies with CHD.[13] The standard requirement for these babies was taken as per the revised recommended dietary allowance 2010 for normal Indian infants @ 92 kcal/kg body wt/day as there was no clarity about the exact requirement. In both groups, the targeted calorie was 30–90 kcal/kg body weight. This calculated amount of calorie was provided from day 1 (at 30 kcal/kg) and gradually increased on subsequent days till day 10 to achieve target goal of all macro- and micro-nutrients in the early postoperative period. The required fluid volume for these infants would be around 100–120 ml/kg/day of formula containing 24–27 kcal/30 ml. However, this requirement of targeted calorie and volume of feed could not be met due to fluid restriction (30–50 ml/kg/day) and cardiovascular function in both groups. The feed was interrupted (feed interruption [FI]) sometimes. The reasons and parenteral supplementation were recorded. Prolonged FI (more than 24 h at a stretch with addition of PN) was an exclusion as it affected the nutritional therapy which we envisaged. Furthermore, we studied the calorie consumption in both groups, the maximum percentage of calculated macronutrients, that was possible to supplement enterally were calculated. This depended upon the cardiovascular status of the infant and was variable.
Data collection was done on daily basis for macronutrient consumption. The amount of energy consumption per day by each baby was calculated usingDietCal software version 5.0 (Profound Tech solution; http//dietcal.in/) which is based on values from the Nutritive values of Indian foods by providing the amount of protein, fat, and carbohydrates, fed to the baby in toto. Anthropometric measurements and hematological parameters were recorded on alternate days. Any suspicion of probable sepsis in a baby was closely monitored and the routine standard screening protocol of the ICU was followed. ICU and hospital death rate were recorded for both groups. The results were analyzed in terms of feasibility of nutritional therapy in postoperative setting, with EBM alone and EBM + energy supplement/fortification independently and their efficacy on the recovery of the patient.
Statistical analysis
Quantitative variables such as age, height, weight, and macronutrients were summarized as mean ± standard deviation and were compared using Student's t-test. Qualitative characteristics were summarized as proportion (%) and were compared using Fisher's exact test. Day-wise macronutrient consumption between the two groups was compared using a generalized estimating equations analysis. Analysis was done as per the protocol. P < 0.05 was considered statistically significant. All analyses were carried out using Stata 14.1 (STATA Corp, College station, Tx, USA).
RESULTS
The baseline characteristics of the infants in control (n = 25) and intervention (n = 25) groups were comparable in terms of their age, length, and weight [Table 1]. One baby from control group had to be excluded in view of prolonged FI on day 1, could not be initiated on breast milk feeding and necessitating PN later. Thirty-nine patients had cyanotic CHD comprising TOF, TAPVCs, and TGAs while ten patients had acyanotic CHD comprising ASD and VSD. Peroperative factors in terms of the CPB and AOXL times [Table 2] were comparable between the groups.
Table 1.
The baseline characteristics of infants undergoing surgical repair of congenital cardiac malformations
| Characteristics | Control group (n=25) | Intervention group (n=25) | P |
|---|---|---|---|
| Age (days) | 67.6±37.5 | 79.5±51.4 | 0.59 |
| Male/female (n) | 20/4 | 23/2 | - |
| Length (cm) | 55.1±4.48 | 55.76±5.5 | 0.658 |
| Weight (kg) | 3.6±0.8 | 3.5±0.91 | 0.452 |
| Cyanotics/acyanotics | 21/4 | 20/5 | |
| TOF | 8 | 5 | |
| TAPVC | 3 | 4 | |
| TGA | 10 | 11 | |
| VSD | 2 | 4 | |
| ASD | 2 | 1 |
Data are expressed as mean±SD. TOF: Tetralogy of Fallot, TAPVC: Total anomalous pulmonary venous connection, TGA: Transposition of great arteries, VSD: Ventricular septal defect, ASD: Atrial septal defect, SD: Standard deviation
Table 2.
