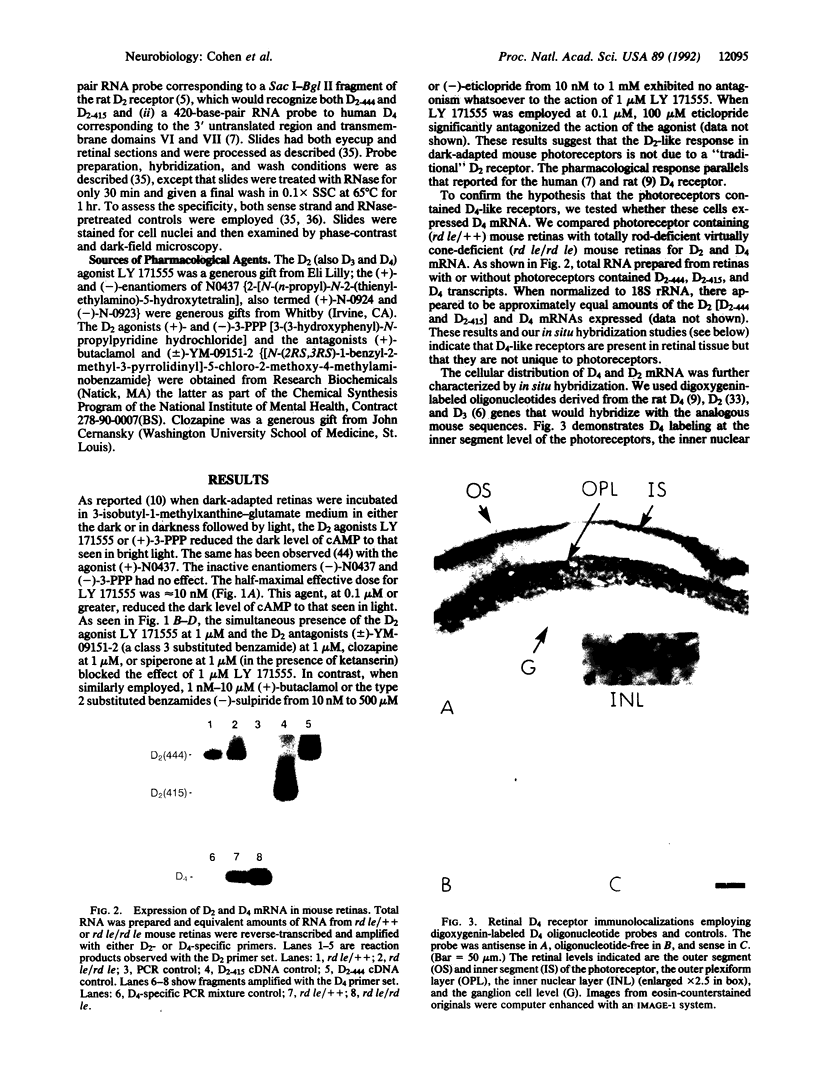
Abstract
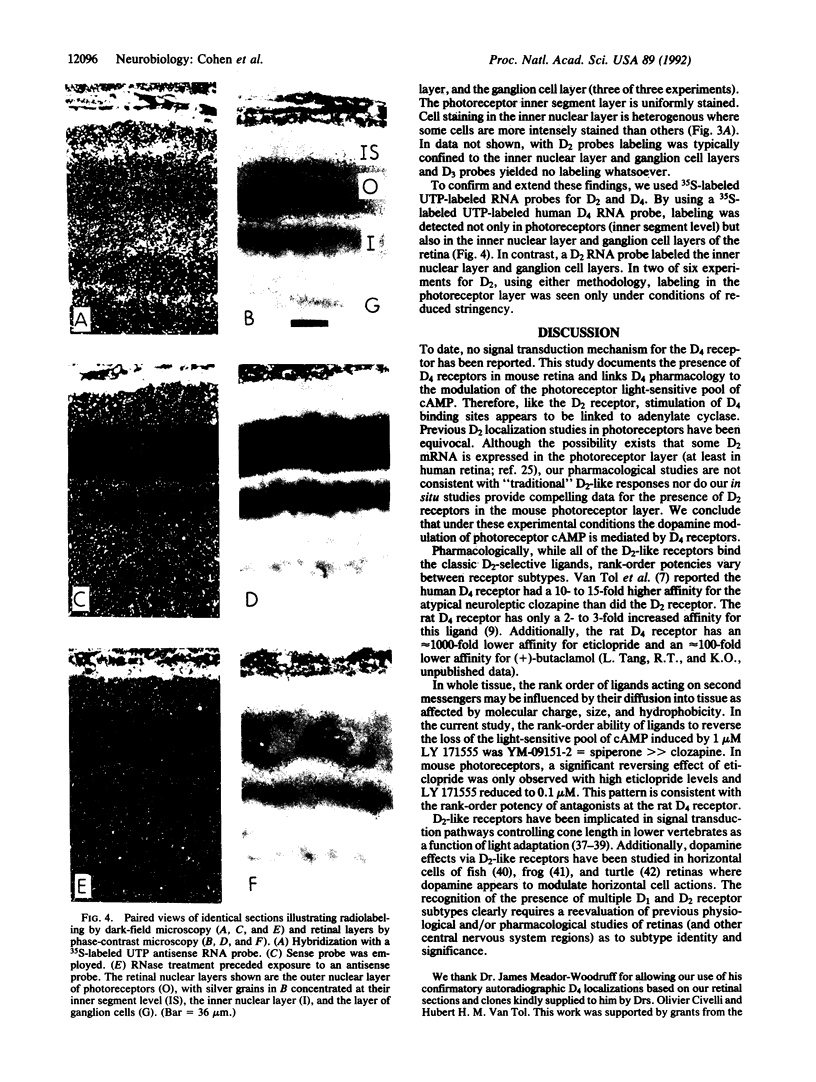
In the mouse, the light-sensitive pool of cAMP can be eliminated in the dark by application of the dopamine D2-like receptor agonists LY 171555 (quinpirole), (+)-N0437 (2-[N-(n-propyl)-N-2-(thienylethylamino)-5-hydroxytetralin]) , or (+)-3-PPP [3-(3-hydroxyphenyl)-N-propylpiperidine hydrochloride]. The rank-order affinity of the ability of the D2-like antagonists to block the action of LY 171555 matched that of the rat D4 receptor. Reverse transcription of retina mRNA followed by DNA amplification using D4-specific nucleotides demonstrates the presence of D4 mRNA in retina. In situ hybridization studies using D4-specific digoxygenin-labeled oligonucleotides or 35S-labeled UTP RNA probes demonstrate the presence of D4 mRNA in the photoreceptor cell layer and in the inner nuclear and ganglion cell layers. The modulation by D4 ligands of the dark level of light-sensitive cAMP in photoreceptors demonstrates the physiological coupling of the D4 receptor subtype.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brann M. R., Jelsema C. L. Dopamine receptors on photoreceptor membranes couple to a GTP-binding protein which is sensitive to both pertussis and cholera toxin. Biochem Biophys Res Commun. 1985 Nov 27;133(1):222–227. doi: 10.1016/0006-291x(85)91864-9. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Young W. S., 3rd Dopamine receptors are located on rods in bovine retina. Neurosci Lett. 1986 Sep 12;69(3):221–226. doi: 10.1016/0304-3940(86)90483-0. [DOI] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen A. I., Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Vis Neurosci. 1990 Jan;4(1):43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- Cohen A. I., McDaniel M., Orr H. Absolute levels of some free amino acids in normal and biologically fractionated retinas. Invest Ophthalmol. 1973 Sep;12(9):686–693. [PubMed] [Google Scholar]
- Dearry A., Edelman J. L., Miller S., Burnside B. Dopamine induces light-adaptive retinomotor movements in bullfrog cones via D2 receptors and in retinal pigment epithelium via D1 receptors. J Neurochem. 1990 Apr;54(4):1367–1378. doi: 10.1111/j.1471-4159.1990.tb01971.x. [DOI] [PubMed] [Google Scholar]
