Abstract
Aim
The involvement of various penile structures in radiotherapy (RT)-induced sexual dysfunction among prostate cancer survivors remains unclear and domains beyond erectile dysfunction such as orgasm, and pain have typically not been considered. The purpose of this study was to investigate sexual dysfunction post-RT for localized prostate cancer and to examine whether radiation dose to different penile structures can explain these symptoms.
Methods
We investigated sexual dysfunction in two treated prostate cancer cohorts and in one non-pelvic-irradiated cohort, 328 sexually active men part of an unselected, population-based study conducted in 2008. The treated subjects were prescribed primary/salvage external-beam RT to 70 Gy@2.0 Gy/fraction. Absorbed RT doses (Dmean and Dmax) of the corpora cavernosa (CC), the penile bulb (PB), and the total penile structure (CC + PB) were related to 13 patient-reported symptoms on sexual dysfunction by means of factor analysis (FA) and logistic regression.
Results
Three distinct symptom domains were identified across all cohorts: “erectile dysfunction” (ED, two to five symptoms), “orgasmic dysfunction” (OD, two to four symptoms), and “pain” (two to three symptoms). The strongest predictor for ED symptoms was CC + PB Dmax (P = 0.001–0.03), CC and PB Dmean predicted OD symptoms equally well (P = 0.03 and 0.02–0.05, respectively), and the strongest predictor for pain symptoms was CC + PB Dmean (P = 0.02–0.03).
Conclusion
Sexual dysfunction following RT was separated into three main domains with symptoms related to erectile dysfunction, orgasmic dysfunction, and pain. Chances for intact sexual functionality may be increased if dose to the total penile structure can be restricted for these domains in the planning of RT.
Keywords: Prostate Cancer, Radiotherapy, Sexual Dysfunction
Introduction
Prostate cancer cure rates following radiotherapy (RT) are constantly improving, but RT-induced sexual dysfunction is observed in approximately one of two prostate cancer survivors [1,2]. The involvement of various penile structures for RT-induced sexual dysfunction remains unclear and studied symptom domains are typically restricted to erectile dysfunction. State-of-the-art planning and delivery of RT can offer avoidance of dose to structures involved in sexual dysfunction if “RT-dose-and-sexual-dysfunction relationships” are known. Improving predictive RT dose–response models of all domains, e.g., orgasmic dysfunction, can thus increase possibilities for intact post-RT sexual function with maintained prostate cancer cure rates.
Sexual dysfunction following RT involves the interplay between various structures and symptoms [1,3,4]. In general, predictive dose–response modeling has focused on relationships between doses to individual structures, predominantly the penile bulb (PB), and single erectile dysfunction symptoms [3–6]. However, according to the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) review, which currently provides the most clinically useful data on RT dose–volume responses, the relevance of PB dose for erectile dysfunction is inconclusive between studies [4]. The QUANTEC review also emphasizes the importance of adding the patient’s perspective when establishing links between dose and morbidity. Patient-reported outcomes (PROs), reflecting an individual’s perception of a certain symptom, are particularly suitable to capture delicate symptoms such as those reflecting sexual dysfunction [7,8]. Using PROs as response variables for quantitative modeling of RT-induced morbidity, however, is challenging because of the level of detail in comprehensive questionnaires.
Aims
The aim of the current study was to investigate different domains of sexual dysfunction post-RT for localized prostate cancer and to examine their relationships with various penile structure RT doses.
Methods and Main Outcome Measures
With our approach, we first identify interacting patient-reported sexual dysfunction symptoms and then explore whether symptoms share a common RT-related causal component. To group interacting symptoms, we applied factor analysis (FA). To test if this approach could add to current knowledge regarding RT-related sexual dysfunction, we then investigated relationships between RT dose to the corpora cavernosa (CC), the PB, or the total penile structure (CC + PB), and the interacting symptoms. An overview of our methodological approach is illustrated in Figure 1. Information on the investigated three cohorts and the study-specific questionnaire is summarized below; details on the complete study population and information about the development/validation of the questionnaire can be found in [9–11].
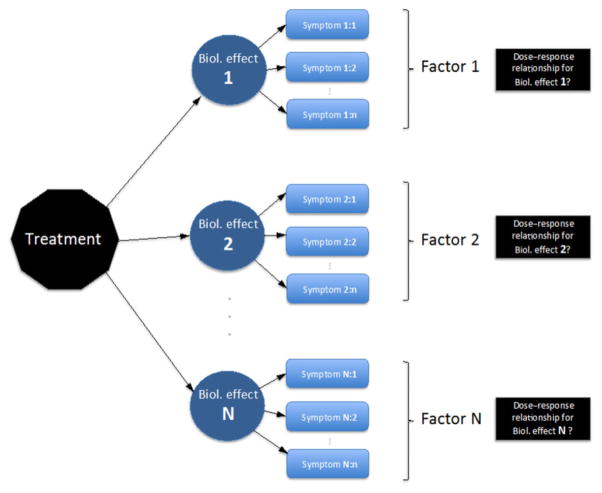
Figure 1.
Overview of our methodological approach to identify symptom domains (reflecting various biological effects), “factors,” and to ultimately identify underlying causal injuries by investigating the relationships between radiotherapy (RT) dose in various critical structures and these “factors.”