Comparison of per-and post-operative parameters of infants undergoing corrective cardiac surgery
| Variables | Control group (n=25) | Intervention group (n=25) | P |
|---|---|---|---|
| CPB duration (min) | 118.5±53.1 | 110.4±54.4 | 0.50 |
| AoXCl (min) | 85.0±97.7 | 70.1±43 | 0.24 |
| Mortality (n) | 2/25 | 1/25 | - |
| Ventilation duration (h) | 153.6±149 | 123.2±107 | 0.20 |
| LOIS (days) | 13.2±8.9 | 11±6.13 | 0.14 |
| LOHS (days) | 16.5±9.8 | 14.1±7.03 | 0.17 |
| Sepsis (culture positives) (n) | 5/25 | 3/25 | - |
Data are expressed as mean±SD. CPB: Cardiopulmonary bypass, AOXCL: Aortic cross clamp, LOIS: Length of ICU stay, LOHS: Length of hospital stay, ICU: Intensive Care Unit
Table 3 shows the consumption of macronutrients, i.e., energy, protein, fat, carbohydrate in both groups. Our result showed a marked difference in macronutrient consumption among the infants between both groups. We started feeding the babies as early as 6 h after surgery in both groups. We could provide 19 kcal/kg bw in control group and 25 kcal/kg bw in the intervention group on day 1. Day by day, the calorie consumption was increased in both groups, and on day 10, the total calorie consumption was 30 kcal/kg in control group and 38 kcal/kg in the intervention group (again very low calorie consumption according to the set target). However, by supplementing the extra energy like HMF/Simyl MCT oil along with the EBM could help achieve at least about 42.2% of the calculated target energy in the intervention group and up to 33.3% of energy consumption in control group patients by the 10th postoperative day, which is still very low as per the estimated energy requirement set target. Our data demonstrated that actual calorie intake during the CSICU stay was substantially below what was recommended. However, there were no hypoglycemic episodes in any infant in either group on routine monitoring as per the unit protocol. However, the infants in the intervention group consumed 40 kcal more (i.e., 127.2 ± 56.1 kcal vs. 87.1 ± 38.3 kcal) than that of control group infants on the 10th postoperative day and this was possible by enriching EBM despite same fluid volume restrictions.
Table 3.
Comparison of macronutrient consumption by infants postoperatively in Cardiac Surgical Intensive Care Unit for 10 days
| Macronutrients | Energy (kcal/day) | Proteins (g/day) | Fat (g/day) | Carbohydrates (g/day) | ||||
|---|---|---|---|---|---|---|---|---|
| Group | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention |
| Day 1 | 67.6±18.5 | 87.8±29.6 | 1±0.3 | 1.3±0.5 | 3.1±1 | 3.8±1.4 | 8.7±2.4 | 12.4±3.5 |
| Day 2 | 68.13±9.6 | 98.8±28.2 | 1±0.3 | 1.5±0.5 | 3.1±1 | 4.4±1.5 | 8.8±2.3 | 13.2±3.1 |
| Day 3 | 65.6±26.5 | 110.2±36.4 | 0.96±0.4 | 1.7±0.67 | 2.9±1.5 | 5±1.9 | 8.6±2.8 | 14.4±4.1 |
| Day 4 | 77.5±31.7 | 100±41 | 1.1±0.5 | 1.7±0.78 | 3.5±1.7 | 5±2.3 | 10±3.5 | 14±4.5 |
| Day 5 | 75.5±36.5 | 117.7±46.5 | 1.1±0.6 | 1.9±0.89 | 3.5±2 | 5.5±2.5 | 4.5±3.9 | 15.1±5 |
| Day 6 | 83.9±27.0 | 124.1±51.8 | 1.26±0.5 | 2±0.9 | 3.8±1.7 | 6±2.7 | 10.8±2.8 | 16.1±5.4 |
| Day 7 | 74.3±32.9 | 124.1±57.6 | 1.2±0.5 | 2.1±1 | 3.9±1.6 | 6.1±2.9 | 9.3±3.4 | 15.8±6.4 |
| Day 8 | 78±33.2 | 128.6±48.6 | 1.3±0.4 | 2.1±0.9 | 4±1.5 | 6.1±2.6 | 9.9±3.5 | 16.2±5.3 |
| Day 9 | 83±32.7 | 138.3±48 | 1.4±0.4 | 2.3±0.8 | 4.4±1.4 | 6.6±2.5 | 10.4±3.3 | 17.2±5.5 |
| Day 10 | 87.1±38.3 | 127.2±56.1 | 1.5±0.5 | 2.1±1 | 4.7±1.6 | 5.9±3 | 10.8±3.9 | 16.2±6.3 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | ||||
Data are expressed as mean±SD. SD: Standard deviation
The following trends (P > 0.05) were observed in the postoperative course of infants in both groups [Table 2]: Infants in control group needed mechanical ventilation for about a day more (i.e., 153.6 ± 149.0 h vs. 123.2 ± 107.0 h; P = 0.20) than infants in the intervention group. Similarly, infants in control group stayed for longer duration in ICU (13.2 ± 8.9 days) and hospital (16.5 ± 9.8 days) as compared to the intervention group (11.0 ± 6.1 days; 14.1 ± 7.0 days) (P = 0.14 and P = 0.17, respectively). The LOIS and LOHS were decreased by 2.2 and 2.4 days, respectively, in the intervention group compared to control group.