- Dearry A., Falardeau P., Shores C., Caron M. G. D2 dopamine receptors in the human retina: cloning of cDNA and localization of mRNA. Cell Mol Neurobiol. 1991 Oct;11(5):437–453. doi: 10.1007/BF00734808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A., Gingrich J. A., Falardeau P., Fremeau R. T., Jr, Bates M. D., Caron M. G. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990 Sep 6;347(6288):72–76. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E. Retinal neuromodulation: the role of dopamine. Vis Neurosci. 1991 Jul-Aug;7(1-2):87–97. doi: 10.1017/s0952523800010968. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. First-strand cDNA synthesis primed with oligo(dT). Methods Enzymol. 1987;152:316–325. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mack K. J., Todd R. D., O'Malley K. L. The mouse dopamine D2A receptor gene: sequence homology with the rat and human genes and expression of alternative transcripts. J Neurochem. 1991 Sep;57(3):795–801. doi: 10.1111/j.1471-4159.1991.tb08221.x. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff J. H., Mansour A., Bunzow J. R., Van Tol H. H., Watson S. J., Jr, Civelli O. Distribution of D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7625–7628. doi: 10.1073/pnas.86.19.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Mahan L. C., McVittie L. D., Gerfen C. R., Sibley D. R. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley K. L., Harmon S., Tang L., Todd R. D. The rat dopamine D4 receptor: sequence, gene structure, and demonstration of expression in the cardiovascular system. New Biol. 1992 Feb;4(2):137–146. [PubMed] [Google Scholar]
- O'Malley K. L., Mack K. J., Gandelman K. Y., Todd R. D. Organization and expression of the rat D2A receptor gene: identification of alternative transcripts and a variant donor splice site. Biochemistry. 1990 Feb 13;29(6):1367–1371. doi: 10.1021/bi00458a003. [DOI] [PubMed] [Google Scholar]
- POTTS A. M., MODRELL R. W., KINGSBURY C. Permanent fractionation of the electroretinogram by sodium glutamate. Am J Ophthalmol. 1960 Nov;50:900–907. doi: 10.1016/0002-9394(60)90342-1. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Brown P. K., Lurie M., Dowling J. E. Visual pigment and photoreceptor sensitivity in the isolated skate retina. J Gen Physiol. 1978 Apr;71(4):369–396. doi: 10.1085/jgp.71.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A., Hanitzsch R. Der Nachweis von Subkomponenten von PIII im ERG der isolierten Kaninchennetzhaut unter Verwendung von Natriumasparaginat. Acta Biol Med Ger. 1975;34(5):857–863. [PubMed] [Google Scholar]