Original Study Design and Treatment
The study, which had been approved by the regional ethical review board in Gothenburg, Sweden, consisted of patients consecutively treated with RT for localized prostate cancer at the Sahlgrenska University Hospital in 1993–2006. The patients completed the study-specific questionnaire in 2008 (874/985 returned a completed questionnaire) and among those with retrievable RT treatment plans, 302 patients had been treated with primary external beam radiotherapy abbreviates (EBRT) and 199 patients with surgical prostatectomy followed by salvage EBRT (POSTOP) to a total dose of 70 Gy delivered in daily fractions of 2 Gy, five fractions/week. The study-specific questionnaire had previously been developed and validated according to the methodology at the Divisions of Clinical Epidemiology at the University of Gothenburg and Karolinska institutet in Sweden [11,12]. In short, symptoms are identified after in-depth interviews and operationalized into detailed questions (one atomized, “conceptually clean,” symptom per question with answering categories of symptom frequency or associated degree of severity). In this, and for many questions in previous studies, the questionnaire has undergone extensive face-to-face validation, as well as pilot studies to assess the rate of missing information. Information from a non-pelvic-irradiated reference cohort (REF), which had been matched with respect to age and area of residence, was also available.
Primary and salvage EBRT were planned/delivered with the patient in supine position. The majority of patients were treated with a conformal three-field technique using 15 MV photon beam quality. RT was prescribed consistently according to the International Commission on Radiation Units and Measurements recommendations [13]. The planning target volume (PTV) margin, added to the prostate volume was 20 mm in all directions, except posteriorly where the margin was the lesser of 15 mm or half of the rectal cross-sectional area. For the patients in the POSTOP cohort, the PTV was defined as the postoperative prostatic region with the same applied margins as in the EBRT cohort. For each patient, the CC and the PB were consistently delineated based on their outer borders using previously established delineation procedures [14]. The ventral border was defined at the elongated extension of the corpus spongiosum for PB and at the exit from the abdomen for CC. The CC included also the crus (Figure 2). For the purpose of this study, the total penile structure was defined as CC + PB.
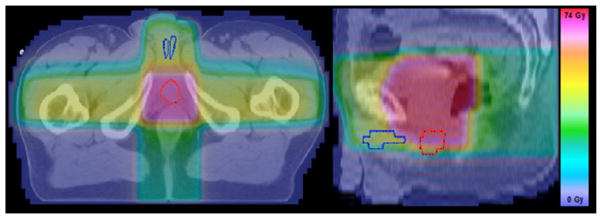
Figure 2.
The corpora cavernosa (blue) and the penile bulb (red) displayed for one prostate cancer patient with the dose distribution overlaid (for a central slice in axial (left) and sagittal (right) view).
The questionnaire contained 165 questions on physical symptoms originating from the pelvis and questions on quality of life, demographics, additional treatments and comorbidities [9]. In this study, we focus on 16 questions that directly reflected physical symptoms of potentially RT-induced sexual dysfunction: erectile dysfunction, orgasmic dysfunction, pain, seminal fluid, and libido (Table S1). Symptom occurrence was assessed “during the previous six months” to balance the noise because of day-by-day events against the risk of not remembering a symptom to be persisting. For the purpose of this study, we focus on sexually active men; the men who responded as being sexually inactive (“Not relevant, I have not been sexually active including self-stimulation” to either of the questions Q3, Q5, Q12–Q15) were excluded from all analyses.
FA to Identify Symptom Domains
Computational details are presented in the Appendix. In brief, results from FA are presented as a set of factors with corresponding factor loadings [15,16]. A factor defines a symptom group and can, when the input is questionnaire-based data, be thought of as a higher order question, a linear combination of questions/symptoms. A factor loading reflects the correlation between a symptom and the factor of a specific symptom group. An FA-based symptom group, thus, includes mathematically constructed co-varying/interacting symptoms, and will henceforth be referred to as a symptom domain.
We applied our recently proposed FA approach in each cohort to identify sexual dysfunction symptom domains [17]. After a number of preprocessing steps (symptom prevalence, missing data, symptom/question-specific answering frequency), symptom groups are identified by exploratory FA (EFA) followed by a confirmatory FA (CFA). In the EFA, we explored two to eight symptom domains, and in the CFA, we retained the symptoms found in the EFA with factor loadings >0.30–0.50 [18]. Agreement between results by EFA and CFA was assessed by the comparative fit index (CFI) ≥ 0.90 (CFI ranges between 0.00 and 1.00; CFI ≥ 0.90 indicates a very good agreement) [19]. Generalizability of the identified symptom domains between EBRT and POSTOP was explored by investigating each cohort-specific final factor model in the other cohort, again using an EFA–CFA approach. All analyses were performed using the R language packages “Psych” [20] and “Lavaan” [21].
Relationships between Dose Parameters and Symptom Domains
A dose–response outcome variable was defined as any combination of symptoms in an identified symptom domain (Boolean “or condition” used when combining symptoms). Suitable dose parameters were then tested as predictors for each outcome variable using logistic regression (statistical significance: two-sided P value ≤ 0.05). The produced model’s discriminative ability to separate patients with and without any of the symptoms in question was then decided by the highest Area under the receiver operating curve value, Az. The strongest “dose–symptom–domain” relationship was typically given by the dose parameter, which generated the highest Az/lowest P value. The symptom cut-off was dichotomized and the analyses were performed separately for mild, moderate, and severe symptom severities, i.e., ≥ answering category 1 (e.g., “any occurrence during the last six months”), 2 (e.g., “occurrence at least on a monthly basis during the last six months”), and 3 (e.g., “occurrence at least on a weekly basis during the last six months”), respectively. To determine suitable dose parameters, we calculated Pearson’s correlation coefficients (Pr) between the mean absorbed dose (Dmean) in CC or PB and various partial relative CC and PB volumes, respectively, receiving ×Gy (Vx), given by the population range of Dmean, in steps of 5 Gy [22].