Table 2 also shows the microbiologically confirmed cultures positives (from tracheal aspirates, blood, wound discharge and urine). A total of 28 patients were suspected to have infection in both groups, and the samples from different sources in 17 patients were sent for culture to microbiology laboratory. Of 17 patients, pathogenic microorganisms were isolated from different samples of eight patients and rest of the nine patients produced negative results. Of the eight culture positive patients, five were from control group and three patients were from the intervention group. Two patients from the control group and one patient from the intervention group died within the 10 days of study period [Table 2]. One child with TAPVC repair from control group died due to severe sepsis with multiorgan dysfunction syndrome on day 7 postoperatively, and another infant with arterial switch operation for TGA succumbed to severe low cardiac output syndrome and arrhythmias; the death of one baby with intracardiac repair for TOF in the intervention group occurred because of severe right ventricular dysfunction, prolonged ventilation, and ventilator-associated pneumonia (VAP).
Table 4 shows the anthropometric and hematological parameters. There was no significant difference in length, weight, and hematologic parameters statistically, but the data show that there is a continuous weight gain in the intervention group infants throughout the study period and also the PO4 levels in blood were higher in these children (P = 0.05). Serial albumin levels during the study period were found to be higher in the intervention group but were not statistically significant. Rest of the other parameters did not show any major difference between both groups. The CRP level was found to be inversely proportional to the albumin levels in the intervention group, compared to that of the control group [Table 5].
Table 4.
Postoperative clinical and laboratory variables of infants undergoing corrective cardiac surgery
| Group | Length (cm) | Weight (kg) | WBC count (/µL) | Urea (mg %) | Creatinine (mg %) | Bilirubin (mg %) | SGPT (IU/l) | PO4 (mg%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | |
| D 1 | 55.1±4.4 | 55.7±5.5 | 3.5±0.83 | 3.5±0.9 | 10.6±3.5 | 10.5±4.9 | 35±11.6 | 32.3±13.6 | 0.48±0.15 | 0.45±0.14 | 1.6±1.0 | 1.6±1.7 | 65±69 | 79.6±77 | 4.2±0.7 | 4.6±0.7 |
| D 3 | 55.1±4.4 | 55.8±5.6 | 3.5±0.82 | 3.7±0.9 | 8.0±2.7 | 7.3±3.0 | 43.2±25 | 39.7±14.5 | 0.57±0.42 | 0.44±0.13 | 0.96±0.41 | 1.1±1.25 | 50.8±72 | 41.6±33.8 | 4.4±0.8 | 4.6±0.63 |
| D 5 | 54.9±4.6 | 55.9±5.5 | 3.4±0.8 | 3.7±0.91 | 8.6±4.1 | 7.3±2.6 | 44.4±37.6 | 27.2±16.2 | 0.76±0.98 | 0.41±0.18 | 0.8±0.26 | 0.75±0.3 | 42.7±45 | 32.5±19.5 | 3.8±0.57 | 3.8±1.1 |
| D 7 | 54.6±4.6 | 55.9±5.6 | 3.3±0.75 | 3.8±0.81 | 7.8±2.9 | 8.3±2.0 | 36.2±29 | 23.2±16 | 0.59±0.45 | 0.45±0.16 | 0.76±0.25 | 1.24±1.36 | 44±43 | 29.2±18.2 | 3.9±0.8 | 4.85±1.7 |
| D 9 | 55±4.7 | 54.7±5.2 | 3.3±0.7 | 3.6±0.80 | 9.3±3.3 | 8.8±2.8 | 29±20 | 28.5±24.6 | 0.54±0.43 | 0.44±0.18 | 0.63±0.19 | 1.1±1.4 | 31.3±12.2 | 48.2±62.8 | 3.8±1.12 | 4.12±0.60 |
| D 11 | 55±4.6 | 54.7±5.2 | 3.1±0.5 | 3.6±0.7 | 10.7±4.1 | 9.8±3.3 | 23.1±10.7 | 22±11.6 | 0.45±0.27 | 0.43±0.18 | 0.79±0.33 | 0.63±14 | 30±9 | 85.7±12.8 | 3.5±0.47 | 4.16±1.1 |
| P | 0.66 | 0.64 | 0.78 | 0.32 | 0.19 | 0.89 | 0.99 | 0.05 | ||||||||
Data are expressed as mean±SD. D: Days (postoperative), WBC: White blood count, SGPT: Serum glutamate-pyruvate transaminase, SD: Standard deviation
Table 5.
