Abstract
Decorin, a small leucine-rich proteoglycan, regulates extracellular matrix organization, growth factor-mediated signaling and cell growth. As decorin may directly modulate immune responses, we investigated its role in a mouse model of contact allergy (oxazolone-mediated delayed-type hypersensitivity, DTH) in decorin-deficient (Dcn−/−) and wild-type mice. Dcn−/− mice showed a reduced ear swelling 24 hours after oxazolone treatment with a concurrent attenuation of leukocyte infiltration. These findings were corroborated by reduced glucose metabolism as determined by 18FDG uptake in positron emission tomography scans. Unexpectedly, polymorphonuclear leukocyte numbers in Dcn−/− blood vessels were significantly increased, accompanied by large numbers of flattened leukocytes adherent to the endothelium. Intravital microscopy, flow chamber and static adhesion assays confirmed increased adhesion and reduced transmigration of Dcn−/− leukocytes. Circulating blood neutrophil numbers were significantly increased in Dcn−/− mice 24 hours after DTH elicitation, but only moderately increased in wild-type mice. Expression of the pro-inflammatory cytokine TNF-α was reduced, while syndecan-1 and ICAM-1 were overexpressed in inflamed ears of Dcn−/− mice, indicating that these adhesion molecules could be responsible for increased leukocyte adhesion. Decorin treatment of endothelial cells increased tyrosine phosphorylation and reduced syndecan-1 expression. Notably, absence of syndecan-1 in a genetic background lacking decorin rescued the attenuated DTH phenotype of Dcn−/− mice. Collectively, these results implicate a role for decorin in mediating DTH responses by influencing polymorphonuclear leukocyte attachment to the endothelium. This occurs via two non-mutually exclusive mechanisms that involve a direct anti-adhesive effect on polymorphonuclear leukocytes and a negative regulation of ICAM-1 and syndecan-1 expression.
Introduction
Delayed-type hypersensitivity (DTH) is a common mouse model for allergic contact dermatitis (1), which allows studying cell-mediated immune responses in vivo (2). The sensitization phase is characterized by covalent modification of surface proteins with the hapten oxazolone, followed by the uptake and processing by dendritic and Langerhans cells which migrate to lymph nodes and prime oxazolone-specific T cell populations (1, 2). During the elicitation phase, oxazolone exposure evokes recruitment and activation of primed T cells followed by synthesis and local release of chemokines and cytokines, and mast cell degranulation. The release of vasoactive mediators leads to a massive leukocytic infiltration of the skin, a process that depends on cytokine gradients, and the synthesis and activation of adhesion molecules of the integrin, selectin and cell adhesion molecule (CAM) families (1, 2).
During inflammation, polymorphonuclear leukocytes follow chemotactic gradients to attach to activated endothelial cells resulting in leukocyte diapedesis, penetration of the subendothelial matrix and migration into areas of tissue damage (3, 4). This process involves coordinated signaling events mediated by pro-inflammatory cytokines and chemokines, and sequential interactions with multiple adhesion molecules including selectins and their carbohydrate ligands, and integrins (3, 4). All of these steps are modulated by various types of proteoglycans (4, 5). Biochemical data have demonstrated sequence-specific interactions of glycosaminoglycans with a variety of ligands relevant to inflammation (6). For example, mice deficient in syndecan-1 (Sdc1−/−) show increased leukocyte recruitment upon intraperitoneal TNF-α stimulation, during kidney inflammation, contact allergies and colitis (7–9). Although the role for heparan sulfate in inflammation is increasingly recognized, the role of dermatan/chondroitin sulfate is less well investigated. Several observations indicate that dermatan sulfate can interact with various cytokines and chemokines (10), including FGF family members (11), and heparin cofactor II (6). Moreover, injection of mice with dermatan sulfate increases the soluble levels of circulating ICAM-1 (12). From a clinical perspective, the use of dermatan sulfate may be advantageous in comparison to heparin, due to the lack of anticoagulant side effects. The most prominent dermatan sulfate proteoglycan in the skin is decorin, which is involved in collagen fibrillogenesis and extracellular matrix organization (13–15). Decorin also acts as a key signaling molecule that can modulate the activity of several tyrosine kinase receptors (16–18) and integrins (19). Furthermore, decorin inhibits growth of different tumor cell types in vitro (20) and in vivo (21), via interactions with EGFR. In vitro, endothelial cells synthesize decorin under inflammatory conditions (22) and Dcn−/− mice (23) show a delayed wound healing with enhanced blood vessel formation (24). In tubulointerstitial kidney fibrosis, decorin deficiency enhances apoptosis and increases inflammation (25). Based on these findings and considering the structural homology of heparan and dermatan sulfate, we hypothesized that decorin could play a role in modulating contact allergy. Thus, we investigated the role of decorin in delayed-type inflammation utilizing an in vivo model of contact allergy and in vitro models of leukocyte recruitment like intravital microscopy and flow chamber assays on P-selectin, ICAM-1 and CXCL-1. Our results show for the first time that decorin is expressed by polymorphonuclear leukocytes and mononuclear cells, and that it influences the expression of adhesion molecules like ICAM-1 and SDC1. Combined with the anti-adhesive properties of decorin, this regulation of adhesion molecules promotes leukocyte extravasation into the tissue.
Material and Methods
Decorin-null mice and Decorin/Syndecan-1 double-deficient mice
Decorin-deficient mice (Dcn−/−) (23) and Syndecan-1 deficient mice (Sdc1−/−) (26) were bred in the animal facility in accordance with the German Animal Protection Act and were approved by the responsible Ethics Review Committee. Decorin/Syndecan-1 double-deficient mice (Dcn−/−/Sdc1−/−) were generated by breeding and genotyped by genomic PCR as previously described (23, 26).
Delayed-type hypersensitivity assay
DTH was carried out with 8 to 12 weeks old male Dcn−/− mice and the respective controls as described previously (8). Briefly, mice were sensitized on abdominal shaved skin with 150 µl 2.5 % oxazolone (Sigma, Deisenhofen, Germany) dissolved in acetone/ethanol [3:1 (vol/vol)]. Mice were challenged 7 days later with 10 µl of 1% oxazolone topically administrated to the ear twice. Thickness of a constant area (1 cm2) of the ear was measured with Mitutoyo engineer’s micrometer, immediately before challenging, or at 24, 48, 72 hours and 7 days. Five mice per time point were used for each experimental condition. Experiments were carried out 4 times with similar results. In total we used 32 male wild-type and Dcn−/− mice, and excluded 3 mice because they did not respond to the oxazolone treatment.
Positron emission tomography (PET), quantification and CT Scanning
8 to 12 weeks old male Dcn−/− mice (n=9) and the respective controls were subjected to DTH and investigated 24 hours later. Animals were anesthetized with isoflurane (1.8 %) and placed on a heating pad to maintain the body temperature. 10 MBq of 18F-Fluordeoxyglucose ([18F]-FDG) in 100 µl 0.9% saline were injected intravenously 1 hour prior to each PET analysis. For PET acquisition animals were placed on a heat controlled multimodal scanning bed and PET list mode data were acquired for 15 minutes using the 32-module quadHIDAC scanner (Oxford Positron Systems, Weston-on-the-Green, UK) dedicated to small animal imaging. The scanner has an effective resolution of 0.7 mm (FWHM) in the transaxial and axial directions when using an iterative resolution recovery reconstruction algorithm. Subsequently the scanning bed was transferred to the computed tomography (CT) scanner (Inveon, Siemens Medical Solutions, USA) and a medium resolution (25 µm) CT acquisition was performed for each mouse. PET data were reconstructed into a single image volume for each mouse with a voxel size 0.4x0.4x0.4 mm³. CT was reconstructed into a volume data set with a voxel size 0.007x0.007x0.007 mm³. Image data sets were coregistered using extrinsic markers attached to the multimodal scanning bed and the image analysis software (Inveon Research Workplace, Siemens Medical Solutions, USA). For quantification of regional FDG uptake the outer ears were segmented three-dimensionally using CT data and the resulting volume of interest was applied to the co-registered PET data set. Measured data were corrected for partial volume effects, scatter, and background using a model-based approach. The FDG uptake was calculated as percentage of injected dose per volume (%ID/mL) for each ear and the net uptake in the inflamed left ear (Δ%ID/mL) was computed as (%ID/mL left ear) - (%ID/mL right ear).