Results
Symptom Domains Based on FA
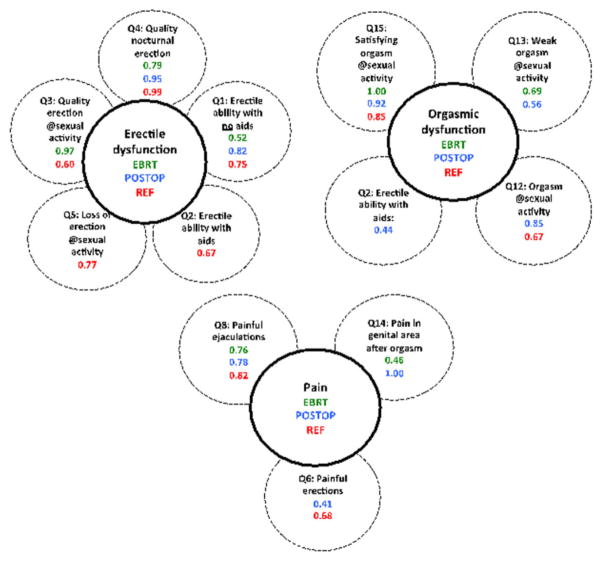
In total, 118/284 (42%), and 70/192 (36%) sexually active men with complete treatment and questionnaire information in the EBRT and POSTOP cohorts, respectively, were retained for analysis because of high symptom/question-specific answering frequencies (Table S2). Altogether, 13 questions were submitted to FA (Q16 excluded because of not directly reflecting a RT-related symptom; Q9, and Q11 excluded due to low symptom prevalence (0–5%); Tables 1 and S1). Three distinct domains were identified for both cohorts: “erectile dysfunction” (two to three symptoms), “orgasmic dysfunction” (two to four symptoms), and “pain” (two to three symptoms; Table S3; Figure 3). The CFI was high within (CFI = 1.00) and between the cohorts (CFI = 0.91 and 0.95 for the POSTOP model in EBRT and vice versa).
Table 1.
Proportions for questions/symptoms considered for factor analysis-based symptom grouping in each cohort
| Proportion (n/N)
|
Question phrasing (status within the last six months) |
Answering category
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study-specific questionnaire | |||||||||||||||||||
| Question ID | Mild
|
Moderate
|
Severe
|
>0
|
|||||||||||||||
| 1
|
2
|
3
|
4
|
5
|
|||||||||||||||
| EBRT | POSTOP | REF | EBRT | POSTOP | REF | EBRT | POSTOP | REF | EBRT | POSTOP | REF | EBRT | POSTOP | REF | EBRT | POSTOP | REF | ||
| 1 | Ability to get an erection without medication or technical aids | 0.09 | 0.07 | 0.46 | 0.33 | 0.23 | 0.35 | 0.44 | 0.29 | 0.11 | — | — | — | — | — | — | 0.90 | 0.96 | 0.54 |
| 2 | Ability to get an erection with medication or technical aids | 0.05 | 0.26 | 0.04 | 0.17 | 0.13 | 0.07 | 0.04 | 0.17 | 0.02 | — | — | — | — | — | — | 0.25 | 0.36 | 0.11 |
| 3 | Quality of erection at sexual activity | 0.17 | 0.20 | 0.49 | 0.18 | 0.11 | 0.24 | 0.20 | 0.13 | 0.07 | 0.17 | 0.17 | 0.11 | — | — | — | 0.83 | 0.81 | 0.50 |
| 4 | Quality of nocturnal erection | 0.07 | 0.07 | 0.31 | 0.07 | 0.06 | 0.18 | 0.10 | 0.07 | 0.11 | 0.16 | 0.09 | 0.09 | — | — | — | 0.47 | 0.43 | 0.41 |
| 5 | Loss of erection during sexual activity | 0.29 | 0.37 | 0.49 | 0.32 | 0.27 | 0.31 | 0.16 | 0.10 | 0.10 | 0.09 | 0.13 | 0.05 | — | — | — | 0.71 | 0.60 | 0.51 |
| 6 | Painful erections | 0.76 | 0.76 | 0.91 | 0.03 | 0.06 | 0.01 | 0.03 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 | 0.01 | 0.00 | 0.00 | 0.06 | 0.10 | 0.04 |
| 7 | Amount of sexual desire | 0.06 | 0.10 | 0.16 | 0.63 | 0.64 | 0.69 | 0.31 | 0.23 | 0.13 | — | — | — | — | — | — | 0.95 | 0.93 | 0.84 |
| 8 | Painful ejaculations | 0.88 | 0.80 | 0.96 | 0.08 | 0.11 | 0.02 | 0.00 | 0.00 | 0.01 | 0.03 | 0.01 | 0.00 | — | — | — | 0.11 | 0.17 | 0.03 |
| 9 | Discomfortable but not painful, ejaculations | 0.93 | 0.89 | 0.95 | 0.03 | 0.01 | 0.01 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | — | — | — | 0.05* | 0.01* | 0.02* |
| 10 | Amount of secretion or expulsion of semen following ejaculation | 0.00 | 0.03 | 0.09 | 0.12 | 0.03 | 0.72 | 0.76 | 0.06 | 0.16 | — | — | — | — | — | — | 0.98 | 0.94 | 0.91 |
| 11 | Blood in semen | 0.92 | 0.23 | 0.96 | 0.02 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | — | — | — | 0.02* | 0.00* | 0.01* |
| 12 | Orgasm at sexual activity | 0.35 | 0.46 | 0.58 | 0.31 | 0.30 | 0.22 | 0.11 | 0.11 | 0.08 | 0.15 | 0.13 | 0.11 | — | — | — | 0.65 | 0.57 | 0.42 |
| 13 | Sensation of orgasm at sexual activity weaker than standard | 0.39 | 0.46 | 0.67 | 0.19 | 0.20 | 0.17 | 0.19 | 0.11 | 0.06 | 0.08 | 0.11 | 0.06 | — | — | — | 0.60 | 0.56 | 0.33 |
| 14 | Pain in genital area after orgasm | 0.92 | 0.94 | 0.96 | 0.04 | 0.03 | 0.04 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | — | — | — | 0.07 | 0.06 | 0.04 |
| 15 | Sensation of orgasm at sexual activity satisfying | 0.19 | 0.33 | 0.51 | 0.28 | 0.20 | 0.24 | 0.19 | 0.23 | 0.09 | 0.06 | 0.04 | 0.07 | — | — | — | 0.81 | 0.67 | 0.49 |
Bold styling indicates prevalence ≥10%;—Missing answering category (cf. Table S1);
Exclusion due to prevalence <2% (Q9 and Q11 had an overall low prevalence and were excluded from all cohorts).