Correlation between serum C-reactive protein and albumin values in the postoperative period
| Variables | Albumin (g %) | CRP (mg/L) | ||
|---|---|---|---|---|
| Groups | Control | Intervention | Control | Intervention |
| D 1 | 2.7±0.4 | 2.8±0.4 | 83.7±42.6 | 83.8±34.4 |
| D 3 | 2.6±0.5 | 2.79±0.5 | 111.1±19.6 | 97.5±44.7 |
| D 7 | 2.2±0.21 | 3.05±0.34 | 70.2±27.8 | 33.8±19.0 |
| P | 0.41 | 0.3 | ||
Data are expressed as mean±SD. D: Days (postoperative), SD: Standard deviation, CRP: C-reactive protein
DISCUSSION
Adequate nutritional support for infants with congenital cardiac malformations after surgical repair remains a significant challenge. The immediate postoperative period after cardiopulmonary bypass is characterized by an intense stress response with activation of the immune-neuroendocrine axis and the inflammatory cascade and is associated with substrate rerouting and hypercatabolism which leads to increased nutritional requirements.[4] This state is further compromised by fluid restriction, digestive intolerance, and postoperative complications. Thus, therapies directed to modulate these stress-related alterations in the metabolic and immune responses could potentially lead to improved patient outcomes. The data from our randomized controlled trial showed that the nutrition therapy (calorie supplementation with breast milk fortification) that we envisaged is feasible and beneficial to such infants. We also defined the beneficial effect in terms of gain in babies’ weight, decrease in mechanical ventilation duration, LOIS and LOHS. The positive trend in each of the parameters in the intervention group compared to control group, reflects the effect of caloric supplementation and fortification of breast milk feeding. The primary outcome was reduction in mortality in babies receiving higher calorie feeds through breast milk and fortification. In our study, the overall mortality rate was also lesser in babies (both groups) who received nutrients early through mother's milk. The death rate was comparatively lesser in the higher caloric-fed intervention group. The trend of infection was higher in the control group. Further, benefits of early feeding with EBM are supplemented by caloric enhancement and fortification of feeds (intervention group) of these critically sick infants in the postoperative period after cardiac surgery.
We could at best achieve up to one-third to half of calculated target nutrition goal in both groups. Only up to 42.2% could be supplemented in the intervention group, which was significantly different from the control group (who got 33.3% of requirement by 10th postoperative day). The baseline calorie intake on day 1 in both groups was very low. Only by postoperative day 10, we could provide 30 kcal/kg bw in control group and 38 kcal/kg bw in intervention group. Our findings of inadequate nutrition consumption are similar to those reported by Kelleher et al.[1] from the Boston Children's Hospital and Schwalbe-Terilli et al. from New York.[13] Delivery of energy and protein was also inadequate in the first 8 days of PICU stay in critically ill children as reported by Kyle et al.[14] However, there was no significant increase in either leukocyte count, CRP, or culture yield in either group after early initiation of breast milk. The decreasing CRP levels were correlating with the gradual increase in the serum albumin levels, confirming convalescence in these babies [Table 5].[15] Reports[15] suggested that a serum CRP level is inversely related to the levels of visceral proteins and a decreasing serum CRP level when associated with an up-going trend in visceral protein might signify reversal of catabolism toward anabolism in response to feeding.
The following factors describe the limitations of exclusive enteral feeding in the postoperative period.[16,17,18,19] There were multiple FI (avoidable and unavoidable) in most of the babies during the 10 days course (due to congestive heart failure, fluid restrictions, feed intolerance, and interruption of feeding for diagnostic and therapeutic procedures or feed aversion). Abdominal distension and gut wall ischemia/edema predisposing to microbial transmigration across intestinal barrier was also quite common in these babies postoperatively. On postcardiopulmonary bypass, alimentation is impaired by mucosal edema, gut ischemia, and ileus.