Decorin purification
Decorin was purified from conditioned cell culture medium of human fibroblasts by anion exchange chromatography and analyzed for purity on a silver gel as described previously (27, 28).
Adhesion assay of polymorphonuclear leukocytes on endothelial cells
The murine endothelial cell line bEnd.3 was employed for static leukocyte adhesion assays as previously described (9). About 20.000 endothelial cells per 96-well plate were cultured over night followed by either treatment with 5 nM TNF-α in order to stimulate endothelial cells or used directly for the experiment. Under these conditions, TNF-α treatment did not result in increased cytotoxicity (not shown). Polymorphonuclear leukocytes of wild-type and Dcn−/−mice were prepared from bone marrow of tibias and femurs as previously described (8). 2x106 cells/mL polymorphonuclear leukocytes in PBS/1 % FCS were incubated with 1 µM fluorescent marker 2`7`-bis-(2 carboxyethyl)-5 carboxyfluorescein acetoxymethyl ester (Molecular Probes, Eugene, OR) in DMSO for 20 minutes at 37°C. Equal labeling efficiency was controlled using standard curves. Labeled samples were centrifuged for 5 minutes at 1500 rpm and the pellet was resuspended in medium. Polymorphonuclear leukocytes were treated at 37°C for 30 minutes with 5 µg/mL decorin and the respective solvent controls. Afterwards, endothelial cells were incubated with pre-treated polymorphonuclear leukocytes (2x106/ mL, 50µl/well) for 10 min at 37°C. Wells were washed two times with PBS and adherent polymorphonuclear leukocytes were lysed with lysis buffer (10 mM Tris/HCl, 0.1% SDS, pH8.5). The fluorescence signal was quantified in a Spectramax Fluorimeter (excitation, 485 nm; emission, 535 nm). The adhesion was reported as the number of adherent cells/mm2. The results were expressed as mean ±SEM.
Histology and immunohistochemistry
Paraffin embedded ears were cut in 5-µm sections and every 20–30th section was stained with H&E. Sections were analyzed for polymorphonuclear leukocyte distribution in the tissue and in the blood vessels. The blood vessel size was also evaluated for wild-type and Dcn−/− mice. Free, round, flattened and transmigrated polymorphonuclear leukocytes were analyzed by scoring adapted from Bixel and co-workers (29). Briefly, free or non-adherent, and adherent polymorphonuclear leukocytes per 104 µm2 of blood vessel surface area were evaluated by light microscopy with a color view soft imaging system (Sis, Münster, Germany). Furthermore, extravasated polymorphonuclear leukocytes per 104 µm2 of inflamed tissue surface in wild-type and Dcn−/− were counted.
For immunohistochemistry, after rehydration the PFA-fixed sections were blocked with 10% BSA for 30 minutes at room temperature, following incubation with either rat anti-mouse syndecan-1 monoclonal antibody (BD Biosciences, Franklin Lakes, NJ) or rat anti-mouse ICAM-1 monoclonal antibody (Biolegend, Uithoorn, NL) both 1/100 with PBS containing 1% BSA over night at 4°C; omission of the primary antibody served as a negative control. SDC1 staining and development was carried out with secondary anti-Rat IgG biotinylated antibody (Vectastain ABC, Vector Labs, Burlingame, MA) diluted as described by the manufacture and counterstained with Mayer`s hemalum (Merck, Darmstadt, Germany) and mounting in Kaiser`s glycerol gelatine (Merck) (8). For ICAM-1, a secondary peroxidase conjugated affinity purified anti-Rat IgG (Dako, Glostrup, DK) diluted 1/1000 with PBS containing 1% BSA, anti-rabbit IgG (Amersham, Braunschweig, Germany) was applied for 30 minutes at room temperature. After washing with PBS, sections were incubated for 5 minutes with DAB reagent (Dako) and hematoxylin counterstained. Sections were analyzed with an Olympus photomicroscope.
For immunofluorescence staining the tissue was embedded in Tissue Tek OCT compound (Sakura Finetek, Japan). 5 µm tissue sections were fixed with methanol for 10 min at −20 °C, blocked with 1% BSA (Serva, Heidelberg, Germany)/PBS for 30 min at RT, followed by incubation with rat anti-mouse CD4 (clone L3T4, BD Pharmingen, Heidelberg, Germany), anti-mouse CD8 (clone 53–6.7, BD Pharmingen, Heidelberg, Germany) rat-anti human F4/80 (Abcam, Cambridge, UK) or rabbit-anti human decorin antiserum LF-113 (kindly provided by Dr. Larry Fisher, NIH) for 1 h at RT. Positive cells were detected using Cy3-labeled donkey anti-rat IgG (H+L) (Dianova, Hamburg, Germany) or Alexa488-labeled goat-anti rabbit IgG (Invitrogen, Karlsruhe, Germany), respectively.
Auto-perfused flow-chamber
In order to investigate neutrophil arrest, we used a previously published flow chamber system (31, 32), Briefly, rectangular glass capillaries (20 x 200 µm, VitroCom) were coated with P-selectin (40 µg/ml, R&D Systems), ICAM-1 (25 µg/ml, R&D Systems), and CXCL1 (25 µg/ml, R&D Systems) for 2 h, and blocked for 1 h with casein (Thermo Fisher Scientific). The chamber was connected at one side to a PE 50 tubing and used to control the wall shear stress. The other side of the chamber was connected to a syringe filled with isolated PMNs resuspended in PBS containing 1mM Mg2+ and 1mM Ca2+. We perfused each chamber with PMNs for 6 min before we recorded representative fields of view for 30s using an SW40/0.75 objective (Axio Scope, Carl Zeiss).
Intravital microscopy
We prepared cremaster muscle for intravital microscopy as described previously (32, 33) and investigated leukocyte rolling flux fraction, rolling velocity, adhesion and extravasation in postcapillary venules (20–40 µm diameter) 4 h after an intrascrotal injection of 50 ng Il-1β.
Reverse transcription and quantitative Taqman real-time PCR (qPCR)
After euthanasia of the animals, ears were excised and either snap-frozen in liquid nitrogen, followed by preparation of total RNA using the RNAeasy kit (Qiagen, Hilden, Germany). One microgram of total RNA was transcribed using the First Stand cDNA Synthesis Kit (Fermentas, St. Leon Rot, Germany). To detect Dcn, Sdc1 and Gapdh expression, conventional PCR was used with primer pairs for Dcn-sense 5’ CCT TCT GGC ACA AGT CTC TTG G 3’ Dcn-antisense 5’ TCG AAG ATG ACA CTG GCA TCG G 3’ (24), Sdc1-sense 5’ GAC TCT GAC AAC TTC TCT GGC TCT 3’, Sdc1-antisense 5’ GCT GTG GTG ACT CTG ACT GTT G ‘3 and Gapdh. cDNA corresponding to 25 ng of total RNA was used as a template in the PCR consisting of Applied Biosystems MasterMix and predesigned TaqMan gene expression systems (Applied Biosystems, Darmstadt, Germany) according to the manufacturer’s instructions. For detection of Sdc1, Tnfα, and Icam1 mRNA, primers Mm00448918_m1 (Sdc1 exons 2 and 3), Mm00443258_m1 (Tnfα exons 1 and 2), and Mm00516023_m1 (Icam1 exons 2 and 3) were used and normalized to the expression of mammalian 18S rRNA (Hs99999901_s1, all primers from Applied Biosystems). qPCR was performed with an Applied Biosystems PRISM 7300 Sequence Detection System using the default thermal cycling conditions (10 minutes at 95°C and 40 cycles of 15 seconds at 95°C plus 1 minute at 60°C). Relative quantification was performed using the comparative cycle threshold method (34). Three to five biological replicates were used for each time point investigated. For statistical analysis, the Mann-Withney U-test was used. A P < 0.05 was considered statistically significant.