Figure 3.
The symptoms (question id, phrasing, factor loading) in the three symptom domains identified in each cohort (EBRT: green; POSTOP: blue; REF: red).
In the REF cohort, 140 (58%) sexually active men and the same 13 questions were retained for the same reasons as for the treated cohorts (Tables 1 and S1). “Erectile dysfunction” (two to three symptoms), “orgasmic dysfunction” (two symptoms), and “pain” (two symptoms) were also identified for REF, but “erectile dysfunction” consisted of two separate groups (CFI = 0.97; Table S3; Figure 3). In general, the symptom prevalence in the identified domains was up to four times lower for corresponding symptoms in REF than in the treated cohorts.
Relationships between Dose Parameters and Identified Symptom Domains
For CC and PB in both EBRT and POSTOP, Pr between the population range of Dmean and various Vx was high: Pr = 0.75–0.95 (V10–V40) for CC, and Pr = 0.86–0.96 (V30–V65) for PB. We, therefore, used Dmax (maximum dose) and Dmean of CC, PB, and CC + PB to investigate relationships with the identified symptom domains (Table 2).
Table 2.
Population median values (range) from EBRT and POSTOP for the investigated structures and dose metrics
| Cohort | Structure | Volume (cm3) | Dmax (Gy) | Dmean (Gy) |
|---|---|---|---|---|
| EBRT | CC | 11.7 (3.6–24.6) | 68.8 (27.3–75.7) | 39.7 (5.7–50.8) |
| PB | 7.2 (4.4–12.1) | 70.7 (55.7–76.9) | 65.7 (25.9–74.3) | |
| CC + PB | 19.2 (9.4–32.2) | 70.8 (55.7–76.9) | 49.4 (18.7–60.2) | |
| POSTOP | CC | 19.0 (10.8–34.4) | 69.6 (44.1–72.9) | 41.0 (13.1–52.3) |
| PB | 7.9 (5.0–13.0) | 70.6 (65.5–73.7) | 67.2 (38.4–71.1) | |
| CC + PB | 11.5 (3.1–24.4) | 70.9 (65.5–73.7) | 51.7 (26.4–51.7) |
CC = corpora cavernosa; Dmax = maximum absorbed dose; Dmean = mean absorbed dose; PB = penile bulb.
For mild symptom severity, dose to all investigated structures significantly predicted “erectile dysfunction” (EBRT: PB Dmax/Dmean, and CC + PB Dmean; POSTOP: CC and PB Dmean, and CC + PB Dmax/Dmean) and “pain” (EBRT: CC, PB and CC + PB Dmean, and CC + PB Dmax; POSTOP: CC Dmax) with the strongest relationship observed for CC + PB Dmean (“erectile dysfunction” (EBRT/POSTOP): P = 0.004/0.02; Az = 0.81/0.67 and “pain” (EBRT): P = 0.02; Az = 0.68, respectively, Table 3). For moderate symptoms, dose to all structures (EBRT: PB Dmax/Dmean; POSTOP: CC Dmax, PB and CC + PB Dmax/Dmean) again predicted “erectile dysfunction” with CC + PB Dmax being the best predictor (POSTOP: P = 0.001; Az = 0.78; Figure S1), whereas only PB Dmean predicted “orgasmic dysfunction” (POSTOP: P = 0.02–0.05; Az = 0.65–0.67). For severe symptoms, CC + PB Dmax also showed the strongest relationship with “erectile dysfunction” (POSTOP: P = 0.004; Az = 0.72), but relationships were additionally found with dose to CC or PB (EBRT: CC Dmean; POSTOP: CC and PB Dmax). Only one relationship was observed for “orgasmic dysfunction” (EBRT: CC Dmean).
Table 3.