The study confirmed the nutritional deficit in this group of vulnerable babies after CPB which increases the risk for developing protein energy malnutrition. It further demonstrated that nutrition support was insufficient on several levels, including delayed initiation and under feeding and physical obstacles, including gastrointestinal intolerance, frequent procedures requiring interruption of feeding and hemodynamic instability, as reported previously also.[18,19,20,21]
The practice implication of this result in terms of ventilation duration, LOIS, and LOHS has relevance for reduction in rate of nosocomial infection (HCAI, VAP, etc.) and postoperative growth of babies. However, the differences in these parameters between the two randomized groups are not statistically significant. The study was not powered to detect this difference. To explain this, no previous prospective assessment has been done in this extremely vulnerable group of babies in a randomized control manner. Other investigators have reported, in their retrospective record analysis, significant weight loss with either total PN or early EN initiated after around 3 days of ICU stay postoperatively.[13,19] Our sample size was also determined by the availability of adequate breast milk in consenting mothers. Hence, our observation of no weight loss in either group (control and intervention) with weight gain in the intervention group with caloric supplementation and fortification is a significant finding which needs to be further explored. The underlying cardiac malformation and gut priming preoperatively may be significant determinants of the effect of early nutrition therapy postoperatively.
Limitations of the study
The availability of breast milk for consenting mothers and the avoidable FIs due to various reasons were determining factors in enrollment and continuation of the babies in the study. This impacted our sample size and power of the study. However, the prospective nature of the study and daily assessment of fluid volumes received and flexible caloric consumption enterally enabled us to perform the study rigorously. Besides, the risk of microbial transmigration and gut ischemia was a major influence in our conservative EN. Although we could not demonstrate statistical significance in the improvement in weight and reduction in infection, it had beneficial clinical implications in the postoperative care of these babies. We chose not to include PN to supplement the deficit of caloric supplementation in these babies. The reasons were two-fold. Previous experience, published and unpublished, had demonstrated malnutrition and infection to be more prevalent even with total PN in the early postoperative period. Moreover, the culturally accepted and encouraged practice of breast-feeding in our country makes mother's milk (with or without fortification) the most economically viable and acceptable alternative. Supplemental nutrition helps in achieving maximum possible calculated target calorie/protein/energy required by these babies during early postoperative. The use of more aggressive EN early in postoperative period avoids pitfalls of PN, decreases the incidence of infection, duration of mechanical ventilation, LOIS and LOHS.
CONCLUSIONS
Our study essentially demonstrates the feasibility of early EN for babies with congenital cardiac malformations, postoperatively with mother's milk. Providing fortification in the form of a calorie dense EBM to these infants is tolerated and also benefits in better postoperative recovery with less chance of infection and ICU stay, thereby reducing the health-care burden to the individual and the system. Further prospective randomized controlled studies with bigger sample sizes are required to strengthen the evidence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006;22:237–44. doi: 10.1016/j.nut.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JB, Beekman RH, 3rd, Eghtesady P, Kalkwarf HJ, Uzark K, Kehl JE, et al. Predictors of poor weight gain in infants with a single ventricle. J Pediatr. 2010;157:407–13. doi: 10.1016/j.jpeds.2010.04.012. 413.e1. [DOI] [PubMed] [Google Scholar]
- 3.Williams RV, Zak V, Ravishankar C, Altmann K, Anderson J, Atz AM, et al. Factors affecting growth in infants with single ventricle physiology: A report from the Pediatric Heart Network Infant Single Ventricle Trial. J Pediatr. 2011;159:1017–22.e2. doi: 10.1016/j.jpeds.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coss-Bu JA, Klish WJ, Walding D, Stein F, Smith EO, Jefferson LS. Energy metabolism, nitrogen balance, and substrate utilization in critically ill children. Am J Clin Nutr. 2001;74:664–9. doi: 10.1093/ajcn/74.5.664. [DOI] [PubMed] [Google Scholar]