Protein extraction, ELISA and Immunoblotting
For protein extraction excised ears were snap-frozen in liquid nitrogen and homogenised as described previously (8). Briefly, ears were homogenised on ice with 500 µl PBS containing 10 mM EDTA and a cocktail of protease inhibitors. Samples were centrifuged for 10 minutes at 12.000 g and supernatant was collected. Total protein concentration was quantified by BCA-Lowry assay (Pierce, Rockford, IL, USA). Protein extracts were used for ELISA or Western blotting. All protein samples were diluted to 1.5 mg/mL keratinocyte chemoattractant (KC) or 1 mg/mL (TNF-α), and the tissue concentrations of KC and TNF-α immunoassays were determined exactly as described by the manufacturer (R&D Systems, Wiesbaden, Germany). For Western blotting, ∼40 µg protein extracts of ears derived from DTH experiments, or of bEnd.3 cells subjected to 24h of TNF-α (5 nM) and/or decorin (5 µg/ml) stimulation were loaded on a 12 % SDS-gel under non-reducing conditions. After blotting the nitrocellulose membrane was blocked with 5% milk in TBS-T. The membrane was incubated with ICAM-1 antibody rat anti-mouse clone YN1/1.7.4 (Biolegend), or mouse anti P-Tyrosine (P-Tyr-100, Cell Signaling) at 4°C overnight. After washing the sections the horseradisch peroxidase-labeled secondary anti-rat (Pierce, Rockland, PA, USA), or anti-mouse (Calbiochem) antibodies were used to detect ICAM-1 or P-Tyrosine, respectively. Decorin was detected analogously following digestion of tissue extracts with Chondroitinase ABC (Seikagaku, Kogyo, Japan) for 2h at 37°C, using a polyclonal antiserum kindly provided by Dr. Larry Fisher, and HRP-labeled goat-anti-rabbit IgG (Calbiochem) as a secondary antibody. The dot-blot for Sdc-1 was performed as previously described (35) and analyzed densitometrically using Image J software (NIH).
Statistical analysis
Statistical evaluation was performed with GraphPad Prism. If not mentioned we used Student’s t -test and considered P < 0.05 as significant.
Results
Decorin deficiency attenuates edema formation and leukocyte recruitment in oxazolone-mediated delayed-type hypersensitivity
To study the role of decorin in a contact allergy model we used oxazolone as a hapten and followed ear swelling as readout of inflammation-induced edema formation for up to 72 hours after challenging the mice. Dcn−/− mice showed a suppressed response to oxazolone, about 25 % less swelling, compared to wild-type (P < 0.001, Figure 1A). Next, inflammatory activity of the ears was assessed non-invasively by PET/CT scanning, a strategy that allows direct visualization and quantification of tissue metabolic activity by administration of a radioactive sugar, 18FDG, as surrogate marker for cellular glucose metabolism (36). We found that metabolism was reduced in the Dcn−/− ears (Figure 1B). Quantification of 18FDG uptake in the treated ears of different animals, normalized on the signal of the contralateral ear, showed that wild-type mice had an uptake of 18FDG of 1.53 ± 0.58 %ID/mL. In contrast, Dcn−/− mice showed a reduced tracer accumulation of 0.85 ± 0.35 %ID/mL, equivalent to a ∼40% reduction in metabolic activity (P <0.001, Figure 1C). These values are in good agreement with the measured reduction in ear swelling in the Dcn−/− mice vis-à-vis wild-type mice. Additionally, ear volume was identified by CT scanning, confirming the results obtained by caliper measurements (data not shown).
FIGURE 1. Reduced DTH reactivity in Dcn−/− mice compared to wild-type mice.
Left ears of male wild-type and Dcn−/− mice were challenged with oxazolone. Right ears were treated with vehicle alone. (A) Swelling of the left ears is expressed in µm as the increase of baseline thickness of control ears. Dcn−/− ears are shown in red and wild-type in black. Dcn−/− mice show significant less swelling at 24 hours after challenge (*** P < 0.001; n=45 (24 hours); n=30 (48 hours); n=15 (72 hours). Data are expressed as mean ± SEM. (B) To visualize the reduced DTH reaction in three dimensions, combined PET/CT scans were performed 24 hours after challenging to allow detection of maximal differences in metabolic activity between Dcn−/− and control animals. Arrows indicate the areas of interest in the horizontal and arrowheads in the transaxial image. (C) Quantification of [18F]-FDG-uptake of the treated ears showed a significant reduction in metabolic activity in the Dcn−/− mice (n=9; 0.85 ± 0.35 Δ%ID/mL) compared to wild-type (n=9, 1.53±0.58 Δ%ID/mL, P = 0.008). Data are expressed as mean ± SEM. (D–G) Histological examination of oxazolone induced DTH in the ears of wild-type and Dcn−/− mice. (D) Quantification of infiltrated leukocytes in ear tissue (*** P < 0.001, n=5 ears and 100 sections were quantified) shows reduced infiltration in Dcn−/− ears. (E) Representative histology (H&E-staining) of wild-type and Dcn−/− ear section after 24 hours DTH. Bar = 50 µm. Dcn−/− ears have less leukocytes 24 hours after treatment compared to wild-type ears. The right panels show representative H&E-stainings of untreated wild-type and Dcn−/− ears. Bar = 200 µm. (F) Quantification of leukocytes in blood vessels over the DTH experiments reveals more leukocytes in Dcn−/− mice 24 hours after challenging. (D) (P < 0.001 n=5). Bar =50 µm). Data are expressed in mean ± SEM, (G) Representative histology (H&E-staining) of wild-type and Dcn−/− blood vessels in ear sections after 24 hours DTH. Bar = 50 µm.
Next, we examined leukocyte infiltration as invasion of leukocytes into the tissue is a pivotal step during DTH (1, 37). Quantification of total tissue leukocytes showed that at 24 hours post-challenge the Dcn−/− mice contained significantly less as compared to wild-type (P < 0.001, Figure 1D,E). The untreated control and Dcn−/− mouse ear tissues showed no difference (Figure 1D,E right panels). Histological examination of the treated ears revealed a different leukocyte distribution within the tissue of wild-type and Dcn−/− mice, which was particularly prominent in blood vessels (Figure 1F). At 24 hours after oxazolone treatment, the blood vessels of Dcn−/− treated ears contained significantly more leukocytes compared to wild-type (Figure 1F,G). Analysis of the blood vessel size revealed no differences (data not shown). In contrast, 48 hours after elicitation, leukocyte numbers in the blood vessels of Dcn−/− mice significantly increased compared to wild-type (1F), and leukocyte recruitment into the tissue resumed, suggesting a delayed leukocyte infiltration following DTH elicitation in the decorin null background.
Dcn−/− leukocytes show increased adherence to the endothelial cell surface in vivo
The previous results raised the question which mechanisms would prevent leukocytes from leaving the blood vessel lumen during inflammation in the absence of decorin. Therefore, the tissue sections were scored with respect to free luminal, attached and transmigrated leukocytes (29). Quantification of 35 sections from wild-type and Dcn−/− ear sections revealed no significant difference in the number of free leukocytes in the blood vessels (Figure 2A–C). However, we found a significant increase in the number of flattened leukocytes in the Dcn−/− ears (Figure 2C, P < 0.001, n=5). While Dcn−/− leukocytes adhered more frequently to the endothelial cells, the number of transmigrated leukocytes decreased as compared to wild-type 24 hours after DTH elicitation (Figure 2C). To investigate the molecular mechanism of this observation, we performed intravital microscopy of the M. cremaster using chimeric mice reconstituted with wild-type or Dcn−/− bone marrow cells. We observed no differences in the rolling velocity of the leukocytes from both populations (data not shown), but the rolling flux fraction was increased in chimeric mice reconstituted with bone marrow from Dcn−/− mice (Figure 2D, P < 0.05). Furthermore, these chimeric mice showed an increased number of adherent and transmigrated leukocytes compared to chimeric mice reconstituted with wild-type bone marrow leukocytes (Figure 2E+F, P < 0.05). Moreover, detailed analysis of leukocyte subsets extravasated into the tissue was performed (supplementary figure 1). Upon measuring peroxidase activity as a readout of neutrophil activity in ear protein extracts (8), no differences between genotypes were observed 24h after DTH elicitation, however, after 48h, a significant increase was observed in Dcn−/− compared to WT mouse ears (supplementary figure 1A,B). F4/80 staining revealed no difference in macrophage numbers in the dermis between wild-type and Dcn−/− treated ears (supplementary figure 1C,D). The immune fluorescence staining revealed that the numbers of CD4+ leukocytes were not differently affected in wild-type and Dcn−/− treated ears during DTH (supplementary figure 1E,F). Similarly, the number of CD8+ cells was not significantly changed 24 h after oxazolone treatment (supplementary figure 1G,H). Analysis of blood leukocyte numbers did not reveal a significant difference between Dcn−/− and wild-type mice (data not shown), and also no changes in the differential blood analysis (Figure 2G; n=4). Interestingly, 24 hours after oxazolone treatment, the number of neutrophils increased significantly in Dcn−/− mice compared to wild-type, whereas the number of lymphocytes significantly decreased (Figure 2H, n=5; P < 0.05).