Logistic regression results for “Erectile dysfunction,” “Orgasmic dysfunction,” and “Pain” in EBRT and POSTOP
| Cohort | Symptom severity | Structure | Dose metric | Erectile dysfunction
|
Orgasmic dysfunction
|
Pain
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | AZ | Question ID | Number of Qs | P | AZ | Question ID | Number of Qs | P | AZ | Question ID | Number of Qs | ||||
| EBRT | ≥Mild | CC | Dmax | 0.45 | 0.56 | Q3/Q4 | 2 | 0.51 | 0.55 | Q15 | 1 | 0.13 | 0.62 | Q8/Q14 | 2 |
| Dmean | 0.11 | 0.62 | Q3 | 1 | 0.18 | 0.57 | Q13 | 1 | 0.05 | 0.65 | Q8/Q14 | 2 | |||
| CC + PB | Dmax | 0.09 | 0.62 | Q3 | 1 | 0.63 | 0.53 | Q15 | 1 | 0.03 | 0.67 | Q8/Q14 | 2 | ||
| 0.05 | 0.67 | Q8 | 1 | ||||||||||||
| 0.08 | 0.69 | Q14 | 1 | ||||||||||||
| Dmean | 0.02 | 0.67 | Q3 | 1 | 0.72 | 0.52 | Q13 | 1 | 0.02 | 0.68 | Q8/Q14 | 2 | |||
| 0.03 | 0.73 | Q14 | 1 | ||||||||||||
| PB | Dmax | 0.03 | 0.66 | Q3 | 1 | 0.65 | 0.53 | Q13/Q15 | 2 | 0.06 | 0.64 | Q8/Q14 | 2 | ||
| 0.10 | 0.64 | Q8 | 1 | ||||||||||||
| Dmean | 0.02 | 0.67 | Q3 | 1 | 0.10 | 0.62 | Q13/Q15 | 2 | 0.05 | 0.71 | Q14 | 1 | |||
| ≥Moderate | CC | Dmax | 0.12 | 0.60 | Q1/Q4 | 2 | 0.48 | 0.54 | Q15 | 1 | |||||
| Dmean | 0.45 | 0.55 | Q1/Q4 | 2 | 0.21 | 0.57 | Q15 | 1 | |||||||
| CC + PB | Dmax | 0.17 | 0.57 | Q1 | 1 | 0.79 | 0.49 | Q13/Q15 | 2 | ||||||
| Dmean | 0.09 | 0.60 | Q3/Q4 | 2 | 0.50 | 0.54 | Q15 | 1 | |||||||
| PB | Dmax | 0.04 | 0.61 | Q1 | 1 | 0.36 | 0.55 | Q13/Q15 | 2 | ||||||
| Dmean | 0.05 | 0.61 | Q1 | 1 | 0.78 | 0.52 | Q13 | 1 | |||||||
| 0.08 | 0.62 | Q1/Q3/Q4 | 3 | ||||||||||||
| ≥Severe | CC | Dmax | 0.43 | 0.54 | Q1/Q4 | 2 | 0.08 | 0.60 | Q15 | 1 | |||||
| Dmean | 0.05 | 0.66 | Q1 | 1 | 0.03 | 0.62 | Q15 | 1 | |||||||
| 0.06 | 0.60 | Q13/Q15 | 2 | ||||||||||||
| CC + PB | Dmax | 0.53 | 0.55 | Q1 | 1 | 0.08 | 0.61 | Q13 | 1 | ||||||
| Dmean | 0.12 | 0.63 | Q1 | 1 | 0.14 | 0.58 | Q15 | 1 | |||||||
| PB | Dmax | 0.54 | 0.53 | Q3 | 1 | 0.14 | 0.60 | Q13 | 1 | ||||||
| Dmean | 0.30 | 0.56 | Q3/Q4 | 2 | 0.07 | 0.60 | Q15 | 1 | |||||||
| POSTOP | ≥Mild | CC | Dmax | 0.27 | 0.62 | Q4 | 1 | 0.13 | 0.61 | Q12/Q15 | 2 | 0.05 | 0.80 | Q14 | 1 |
| Dmean | 0.04 | 0.72 | Q4 | 1 | 0.32 | 0.57 | Q15 | 1 | 0.40 | 0.57 | Q6/Q8 | 2 | |||
| 0.40 | 0.57 | Q6/Q8/Q14 | 3 | ||||||||||||
| CC + PB | Dmax | 0.03 | 0.74 | Q4 | 1 | 0.58 | 0.54 | Q12/Q13 | 2 | 0.13 | 0.73 | Q14 | 1 | ||
| 0.09 | 0.75 | Q1/Q4 | 2 | ||||||||||||
| Dmean | 0.004 | 0.81 | Q4 | 1 | 0.31 | 0.57 | Q13 | 1 | 0.15 | 0.62 | Q6/Q8 | 2 | |||
| 0.15 | 0.62 | Q6/Q8/Q14 | 3 | ||||||||||||
| PB | Dmax | 0.08 | 0.69 | Q4 | 1 | 0.37 | 0.57 | Q12/Q13 | 2 | 0.16 | 0.71 | Q14 | 1 | ||
| Dmean | 0.01 | 0.78 | Q4 | 1 | 0.11 | 0.63 | Q13/Q15 | 2 | 0.38 | 0.58 | Q6/Q8 | 2 | |||
| 0.08 | 0.76 | Q1/Q4 | 2 | 0.11 | 0.63 | Q12/Q13/Q15 | 3 | 0.38 | 0.58 | Q6/Q8/Q14 | 3 | ||||
| ≥Moderate | CC | Dmax | 0.02 | 0.71 | Q1/Q4 | 2 | 0.51 | 0.45 | Q12 | 1 | |||||
| 0.02 | 0.70 | Q4 | 1 | ||||||||||||
| Dmean | 0.10 | 0.65 | Q1/Q4 | 2 | 0.41 | 0.56 | Q2/Q13/Q15 | 3 | |||||||
| 0.41 | 0.56 | Q2/Q12/Q13/Q15 | 4 | ||||||||||||
| CC + PB | Dmax | 0.001 | 0.78 | Q4 | 1 | 0.50 | 0.55 | Q13 | 1 | ||||||
| 0.002 | 0.77 | Q1/Q4 | 2 | ||||||||||||
| 0.01 | 0.69 | Q1 | 1 | ||||||||||||
| Dmean | 0.03 | 0.69 | Q4 | 1 | 0.10 | 0.62 | Q2/Q12/Q13 | 3 | |||||||
| 0.03 | 0.69 | Q1/Q4 | 2 | ||||||||||||
| PB | Dmax | 0.004 | 0.74 | Q4 | 1 | 0.30 | 0.58 | Q13 | 1 | ||||||
| 0.01 | 0.73 | Q1/Q4 | 2 | ||||||||||||
| 0.05 | 0.65 | Q1 | 1 | ||||||||||||
| Dmean | 0.02 | 0.70 | Q4 | 1 | 0.02 | 0.67 | Q2/Q12/Q13 | 3 | |||||||
| 0.07 | 0.66 | Q1/Q4 | 2 | 0.03 | 0.66 | Q2/Q13/Q15 | 3 | ||||||||