- 5.Medoff-Cooper B, Irving SY, Marino BS, García-España JF, Ravishankar C, Bird GL, et al. Weight change in infants with a functionally univentricular heart: From surgical intervention to hospital discharge. Cardiol Young. 2011;21:136–44. doi: 10.1017/S104795111000154X. [DOI] [PubMed] [Google Scholar]
- 6.Peterson RE, Wetzel GT. Growth failure in congenital heart disease: Where are we now? Curr Opin Cardiol. 2004;19:81–3. doi: 10.1097/00001573-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Vaidyanathan B, Nair SB, Sundaram KR, Babu UK, Shivaprakasha K, Rao SG, et al. Malnutrition in children with congenital heart disease (CHD) determinants and short term impact of corrective intervention. Indian Pediatr. 2008;45:541–6. [PubMed] [Google Scholar]
- 8.Okoromah CA, Ekure EN, Lesi FE, Okunowo WO, Tijani BO, Okeiyi JC. Prevalence, profile and predictors of malnutrition in children with congenital heart defects: A case-control observational study. Arch Dis Child. 2011;96:354–60. doi: 10.1136/adc.2009.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris MK, Hill CS. Nutritional issues in infants and children with congenital heart disease. Crit Care Nurs Clin North Am. 1994;6:153–63. [PubMed] [Google Scholar]
- 10.Anderson JB, Kalkwarf HJ, Kehl JE, Eghtesady P, Marino BS. Low weight-for-age z-score and infection risk after the Fontan procedure. Ann Thorac Surg. 2011;91:1460–6. doi: 10.1016/j.athoracsur.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie M, Kuijpers M, Van Rossem M, Ravishankar C, Gaynor JW, Spray T, et al. Determinants of intensive care unit length of stay for infants undergoing cardiac surgery. Congenit Heart Dis. 2006;1:152–60. doi: 10.1111/j.1747-0803.2006.00027.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JB, Beekman RH, 3rd, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, et al. Lower weight-for-age Z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009;138:397–404.e1. doi: 10.1016/j.jtcvs.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Schwalbe-Terilli CR, Hartman DH, Nagle ML, Gallagher PR, Ittenbach RF, Burnham NB, et al. Enteral feeding and caloric intake in neonates after cardiac surgery. Am J Crit Care. 2009;18:52–7. doi: 10.4037/ajcc2009405. [DOI] [PubMed] [Google Scholar]
- 14.Kyle UG, Jaimon N, Coss-Bu JA. Nutrition support in critically ill children: Underdelivery of energy and protein compared with current recommendations. J Acad Nutr Diet. 2012;112:1987–92. doi: 10.1016/j.jand.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R. Parenteral Nutrition Guidelines Working Group; European Society for Clinical Nutrition and Metabolism; European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN); European Society of Paediatric Research (ESPR). 1. Guidelines on paediatric parenteral nutrition of the European Society of paediatric gastroenterology, hepatology and nutrition (ESPGHAN) and the European Society for clinical nutrition and metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR) J Pediatr Gastroenterol Nutr. 2005;41(Suppl 2):S1–87. doi: 10.1097/01.mpg.0000181841.07090.f4. [DOI] [PubMed] [Google Scholar]
- 16.Forchielli ML, McColl R, Walker WA, Lo C. Children with congenital heart disease: A nutrition challenge. Nutr Rev. 1994;52:348–53. doi: 10.1111/j.1753-4887.1994.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 17.Lambe C, Hubert P, Jouvet P, Cosnes J, Colomb V. A nutritional support team in the pediatric intensive care unit: Changes and factors impeding appropriate nutrition. Clin Nutr. 2007;26:355–63. doi: 10.1016/j.clnu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira Iglesias SB, Leite HP, Santana e Meneses JF, de Carvalho WB. Enteral nutrition in critically ill children: Are prescription and delivery according to their energy requirements? Nutr Clin Pract. 2007;22:233–9. doi: 10.1177/0115426507022002233. [DOI] [PubMed] [Google Scholar]
- 19.Mehta NM, McAleer D, Hamilton S, Naples E, Leavitt K, Mitchell P, et al. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN J Parenter Enteral Nutr. 2010;34:38–45. doi: 10.1177/0148607109348065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JB, Marino BS, Irving SY, García-España JF, Ravishankar C, Stallings VA, et al. Poor post-operative growth in infants with two-ventricle physiology. Cardiol Young. 2011;21:421–9. doi: 10.1017/S1047951111000229. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Zhang G, Herridge J, Holtby H, Humpl T, Redington AN, et al. Energy expenditure and caloric and protein intake in infants following the Norwood procedure. Pediatr Crit Care Med. 2008;9:55–61. doi: 10.1097/01.PCC.0000298756.82286.23. [DOI] [PubMed] [Google Scholar]