FIGURE 2. Dcn−/− leukocytes show increased adherence to the endothelial cell surface in vivo.
(A,B) Representative images of blood vessels in wild-type and Dcn−/− blood vessels of DTH ears. Leukocytes were categorized as free, round (arrow in A), flattened (arrow in B), and transmigrated (arrowhead in A). (C) Quantification of leukocytes as digitized with a soft imaging system (SIS, Münster, Germany). Quantitative analysis was performed according to Bixel and co-workers (29). As a reference, leukocytes were counted per 104 µm2 of blood vessel surface area and the number of extravasated leukocytes per 104 µm2 of inflamed tissue surface area. In oxazolone-treated ears of Dcn−/− mice, significantly more flattened leukocytes are present compared to wild-type, which is in line with a decreased number of transmigrated leukocytes (*** P < 0.001, n=5). (D,E,F) Quantification of the intravital microscopy analysis of wild-type mice recipients with wild-type or Dcn−/− bone marrow donors. (* P < 0.05, n=3). (D) Shows the rolling flux fraction and (E) the amount of adherent cells, both results supporting figure A–C. (F) shows that also in vivo there are less emigrated leukocytes if derived from Dcn−/− bone marrow. (G,H) Quantification of blood leukocytes of wild-type and Dcn−/− mice under baseline and DTH conditions. Untreated wild-type and Dcn−/− mice show no quantitative difference in the subsets of circulating leukocytes (G). In contrast, analysis of the subsets of blood leukocytes of treated mice shows a differential count for lymphocytes and neutrophils in Dcn−/− (H) (** P < 0.001; n=4 each). Data are expressed as mean ± SEM.
ICAM-1 and Sdc1 are differentially expressed in Dcn−/− mice during DTH
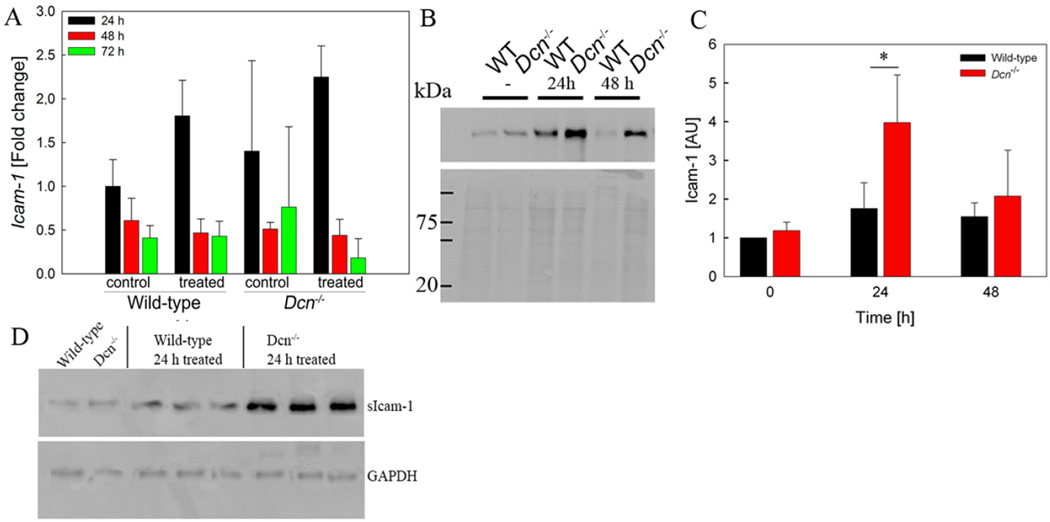
Extravasation is a complex process, which requires sequential steps of leukocyte adhesion to endothelial cells followed by release of leukocytes. The cell surface adhesion molecules ICAM-1 and SDC1 are known to be involved in the multistep leukocyte adhesion cascade (3, 4). Therefore, expression of ICAM-1 was evaluated by qPCR (Figure 3A; n=3–5). No significant differences in ICAM-1 mRNA expression were observed between wild-type and Dcn−/− mice. In unstimulated control ears, ICAM-1 protein was not detectable by immunohistochemistry for both genotypes, however, 24 h after elicitation Dcn−/− ears express more ICAM-1 protein (supplementary figure 2). This observation was confirmed by Western blotting: While ICAM-1 expression increased in wild-type mice during the DTH response, as expected, oxazolone-treated ears of Dcn−/− mice showed an even stronger increase compared to wild-type (Figure 3B,C). ICAM-1 levels in the plasma were also increased in Dcn−/− mice compared to wild-type (Figure 3D).
FIGURE 3. ICAM-1 expression is differentially altered in wild-type and Dcn−/− mice during DTH activity.
(A) Real-time PCR for ICAM-1 expression during the time course of DTH (P = n.s., n=3–5) (B,C) Increased Icam1 expression in Dcn−/− mouse tissues is confirmed by Western blotting 24h after DTH induction. (B) Representative Western blot. Ponceau S staining is used as loading control (lower panel) (C) Densitometric analysis (P = 0.039; n=4). (D) Western blot analysis of ICAM-1 expression in plasma of wild-type and Dcn−/− mice prior to and 24h after DTH elicitation. 20µg of protein/lane were analysed. GAPDH is used as a loading control.
Syndecan-1 is an adhesion molecule with anti-inflammatory properties (9), which has recently been identified as a novel modulator of DTH responses (8). Analysis of Sdc1 mRNA expression showed that the basal level in Dcn−/− mice was about two-fold higher than in wild-type ears (Figure 4A;, n=3–5, P <0.05). In oxazolone-treated ears of Dcn−/− mice the expression of Sdc1 mRNA was significantly increased after 24 h compared to wild-type (Figure 4A; n=3–5; P <0.05). In accordance with previous reports (4, 38, 39), Sdc1 is highly expressed in the epidermis, but also leukocytes were labeled for SDC1 in Dcn−/− and wild-type mice (Figure 4B, arrows and 4C). Within the DTH time course, Dcn−/− ears showed at 24h a significantly increased amount of SDC1-positive leukocytes compared to wild-type (Figure 4C; n=5; P < 0.001). Forty-eight hours after oxazolone application most of the SDC1 signal disappeared in Dcn−/− and wild-type leukocytes (data not shown). Thus, differentially increased expression of the adhesion molecules Icam1 and Sdc1 in the DTH time course may contribute to the increased leukocyte numbers in the Dcn−/− blood vessels.
FIGURE 4. Sdc-1 expression is differentially altered in wild-type and Dcn−/− mice during DTH activity.
(A) Quantitative real-time PCR analysis of Sdc1 mRNA expression in ears of wild-type and Dcn−/− mice during the DTH time course. Sdc1 expression is significantly upregulated in Dcn−/− mice relative to wild-type mice under inflammatory conditions. (n=3–6, * P < 0.05). (B) SDC1 expression was confirmed by staining of ear sections with a specific antibody for syndecan-1. Bar = 100 µm. (C) Quantification of SDC1+ leukocytes shows a significant difference between wild-type and Dcn−/− mice 24 h after elicitation (n=5, ***P <0.001).
Cytokine expression is dysregulated in Dcn−/− mice during DTH
Keratinocyte chemoattractant (KC, CXCL-1) is an early response gene expressed by keratinocytes, monocytes and macrophages. CXCL-1 expression is upregulated by cutaneous contact with allergens (40), and its synthesis can be induced by the pro-inflammatory cytokine TNF-α in keratinocytes (41), leukocytes (30) and during DTH in mice (42). ELISA data showed that KC was upregulated in the DTH time course peaking at 24 hours after DTH. Although Dcn−/− mice showed a lower degree of upregulation compared to wild-type controls, this difference was not significant (Figure 5A). Furthermore, we analysed Ccl2 mRNA expression and obtained for both genotypes a similar increase (supplementary figure 3A). Moreover, lymph nodes were analysed for the occurrence of CD4+ and CD8+ cells by flow cytometry (supplementary figure 3B,C). After sensibilization the amount of CD4+ and CD8+ cells was not significantly different in wild-type and Dcn−/− lymph nodes (supplementary figure 3B,C). In contrast, basal TNF-α levels in Dcn−/− mice were significantly lower compared to wild-type controls (Figure 5B).