| 0.03 | 0.66 | Q2/Q12/Q13/Q15 | 4 | ||||||||||||
| 0.04 | 0.65 | Q2/Q13 | 2 | ||||||||||||
| 0.05 | 0.65 | Q2/Q15 | 2 | ||||||||||||
| 0.05 | 0.65 | Q2/Q12/Q15 | 3 | ||||||||||||
| 0.06 | 0.63 | Q12/Q13 | 2 | ||||||||||||
| 0.07 | 0.63 | Q2/Q12 | 2 | ||||||||||||
| ≥Severe | CC | Dmax | 0.02 | 0.67 | Q4 | 1 | 0.17 | 0.60 | Q2/Q12 | 2 | |||||
| 0.02 | 0.67 | Q1/Q4 | 2 | ||||||||||||
| Dmean | 0.37 | 0.57 | Q4 | 1 | 0.41 | 0.56 | Q2/Q13 | 2 | |||||||
| 0.37 | 0.57 | Q1/Q4 | 2 | 0.41 | 0.56 | Q2/Q12/Q13 | 3 | ||||||||
| CC + PB | Dmax | 0.004 | 0.72 | Q4 | 1 | 0.21 | 0.60 | Q2 | 1 | ||||||
| 0.004 | 0.72 | Q1/Q4 | 2 | ||||||||||||
| Dmean | 0.11 | 0.62 | Q4 | 1 | 0.34 | 0.60 | Q12 | 1 | |||||||
| 0.11 | 0.62 | Q1/Q4 | 2 | ||||||||||||
| PB | Dmax | 0.03 | 0.67 | Q4 | 1 | 0.11 | 0.62 | Q2 | 1 | ||||||
| 0.03 | 0.67 | Q1/Q4 | 2 | ||||||||||||
| Dmean | 0.07 | 0.64 | Q4 | 1 | 0.22 | 0.63 | Q12 | 1 | |||||||
| 0.07 | 0.64 | Q1/Q4 | 2 | ||||||||||||
Note: The model with the lowest P value is given on the top row for each symptom domain; white denotes P ≤ 0.05; dark grey denotes P > 0.10 and light grey denotes P = 0.06–0.10.
Az = area under the receiver operating characteristic curve; CC = corpora cavernosa; CC + PB = total penile structure; Dmax = maximum absorbed dose; Dmean = mean absorbed dose; PB = penile bulb.
Discussion
In this study, we present relationships between dose to the penile structures and patient-reported sexual dysfunction after primary or salvage EBRT for localized prostate cancer. RT dose to the CC, PB, or the total penile structure all predicted the symptom domains “erectile dysfunction,” “orgasmic dysfunction,” and “pain” with the total penile structure particularly providing models with a better discriminative ability for “erectile dysfunction.”
We found typically stronger relationships for mild, moderate, and severe “Erectile dysfunction” using dose to the total penile structure than to the isolated CC or PB. The sexual dysfunction-specific QUANTEC review [4] recognized that the PB is probably only a surrogate for the erectile apparatus, but that keeping specific PB volume cut points below a certain dose may be prudent for intact erectile function post-RT. Nor has accumulated evidence on effects by dose to the CC, or the total penile structure been strong enough to motivate RT avoidance of these structures [6]. Reason for these diverging results is partly appointed to small studies (less than 60 individuals). For example, Selek et al. [5], could not establish relationships between dose to these structures and erectile dysfunction at 2 years follow-up (N = 28). Their population averaged mean dose to the total penile structure was 38 Gy, around 10 Gy lower than in our cohort. In our larger series where wider PTV margins around the prostate/postoperative prostatic region were used, we detected dose–response relationships for all these penile structures.
Late normal tissue effects are typically assessed by physicians and many symptoms are combined into practical, but simplified scores. Among the 13 PRO-based symptoms on sexual dysfunction considered in our analyses, we identified two to three symptoms grouped together as an “erectile dysfunction” domain. A number of PRO-based questionnaires to quantify sexual dysfunction exist [7,23,24]. These questionnaires include one to six symptoms on erectile dysfunction and their levels of detail is similar to the symptoms in our “erectile dysfunction” domain. Our analysis approach could, therefore, be of use in dose–response modeling of erectile dysfunction as quantified by these questionnaires.