FIGURE 5. Differential expression of TNF-α and KC/CXCL11 in ear tissue of Dcn−/− mice compared to wild-type 24–72 hours after DTH induction.
(A) ELISA of KC/CXCL-1 levels in treated and untreated ear tissues of Dcn−/− and wild-type mice 24, 48 and 72 hours after DTH induction. Data are means ± S.E.M (nwild type=3; nDcn−/−=4 each in triplicates, P = n.s.). (B) ELISA of TNF-α level in ear tissues of Dcn−/− and wild-type mice after DTH induction. Data are means ± SD (nwild-type=3; nDcn−/−=4 * P < 0.05).
Dcn−/− polymorphonuclear leukocytes show increased adhesion to endothelial cells
Using RT-PCR, we initially determined that decorin was expressed by both polymorphonuclear leukocytes and monocytes isolated from wild-type mice bone marrow, but not by the corresponding decorin-deficient cells (Figure 6A). We then performed static adhesion experiments with fluorescently-labeled polymorphonuclear leukocytes and murine bEnd.3 endothelial cells (43) with or without TNF-α stimulation. In line with its anti-adhesive effect, decorin proteoglycan purified from skin fibroblasts blocked adhesion of wild-type neutrophils to the unstimulated endothelial cells (Figure 6B, n=10). Interestingly, the Dcn−/− polymorphonuclear leukocytes did not respond to the decorin treatment (Figure 6B, P < 0.001 vs wild-type).
FIGURE 6. Polymorphonuclear leukocytes and mononuclear cells express decorin, and show increased adhesion to endothelial cells in the absence of decorin.
(A) RT-PCR analysis confirms expression of Dcn mRNA in wild-type bone marrow mononuclear cells (upper panel) and polymorphonuclear leukocytes (lower panel) (n=3). Dcn−/− (n=2) mononuclear cells and polymorphonuclear leukocytes did not express decorin. M: 100 bp ladder; neg: negative control with no cDNA. (B) Static adhesion experiments of polymorphonuclear leukocytes to unstimulated bEnd.3 endothelial cells. The adhesion of wild-type polymorphonuclear leukocytes was significantly inhibited by exogenous human decorin (5 µg/mL), whereas decorin treatment had no effect on Dcn−/− leukocyte adhesion. Data represent the mean ± SEM of five experiments. (*** P < 0.001) (C) Adhesion of polymorphonuclear leukocytes purified from Dcn−/− or wild-type mice bone marrow to TNF-α stimulated endothelial cells. Dcn−/− polymorphonuclear leukocytes show significantly increased adhesion compared to wild-type. Data represent the mean ± SEM of five experiments. (* P < 0.05). (D) Adhesion assay under flow condition of wild-type, Dcn−/−, and Dcn−/−/Sdc1−/− cells to the coated ligands ICAM-1 and P-selectin and CXCL-1. Adhesion is significantly increased for Dcn−/− leukocytes compared to wild-type. The leukocytes of the Dcn−/−/Sdc1−/− cells showed a further increased adhesion compared to Dcn−/− cells. Data represent the mean ± SEM (n=3; * P < 0.05).
During DTH, endothelial cells are activated, leading to the expression of adhesion molecules and chemokines promoting leukocyte recruitment to the endothelium (3, 4). To simulate inflammatory conditions in vitro, bEnd.3 endothelial cells were incubated for 16 h with recombinant murine TNF-α (5 nM) followed by adhesion measurements. Under TNF-α-stimulated conditions, adhesion of both wild-type and Dcn−/− polymorphonuclear leukocytes to endothelial cells was higher compared to unstimulated conditions (Figure 6B,C). Of note, Dcn−/−polymorphonuclear leukocytes showed a significantly increased adhesion to TNF-α-activated bEnd.3 cells (P < 0.05, Figure 6C). Addition of exogenous decorin did not result in an inhibitory effect, suggesting a differential modulation of adhesion ligands in unstimulated and TNF-α-activated endothelium. These results could be confirmed under flow conditions (Figure 6D; P < 0.05). The adhesion of Dcn−/− cells to the coated ligands ICAM-1, P-selectin and CXCL-1 is significantly increased compared to wild-type leukocytes.
Sdc1 deficiency rescues the DTH phenotype of Dcn−/− mice
The results described above suggested that Sdc1 expression could directly contribute to the attenuated DTH phenotype of the Dcn−/− mice. To this end, we generated Dcn−/−/Sdc1−/− double knockout mice by mating. The Dcn−/−/Sdc1−/− mice were healthy and viable and showed no overt developmental phenotype. The 8–10 weeks old male Dcn−/−/Sdc1−/− mice had similar weight of 23.2 ± 1.2 g as the wild-type 21.7 ± 1.4 g and the single knock-out (n=8). Genomic PCR showed no Dcn band at 162 bp for the Dcn−/− and the Dcn−/−/Sdc1−/− mice but the knock-out band at 250 bp (Figure 7A). The Sdc1 genomic PCR showed the 450 bp knock-out signal for the Sdc1−/− and the Dcn−/−/Sdc1−/− mice and the wild-type signal at 250 bp (Figure 7B). The absence of DCN and SDC1 protein expression in Dcn−/−/Sdc1−/− mice was confirmed by Western blotting (Figure 7C) and immunohistochemistry (Figure 7D,E), respectively. When we performed another set of DTH experiments, the results showed that the ear swelling phenotype was completely rescued in the Dcn−/−/Sdc1−/− mice, thereby validating our results described in the preceding sections (Figure 7F). Real-time PCR (Figure 7G) and Western blot analysis (Figure 7H,I) revealed that ICAM-1 expression levels in Dcn−/−/Sdc1−/− mice were comparable to wild-type and significantly decreased relative to Dcn−/− mice. In untreated control ears, TNF-α protein expression as determined by ELISA was significantly lower in Dcn−/−mice and Dcn−/−/Sdc1−/− mice compared to wild-type (Figure 7J). Under DTH conditions (24h), TNF-α expression was decreased in Dcn−/−/Sdc1−/−mice compared to wild-type (n=, P = 0.0571), closely resembling the response of Dcn−/−mice (Figure 7J). Interestingly, the adhesion under flow conditions showed for Dcn−/− and Dcn−/−/Sdc1−/−a significant increase compared to wild-type leukocytes (Figure 6D).
FIGURE 7. Absence of Sdc1 rescues the anti-inflammatory phenotype of Dcn−/− mice.
(A,B) Genotyping of Dcn−/−/Sdc1−/− knockout mice. (A) Genomic PCR showed no Dcn signal (162 bp) for the Dcn−/− and the Dcn−/−/Sdc1−/−, but the respective knock-out signal of 250 bp. (B) Genomic PCR showed no Sdc1 signal (250 bp) for the Sdc1−/− and the Dcn−/−/Sdc1−/−, but the respective knock-out signal of 450 bp. (C) Western blotting reveals expression of DCN in extracts or wild-type (WT) and Sdc−/− mouse ear tissue, and absence in Dcn−/− and Dcn−/−/Sdc1−/− mouse ear tissue. (D) Immunohistochemistry demonstrates presence of SDC1 expression in the epithelial layer of wild-type mouse ears, and absence of SDC1 expression in Dcn−/−/Sdc1−/−mouse ears. 20x magnification. (E) Immunofluorescence microscopy demonstrates presence of DCN expression in the interstitial matrix of wild-type mouse ears, and absence of DCN expression in Dcn−/−/Sdc1−/− mouse ears. 20 x magnification. (F) Dcn−/−/Sdc1−/−, wild-type and Dcn−/− mice were employed in a DTH assay as described in Figure 1 to evaluate the contribution of Sdc1 to the phenotype of Dcn−/− mice. Dcn−/− mice show a reduced response to DTH compared to wild-type (cf. Fig. 1). Dcn−/−/Sdc1−/−mice respond to oxazolone like the wild-type mice. Compared to the Dcn−/− mice, the response is significantly different, indicating that Dcn-dependent modulation of Sdc1 contributes to the DTH response in vivo (n=5; ** P < 0.01). (G) Quantitative real-time PCR analysis for ICAM-1 expression in ear tissue of wild-type, Dcn−/− and Dcn−/−/Sdc1−/−mice 24h +/− DTH eliciation. Data represent the mean ± SEM, n≥3. (H,I) Western blot analysis of ICAM-1 expression in ear tissue of wild-type, Dcn−/−, Sdc1−/− and Dcn−/−/Sdc1−/−mice at 24h ± DTH elicitation. (H) Semiquantitative densitometric analysis of ICAM-1 normalized to total protein expression. Data represent the mean ± SEM. (n≥4, P < 0.05, ** P < 0.01) (I) Representative Western blot. (J) TNF-α ELISA of ear tissue extracts of wild-type, Dcn−/− and Dcn−/−/Sdc1−/− mice at 24 h +/− DTH elicitation. Data represent the mean ± SEM. (n≥4, * P < 0.05).
Effect of decorin treatment on endothelial cells
It is well established that decorin can function as a signaling molecule (14, 18) for example by interacting with various tyrosine kinase receptors such as EGFR, IGF-IR and c-Met (16, 18, 19). Thus, we determined the effects of exogenous decorin and TNF-α treatment on overall tyrosine phosphorylation in bEnd.3 endothelial cells. Both decorin and TNF-α significantly increased generalized Tyr phosphorylation (Figure 8A). Furthermore, TNF-α-evoked phosphorylation was significantly potentiated by decorin (Figure 8B, n=3, P < 0.01). Quantitative real-time PCR analysis revealed that Sdc1 mRNA expression was significantly reduced both by TNF-α and decorin treatment (Figure 8C, n=3, P < 0.01) but showed no additive effect for TNF-α + decorin treatment. Dot-blot analysis confirmed significant downregulation of SDC1 protein expression by decorin treatment in TNF-α-stimulated bEnd.3 cells (Figure 8D, n=3, P < 0.05). In contrast to the observed transcriptional downregulation, TNF-α mono treatment did not result in significantly altered SDC1 protein levels (Figure 8D).
FIGURE 8. Analysis of tyrosine phosphorylation and Sdc1 expression in bEnd.3 cells stimulated with human decorin in the presence or absence of TNF-α.
(A) Immunoblots for P-Tyr after treatment of bEnd.3 cells with TNF-α and decorin (5 µg/mL). The migration position of molecular weight markers is indicated at the left margin. (B) Quantification of data as in panel A. Data represent the mean ± SEM of three experiments (** P < 0.01). (C) Quantitative real-time PCR analysis of Sdc1 mRNA expression in bEnd.3 cells treated with TNF-α and decorin (cf panel B). Data represent the mean ± SEM (n=3, * P < 0.05, ** P < 0.01). (D) Dot blot for SDC1 expression in bEnd.3 cells following TNF-α stimulation ± decorin treatment. Upper panel: representative dot-blot. Lower panel: Densitometric quantification of dot blot result. (n=3, P < 0.05, TNF-α-treated samples).
Discussion
Decorin is a well-established regulator of matrix assembly and growth factor activity (14, 44). The function of decorin is diverse and exerts both pro- and anti-fibrotic conditions depending on the experimental system (14, 23, 24, 44). In this study, we expanded the spectrum of decorin functions to the field of allergic skin inflammation, establishing a novel role in leukocyte recruitment during DTH responses. Successful sensitization and upregulated early cytokine expression (Ccl2) showed that decorin-deficient mice respond to the allergen. However, compared to wild-type mice, lack of decorin resulted in an attenuated edema and metabolic activity at the site of inflammation, concomitant with a reduction of polymorphonuclear leukocytes transmigrating into the inflamed tissue. Histological studies showed that the reduced leukocyte diapedesis in the Dcn−/− mice was associated with increased numbers of flattened, adherent polymorphonuclear leukocytes in the blood vessels, and an increased percentage of circulating polymorphonuclear leukocytes. Intravital microscopy experiments demonstrate that chimeric mice reconstituted with Dcn−/− bone marrow cells display an increased leukocyte adhesion and a reduced number of transmigrated leukocytes. Similarly, in vitro, adhesion of Dcn−/− polymorphonuclear leukocytes to endothelial cells was increased compared to wild-type, and wild-type adhesion could be inhibited by addition of human decorin. Overall, our data suggest that decorin directly modulates leukocyte adhesion to the endothelium and affects diapedesis of leukocytes into the inflamed tissue during DTH. As the in vivo adhesion phenotype could be replicated in vitro using Dcn−/− polymorphonuclear leukocytes, part of the phenotype appears to depend on decorin expression by polymorphonuclear leukocytes, in accordance with the anti-adhesive properties of decorin (45). Our flow chamber assays identify increased adhesion of Dcn−/− polymorphonuclear leukocytes to ICAM-1, P-Selectin and CXCL-1 as relevant ligands for this process. Interestingly, polymorphonuclear leukocytes synthesise decorin but obviously not enough to block adhesion completely as we could demonstrate with exogenous decorin. Increased numbers of circulating polymorphonuclear cells in Dcn−/− mice may be indicative of a lack of inhibitory signals on leukocyte recruitment triggered by successful diapedesis, which is inhibited at the 24 h time point.
Under fibrotic conditions, genetic deficiency of decorin results in an increased inflammatory reaction, likely mediated by the compensatory up-regulation of biglycan (25), a known pro-inflammatory proteoglycan that acts by activating TLR4 (46). In our model, biglycan expression did not appreciably change in either wild-type or decorin-null treated ears (not shown) indicating that there are tissue-specific changes. Notably, biglycan can stimulate the synthesis of TNF-α and MIP-2 (46), whereas TNF-α is reduced in our Dcn−/− model. Therefore, we conclude that biglycan is not compensating for decorin under contact allergy conditions at the analysed time points. Moreover, our findings are supported by a recent study on the role of decorin in a mouse model of allergen-induced asthma: Marchica et al (47) demonstrated reduced allergic inflammation in Dcn−/− mice relative to WT, which could partially be attributed to differentially reduced amounts of TGF-β in the bronchoalveolar lavage of the allergen-stimulated decorin-deficient mice.
The resolution of inflammation depends on the localization and activation of lymphocytes and macrophages. Apparently, altered macrophage recruitment was not the cause of the reduced DTH response in Dcn−/− mice. In contrast, it has been shown that lumican, another member of the small leucine-rich proteoglycan family, is necessary for peritoneal polymorphonuclear leukocytes to extravasate into inflamed tissue in a β2 integrin-dependent manner (48).
Increased adhesion of Dcn−/− polymorphonuclear leukocytes resembles previous observations in Dcn−/− embryonic fibroblasts, for which an involvement of increased α2β1 integrin on the cell surface was demonstrated (49, 50). Similarly, an increased expression of adhesion molecules was observed in the present study, as the expression of the endothelial cell adhesion molecule ICAM-1, and of SDC1, a novel player in DTH regulation and leukocyte recruitment (8) were upregulated in Dcn−/− vis-a-vis wild-type mice. Of note, dermatan sulfate, the major carbohydrate moiety of skin-derived decorin, has previously been shown to be involved in the induction of soluble ICAM-1 in an animal model (12). Similarly, under DTH conditions, endothelial cells express ICAM-1 which increased during the DTH time course. In decorin-deficient mice, differential ICAM-1 upregulation during DTH may be a potential compensatory mechanism activated in an attempt to increase the amount of polymorphonuclear leukocytes available for diapedesis. Dressler and co-workers (51) showed that ICAM-1 is increased in endothelial cells near inflammation infiltration during wound healing. Importantly, our data identify ICAM-1 as a relevant ligand for the increased recruitment of Dcn−/− neutrophils compared to wild-type. However, in the absence of decorin, leukocyte diapedesis rather than adhesion appears to be inhibited, suggesting that decorin also modulates downstream steps of the leukocyte adhesion cascade (3, 4). Although speculative at this point, the strong increase of soluble ICAM-1 in the serum of Dcn−/− mice may be linked to this phenomenon, as it has been shown that sICAM-1 depletion from serum results in increased leukocyte diapedesis in vitro (52).