Even though we identified three distinct and similar domains across the two treated cohorts, some differences were detected. For “Erectile dysfunction,” the same two symptoms reflecting both a natural erection (without erectile aids/medical therapy) and the nocturnal erectile quality were included for all cohorts. For EBRT, one additional symptom reflecting erectile quality at sexual activity was included. This suggests that erectile dysfunction could present partially different depending on RT technique. Indeed, it has been suggested that erectile dysfunction following salvage EBRT could be due to changes in neurovascular mechanisms, resulting in corporal smooth muscle damage [25], and penile hypoxia leading to collagen accumulation, smooth muscle atrophy, and fibrosis [26]. Erectile dysfunction after primary EBRT could be due to alterations in erectile hemodynamics and nerve damage in the penile shaft [27]. The same two symptoms reflecting non-satisfaction of orgasm and weak orgasm were included in “orgasmic dysfunction” for both cohorts, but in POSTOP, two symptoms were added (orgasm at sexual activity and ability to get erection with erectile aids/medical therapy). To sum up, one could speculate that symptoms of a FA-based symptom domain, although being constructed mathematically, might share some underlying pathophysiology or that they could be associated through a chain of separate pathophysiological events linking them together.
The main strengths of this work concern the 13 atomized PROs on sexual dysfunction, of which many are not addressed in common scoring systems, and of which the majority were included in our symptom domains. The three investigated cohorts allowed for the testing of generalizability of results with respect to treatment-specific symptom profiles and to background sexual dysfunction given availability to the reference subjects. Together, this facilitated a novel approach to investigate relationships between dose to the CC, the PB, or the total penile structure and three sexual dysfunction symptom domains in sexually active men. In contrast to standard dose–response modeling approaches where single symptoms are pre-selected, our approach has the advantage of addressing multiple symptoms simultaneously and can thereby capture the wider consequences of a specific RT-induced injury. Limitations are that we did not analyze the entire dose distribution in the presence of potential confounders, and our results were based on information from homogeneous patients in terms of age and ethnicity. We did not have information on baseline symptom rates in the treated cohorts. We did, however, test the impact of time to follow-up with respect to the risk of having symptoms in a specific symptom domain (exemplified for mild “Erectile dysfunction,” “Orgasmic dysfunction,” and “Pain” in EBRT; Figure S2). Up to at least 9 years post-RT, the overall risk remained stable regardless of the number of symptoms. Finally, we did not value sexual activity or performance without erectogenic aids from that with aids in this work. Our results suggest that questions specifically addressing the presence of naturally occurring erections can be critical for the “Erectile dysfunction” domain, and, therefore, this distinction may be important to address further.
Conclusions
Our findings support that doses to structures beyond the PB are important for sexual dysfunction post-RT. Dose to the total penile structure was more strongly correlated with erectile dysfunction, orgasmic dysfunction, and pain symptoms than dose to either the isolated CC or the PB. Our data suggest that future dose–response modeling of patient-reported sexual dysfunction needs to consider specific symptoms related to erection (naturally occurring, i.e., without erectogenic aids/medical therapy, nocturnal quality, and quality during sexual activity), and should also acknowledge orgasmic dysfunction. By illustrating the interplay between structures and symptoms related to sexual dysfunction, we hope that this work can contribute to a better understanding of the underlying RT-induced causes for sexual dysfunction.
Supplementary Material
Footnotes
Conflict of Interest: The author(s) report no conflicts of interest.
Statement of Authorship
-
Conception and DesignMaria Thor, Caroline E. Olsson, Jung Hun Oh, Joseph O. Deasy, Gunnar Steineck
-
Acquisition of DataMaria Thor, Caroline E. Olsson, David Alsadius, Niclas Pettersson
-
Analysis and Interpretation of DataMaria Thor, Caroline E. Olsson, Jung Hun Oh, Joseph O. Deasy, Gunnar Steineck
-
Drafting the ArticleMaria Thor, Caroline E. Olsson
-
Revising It for Intellectual ContentMaria Thor, Caroline E. Olsson, Jung Hun Oh, David Alsadius, Niclas Pettersson, Joseph O. Deasy, Gunnar Steineck
-
Final Approval of the Completed ArticleMaria Thor, Caroline E. Olsson, Jung Hun Oh, David Alsadius, Niclas Pettersson, Joseph O. Deasy, Gunnar Steineck
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Figure S1 Relationships between dose and various numbers of symptoms in a symptom domain exemplified for maximum absorbed dose to the total penile structure (CC + PB) and moderate “Erectile dysfunction” in POSTOP. Note: The population averaged dose for 0/1/2 symptoms is denoted in each boxplot.
Figure S2 Time to follow-up (years) with respect to the “risk” (%) of having the symptoms in a symptom domain exemplified for mild “Erectile dysfunction” (low risk (white): 0–1 symptom; moderate risk (grey): 2 symptoms; high risk (black): 3 symptoms), “Orgasmic dysfunction” (low risk (white): 0–1 symptom; intermediate risk (grey): 2 symptoms), and “Pain” (intermediate risk (grey): 2 symptoms) in EBRT. Note: The number of patients in each bar is denoted above the bar.
Table S1 Excerpts from the original study-specific questionnaire including the English-translated symptom categories, question phrasing, and symptoms on sexual dysfunction.
Table S2 Demographical characteristics for the sexually active men in the EBRT, POSTOP, and the REF cohort.
Table S3 Number of questions per symptom domain with altered factor loading thresholds for EBRT, POSTOP, and REF. The final symptom domains (Figure 3) are enclosed within bold squares.
Appendix S1 Detailed description of the FA approach and results for sexual dysfunction.