Part of the phenotype reported in this study could be linked to the increased expression of SDC1 in Dcn−/− mice, as we recently demonstrated increased DTH reactions in Sdc1−/− mice (8). Both Sdc1−/− (9) and Dcn−/− polymorphonuclear leukocytes show increased adhesion to endothelial cells in vitro. However, additional mechanisms, such as the coreceptor role of the heparan sulfate chains of SDC1 in chemokine signalling (4, 5), or the proposed effect of SDC1 on edema formation during inflammation (reviewed in ref. 4) may be of relevance in this context. ICAM-1 expression was similar to wild-type levels in a Sdc1−/− background, suggesting a potential mechanistic contribution of SDC1 to upregulated ICAM-1 expression in the absence of decorin. An important contribution of SDC1 to the inflammatory phenotype of the Dcn−/− mice is further suggested by the observation that genetic ablation of Sdc1 in the Dcn−/− background efficiently rescued the ear swelling phenotype of Dcn−/− mice during DTH at the 24 h time point. As both Sdc1 and Dcn-deficiency promote leukocyte adhesion to endothelium and ICAM-1, reduced leukocyte recruitment does not appear to be the mechanism behind this finding. However, our intravital microscopy data demonstrate that, while Dcn−/− showed increased leukocyte adhesion, leukocyte transmigration was significantly inhibited. In contrast, a previous study showed that Sdc1−/− mice show both increased leukocyte adhesion and transmigration during intravital microscopy (38). Our data therefore strongly suggest that (i) absence of Dcn on the leukocytes plays a pivotal role in reducing allergic inflammation during DTH and (ii) that absence of Sdc1 relieves the block on diapedesis imposed on Dcn−/− leukocytes. Diapedesis phenotypes similar to our findings have been reported in the case of a loss of PECAM-1 or CD99 function (reviewed in (53)). In fact, impaired diapedesis, combined with a lack of feedback regulation indicating the presence of leukocytes in the inflamed tissue, may be a reason for the increased circulating leukocyte numbers in Dcn−/− mice in spite of leukocyte hyperadhesiveness. Sdc1-derived heparan sulfate chains may in fact bind to PECAM-1, possibly at a heparin binding domain located between the IgG-like domains 2 and 3 (54) and may thereby contribute to endothelial cell-endothelial cell integrity (55). Further work will be required to clarify the exact role of glycosaminoglycans in PECAM-1 function. Finally, the differential downregulation of TNF-α expression observed in Dcn−/− mice was also present in a Sdc−/− background, suggesting that decorin may either act upstream of SDC1, or that its effect on TNF-α expression may be independent from SDC1.
Sdc1 is expressed in polymorphonuclear leukocytes and mononuclear cells, indicating that it might play a role in adhesion of these cell types. Consistent with the observed upregulation of Sdc1 in Dcn−/− mice, bEnd.3 cells showed decreased expression of Sdc1 mRNA and protein upon decorin treatment. Of note, decorin treatment of bEnd.3 cells resulted in an activation of general tyrosine phosphorylation, which was enhanced synergistically by TNF-α treatment. These findings are in accordance with the established role for decorin as a modulator of receptor tyrosine kinase activation (16–18), and open up an additional mechanistic level of decorin function in contact allergic reactions. While our bone-marrow transplantation and in vitro adhesion assays suggest a major contribution of decorin-deficient leukocytes to the DTH phenotype, its influence on endothelial RTK signaling suggests that an endothelial contribution can not be fully excluded. TNF-α treatment leads to significant downregulation of Sdc1 mRNA expression, while its protein expression was not significantly altered. We can only speculate if SDC1 expression is subject to posttranscriptional regulation in our experimental system, similar to previous reports on cAMP-dependent modulation of SDC1 levels (56).
In addition to altered adhesion molecule expression, cytokine expression was dysregulated during DTH in Dcn−/− mice. Expression of pro-inflammatory TNF-α was reduced in Dcn−/− ears 24 hours after DTH elicitation. Cellular adhesion molecules and C-X-C chemokines like KC/CXCL-1 and MIP-2 regulate tissue leukocyte accumulation in a multitude of inflammatory states. However, although TNF-α induces KC synthesis in keratinocytes during DTH reactions (41, 42), the reduction of KC expression in Dcn−/− mice did not reach statistical significance. We conclude that primarily the altered levels of TNF-α expression may have contributed to the altered diapedesis, considering its key regulatory role in inflammation.
In summary, we have identified a novel role for decorin as a modulator of contact hypersensitivity reactions. Decorin inhibits the tight adhesion of polymorphonuclear leukocytes to the endothelial layer of blood vessels, facilitates diapedesis in a SDC1-dependent manner, and activates receptor tyrosine kinase-dependent signalling pathways. These events in turn induce transcriptional and posttranscriptional changes in TNFα and adhesion molecule expression. The loss of decorin results in increased expression of ICAM-1 and of anti-inflammatory SDC1, and exerts a protective influence to the allergen oxazolone.
Supplementary Material
Acknowledgments
We thank Margret Bahl, Anne Forsberg, Birgit Pers and Christine Bätza for their expert technical assistance, Dr. Deirdre Coombe for discussions, and Dr. Larry Fisher for the generous gift of decorin antiserum.
This work was financially supported by the Innovative Medizinische Forschung (IMF) of the Medical Faculty Münster (I-SE 12 08 11), the Deutsche Forschungsgemeinschaft DFG SE 1431/3-1 and GRK 1549 International Research Training Group ‘Molecular and Cellular GlycoSciences’ to DGS and MG, and DFG AZ 428/3-1 to AZ, the German Israeli Foundation I-1004-136.11/2008 to MG, the Interdisciplinary Center of Clinical Research (IZKF; Münster, Germany; core unit SmAP) and Za2/001/10 to AZ.
REFERENCES
- 1.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol.Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK. Cell-mediated (type IV) hypersensitivity. In: Kumar V, Abbas AK, Fausto N, editors. Pathologic Basis of Disease. 7th. Philadelphia, PA: Elsevier Saunders; 2005. pp. 216–217. [Google Scholar]
- 3.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol. Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 4.Götte M. Syndecans in inflammation. FASEB J. 2003;17:575–591. doi: 10.1096/fj.02-0739rev. [DOI] [PubMed] [Google Scholar]
- 5.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 6.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. Rev. FASEB. J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 7.Rops AL, Götte M, Baselmans MH, van den Hoven MJ, Steenbergen EJ, Lensen JF, Wijnhoven TJ, Cevikbas F, van den Heuvel LP, van Kuppevelt TH, Berden JH, van der Vlag J. Syndecan-1 deficiency aggravates anti-glomerular basement membrane nephritis. Kidney Int. 2007;72:1204–1215. doi: 10.1038/sj.ki.5002514. [DOI] [PubMed] [Google Scholar]
- 8.Kharabi Masouleh B, Ten Dam GB, Wild MK, Seelige R, van der Vlag J, Rops AL, Echtermeyer FG, Vestweber D, van Kuppevelt TH, Kiesel L, Götte M. Role of the heparan sulfate proteoglycan syndecan-1 (CD138) in delayed-type hypersensitivity. J. Immunol. 2009;182:4985–4993. doi: 10.4049/jimmunol.0800574. [DOI] [PubMed] [Google Scholar]
- 9.Floer M, Götte M, Wild MK, Heidemann J, Gassar ES, Domschke W, Kiesel L, Luegering A, Kucharzik T. Enoxaparin improves the course of dextran sodium sulfate-induced colitis in syndecan-1-deficient mice. Am. J. Pathol. 2010;176:146–157. doi: 10.2353/ajpath.2010.080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawashima H, Atarashi K, Hirose M, Hirose J, Yamada S, Sugahara K, Miyasaka M. Oversulfated chondroitin/dermatan sulfates containing GlcAbeta1/IdoAalpha1-3GalNAc(4,6-O-disulfate) interact with L- and P-selectin and chemokines. J. Biol. Chem. 2002;277:12921–12930. doi: 10.1074/jbc.M200396200. [DOI] [PubMed] [Google Scholar]