References
- 1.Budaus L, Bolla M, Bossi A, Cozzarini C, Crook J, Widmark A, Wiegel T. Functional outcomes and complications following radiation therapy for prostate cancer: A critical analysis of the literature. Eur Urol. 2012;61:112–27. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, Carver B, Coleman J, Lovelock M, Hunt M. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–9. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 3.Mirza M, Griebling TL, Wallace Kazer M. Erectile dysfunction and urinary incontinence after prostate cancer treatment. Semin Oncol Nurs. 2011;27:278–89. doi: 10.1016/j.soncn.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Roach M, 3rd, Nam J, Gagliardi G, El Naqa I, Deasy JO, Marks LB. Radiation dose–volume effects and the penile bulb. Int J Radiat Oncol Biol Phys. 2010;76:S130–4. doi: 10.1016/j.ijrobp.2009.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selek U, Cheung R, Lii M, Allen P, Steadham RE, Vantreese TR, Jr, Little DJ, Rosen II, Kuban D. Erectile dysfunction and radiation dose to penile base structures: A lack of correlation. Int J Radiat Oncol Biol Phys. 2004;59:1039–46. doi: 10.1016/j.ijrobp.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 6.van der Wielen GJ, Mulhall JP, Incrocci L. Erectile dysfunction after radiotherapy for prostate cancer and radiation dose to the penile structures: A critical review. Radiother Oncol. 2007;84:107–13. doi: 10.1016/j.radonc.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui F, Liu AK, Watkins-Bruner D, Movsas B. Patient-reported outcomes and survivorship in radiation oncology: Overcoming the cons. J Clin Oncol. 2014;32:2920–7. doi: 10.1200/JCO.2014.55.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsadius D, Hedelin M, Johansson KA, Pettersson N, Wilderäng U, Lundstedt D, Steineck G. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer. Radiother Oncol. 2011;101:495–501. doi: 10.1016/j.radonc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Olsson CE, Alsadius D, Pettersson N, Tucker SL, Wilderäng U, Johansson KA, Steineck G. Patient-reported sexual toxicity after radiation therapy in long-term prostate cancer survivors. Br J Cancer. 2015;113:802–8. doi: 10.1038/bjc.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steineck G, Bergmark K, Henningsohn L, al-Abany M, Dickman PW, Helgason A. Symptom documentation in cancer survivors as a basis for therapy modifications. Acta Oncol. 2002;41:244–52. doi: 10.1080/02841860260088782. [DOI] [PubMed] [Google Scholar]
- 12.Steineck G, Hunt H, Adolfsson J. A hierarchical step-model for causation of bias-evaluating cancer treatment with epidemiological methods. Acta Oncol. 2006;45:421–9. doi: 10.1080/02841860600649293. [DOI] [PubMed] [Google Scholar]
- 13.International Commissions on Radiation Units and Measurements (ICRU) I. Prescribing, Recording and Reporting Photon Beam Therapy. Bethesda MD: 1999. (Supplement to ICRU Report 50) [Google Scholar]
- 14.Waldenström AC, Alsadius D, Pettersson N, Johansson KA, Holmberg E, Steineck G, Muller M. Variation in position and volume of organs at risk in the small pelvis. Acta Oncol. 2010;49:491–9. doi: 10.3109/02841861003702536. [DOI] [PubMed] [Google Scholar]
- 15.Fabrigar LR, MacCallum RC, Wegener DT, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychol Methods. 1999;4:272–99. [Google Scholar]
- 16.Farnell DJ, Mandall P, Anandadas C, Routledge J, Burns MP, Logue JP, Wylie JP, Swindell R, Livsey J, West CM, Davidson SE. Development of a patient-reported questionnaire for collecting toxicity data following prostate brachytherapy. Radiother Oncol. 2010;97:136–42. doi: 10.1016/j.radonc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Thor M, Olsson CE, Oh JH, Petersen SE, Alsadius D, Bentzen L, Pettersson N, Muren LP, Waldenström AC, Høyer M, Steineck G, Deasy JO. Relationships between dose to the gastro-intestinal tract and patient-reported symptom domains after radiotherapy for localized prostate cancer. Acta Oncol. 2015;4:1–9. doi: 10.3109/0284186X.2015.1063779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skerman HM, Yates PM, Battistutta D. Multivariate methods to identify cancer-related symptom clusters. Res Nurs Health. 2009;32:345–60. doi: 10.1002/nur.20323. [DOI] [PubMed] [Google Scholar]
- 19.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 20.Revelle W. Procedures for personality and psychological research. Evanston, Ill, USA: Northwestern University; 2014. [Google Scholar]
- 21.Rosseel Y. An R package for structural equation modeling. J Stat Softw. 2012;48:21–36. [Google Scholar]
- 22.Vainshtein JM, Griffith KA, Feng FY, Vineberg KA, Chepeha DB, Eisbruch A. Patient-reported voice and speech outcomes after whole-neck intensity modulated radiation therapy and chemotherapy for oropharyngeal cancer: Prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2014;89:973–80. doi: 10.1016/j.ijrobp.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Szymanski KM, Dunn RL, Chipman JJ, Litwin MS, Nguyen PL, Sweeney CJ, Cook R, Wagner AA, DeWolf WC, Bubley GJ, Funches R, Aronovitz JA, Wei JT, Sanda MG. Expanded prostate cancer index composite for clinical practice: Development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J Urol. 2011;186:865–72. doi: 10.1016/j.juro.2011.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: Development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Kendirci M, Bejma J, Hellstrom WJ. Update on erectile dysfunction in prostate cancer patients. Curr Opin Urol. 2006;16:186–95. doi: 10.1097/01.mou.0000193407.05285.d8. [DOI] [PubMed] [Google Scholar]
- 26.Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: A critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–47. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Carrier S, Hricak H, Lee SS, Baba K, Morgan DM, Nunes L, Ross GY, Phillips TL, Lue TF. Radiation-induced decrease in nitric oxide synthase—Containing nerves in the rat penis. Radiology. 1995;195:95–9. doi: 10.1148/radiology.195.1.7534430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.