- 11.Seidler DG, Peter-Katalinic J, Zamfir AD. Galactosaminoglycan function and oligosaccharide structure determination. Scientific World Journal. 2007;7:233–241. doi: 10.1100/tsw.2007.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penc SF, Pomahac B, Eriksson E, Detmar M, Gallo RL. Dermatan sulfate activates nuclear factor-kappab and induces endothelial and circulating intercellular adhesion molecule-1. J. Clin. Invest. 1999;103:1329–1335. doi: 10.1172/JCI4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2003;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J. Biol. Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010;29:248–253. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Schönherr E, Sunderkötter C, Iozzo RV, Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J. Biol.Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 17.Sofeu Feugaing DD, Tammi R, Echtermeyer FG, Stenmark H, Kresse H, Smollich M, Schönherr E, Kiesel L, Götte M. Endocytosis of the dermatan sulfate proteoglycan decorin utilizes multiple pathways and is modulated by epidermal growth factor receptor signaling. Biochimie. 2007;89:637–657. doi: 10.1016/j.biochi.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiedler LR, Schönherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, Eble JA. Decorin regulates endothelial cell motility on collagen I through activation of insulinlike growth factor I receptor and modulation of alpha2beta1 integrin activity. J. Biol. Chem. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 20.Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J. Clin. Invest. 1997;100:149–157. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J. Biol. Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 22.Nelimarkka L, Salminen H, Kuopio T, Nikkari S, Ekfors T, Laine J, Pelliniemi L, Järveläinen H. Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am. J. Pathol. 2001;158:345–353. doi: 10.1016/S0002-9440(10)63975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage EH, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer L, Macakova K, Raslik I, Micegova M, Gröne HJ, Schönherr E, Robenek H, Echtermeyer FG, Grässel S, Bruckner P, Schaefer RM, Iozzo RV, Kresse H. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am. j. Pathol. 2002;160:1181–1191. doi: 10.1016/S0002-9440(10)64937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J. Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 27.Seidler DG, Schaefer L, Robenek H, Iozzo RV, Kresse H, Schönherr E. A physiologic three-dimensional cell culture system to investigate the role of decorin in matrix organisation and cell survival. Biochem. Biophys. Res. Commun. 2005;332:1162–1170. doi: 10.1016/j.bbrc.2005.04.175. [DOI] [PubMed] [Google Scholar]
- 28.Zamfir AD, Flangea C, Sisu E, Serb AF, Dinca N, Bruckner P, Seidler DG. Analysis of novel over- and under-sulfated glycosaminoglycan sequences by enzyme cleavage and multiple stage MS. Proteomics. 2009;9:3435–3444. doi: 10.1002/pmic.200800440. [DOI] [PubMed] [Google Scholar]
- 29.Bixel MG, Petri B, Khandoga AG, Khandoga A, Wolburg-Buchholz K, Wolburg H, März S, Krombach F, Vestweber D. A CD99-related antigen on endothelial cells mediates neutrophil but not lymphocyte extravasation in vivo. Blood. 2007;109:5327–5336. doi: 10.1182/blood-2006-08-043109. [DOI] [PubMed] [Google Scholar]
- 30.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–83. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarbock A, Deem TL, Burcin TL, Ley K. Galphai2 is required for chemokine-induced neutrophil arrest. Blood. 2007;110:3773–3779. doi: 10.1182/blood-2007-06-094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205:2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller H, Stadtmann A, Van Aken H, Hirsch E, Wang D, Ley K, Zarbock A A. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood. 2010;115:3118–3127. doi: 10.1182/blood-2009-11-254185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Vanhoutte D, Schellings MW, Götte M, Swinnen M, Herias V, Wild MK, Vestweber D, Chorianopoulos E, Cortés V, Rigotti A, Stepp MA, Van de Werf F, Carmeliet P, Pinto YM, Heymans S. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115:475–482. doi: 10.1161/CIRCULATIONAHA.106.644609. [DOI] [PubMed] [Google Scholar]
- 36.Stelljes M, Hermann S, Albring J, Köhler G, Löffler M, Franzius C, Poremba C, Schlösser V, Volkmann S, Opitz C, Bremer C, Kucharzik T, Silling G, Schober O, Berdel WE, Schäfers M, Kienast J. Clinical molecular imaging in intestinal graft-versus-host disease: mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood. 2008;111:2909–2918. doi: 10.1182/blood-2007-10-119164. [DOI] [PubMed] [Google Scholar]
- 37.Ahlfors E, Jonsson R, Czerkinsky C. Experimental T cell-mediated inflammatory reactions in the murine oral mucosa. II. Immunohistochemical characterization of resident and infiltrating cells. Clin. Exp. Immunol. 1996;104:297–305. doi: 10.1046/j.1365-2249.1996.967659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Götte M, Joussen AM, Klein C, Andre P, Wagner DD, Hinkes MT, Kirchhof B, Adamis AP, Bernfield M. Role of syndecan-1 in leukocyte-endothelial interactions in the ocular vasculature. Invest. Ophthalmol. Vis. Sci. 2002;43:1135–1141. [PubMed] [Google Scholar]
- 39.McDermott SP, Ranheim EA, Leatherberry VS, Khwaja SS, Klos KS, Alexander CM. Juvenile syndecan-1 null mice are protected from carcinogen-induced tumor development. Oncogene. 2006;26:1407–1416. doi: 10.1038/sj.onc.1209930. [DOI] [PubMed] [Google Scholar]
- 40.Engeman T, Gorbachev AV, Kish DD, Fairchild RL. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. J. Leukoc. Biol. 2004;76:941–949. doi: 10.1189/jlb.0304193. [DOI] [PubMed] [Google Scholar]
- 41.Yamada H, Matsukura M, Yudate T, Chihara J, Stingl G, Tezuka T. Enhanced production of RANTES, an eosinophil chemoattractant factor, by cytokine-stimulated epidermal keratinocytes. Int. Arch. Allergy Immunol. 1997;114(Suppl 1):28–32. doi: 10.1159/000237713. [DOI] [PubMed] [Google Scholar]
- 42.Cunha TM, Verri WA, Jr, Valério DA, Guerrero AT, Nogueira LG, Vieira SM, Souza DG, Teixeira MM, Poole S, Ferreira SH, Cunha FQ. Role of cytokines in mediating mechanical hypernociception in a model of delayed-type hypersensitivity in mice. Eur. J. Pain. 2008;12:1059–1068. doi: 10.1016/j.ejpain.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Montesano R, Pepper MS, Möhle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- 44.Seidler DG, Dreier R. Decorin and its galactosaminoglycan chain: extracellular regulator of cellular function? IUBMB Life. 2008;60:729–733. doi: 10.1002/iub.115. [DOI] [PubMed] [Google Scholar]
- 45.Winnemöller M, Schön P, Vischer P, Kresse H. Interactions between thrombospondin and the small proteoglycan decorin: interference with cell attachment. Eur. J. Cell Biol. 1992;59:47–55. [PubMed] [Google Scholar]
- 46.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankoa M, Marsche G, Young MF, Mihalik D, Götte M, Malle E, Schaefer RM, J .Gröne H. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchica CL, Pinelli V, Borges M, Zummer J, Narayanan V, Iozzo RV, Ludwig MS. A role for decorin in a murine model of allergen-induced asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:L863–L873. doi: 10.1152/ajplung.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S, Bowrin K, Hamad AR, Chakravarti S. Extracellular matrix lumican deposited on the surface of neutrophils promotes migration by binding to beta2 integrin. J. Biol. Chem. 2009;284:23662–23669. doi: 10.1074/jbc.M109.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferdous Z, Wei VM, Iozzo RV, Hook M, Grande-Allen KJ. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J. Biol. Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 50.Ferdous Z, Peterson SB, Tseng H, Anderson DK, Iozzo RV, Grande-Allen KJ. A role for decorin in controlling proliferation, adhesion, and migration of murine embryonic fibroblasts. J. Biomed. Mater. Res. A. 2010;93:419–428. doi: 10.1002/jbm.a.32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dressler J, Bachmann L, Kasper M, Hauck JG, Müller E. Time dependence of the expression of ICAM-1 (CD 54) in human skin wounds. Int. J. Legal Med. 1997;110:299–304. doi: 10.1007/s004140050092. [DOI] [PubMed] [Google Scholar]
- 52.Ohno N, Ichikawa H, Coe L, Kvietys PR, Granger DN, Alexander JS. Soluble selectins and ICAM-1 modulate neutrophil-endothelial adhesion and diapedesis in vitro. Inflammation. 1997;21:313–324. doi: 10.1023/a:1027349900279. [DOI] [PubMed] [Google Scholar]
- 53.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coombe DR, Stevenson SM, Kinnear BF, Gandhi NS, Mancera RL, Osmond RI, Kett WC. Platelet endothelial cell adhesion molecule 1 (PECAM-1) and its interactions with glycosaminoglycans: 2. Biochemical analyses. Biochemistry. 2008;47:4863–4875. doi: 10.1021/bi7024595. [DOI] [PubMed] [Google Scholar]
- 55.DeLisser HM, Van HC, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-I (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J. Biol. Chern. 1993;268:16037–16046. [PubMed] [Google Scholar]
- 56.Yeaman C, Rapraeger AC. Post-transcriptional regulation of syndecan-1 expression by cAMP in peritoneal macrophages. J. Cell Biol. 1993;122:941–950. doi: 10.1083/jcb.122.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.