- Schwartz E. A. Synaptic transmission in amphibian retinae during conditions unfavourable for calcium entry into presynaptic terminals. J Physiol. 1986 Jul;376:411–428. doi: 10.1113/jphysiol.1986.sp016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Monsma F. J., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992 Feb;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. I. General properties of the response. Vision Res. 1969 Dec;9(12):1435–1442. doi: 10.1016/0042-6989(69)90059-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Springer J. E., Robbins E., Gwag B. J., Lewis M. E., Baldino F., Jr Non-radioactive detection of nerve growth factor receptor (NGFR) mRNA in rat brain using in situ hybridization histochemistry. J Histochem Cytochem. 1991 Feb;39(2):231–234. doi: 10.1177/39.2.1846159. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stormann T. M., Gdula D. C., Weiner D. M., Brann M. R. Molecular cloning and expression of a dopamine D2 receptor from human retina. Mol Pharmacol. 1990 Jan;37(1):1–6. [PubMed] [Google Scholar]
- Sunahara R. K., Guan H. C., O'Dowd B. F., Seeman P., Laurier L. G., Ng G., George S. R., Torchia J., Van Tol H. H., Niznik H. B. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991 Apr 18;350(6319):614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Sunahara R. K., Niznik H. B., Weiner D. M., Stormann T. M., Brann M. R., Kennedy J. L., Gelernter J. E., Rozmahel R., Yang Y. L., Israel Y. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990 Sep 6;347(6288):80–83. doi: 10.1038/347080a0. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991 Apr 18;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Stone S., Besharse J. C. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res. 1988 May 24;449(1-2):332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Stone S., Tranchina D. Photoreceptor to horizontal cell synaptic transfer in the Xenopus retina: modulation by dopamine ligands and a circuit model for interactions of rod and cone inputs. J Neurophysiol. 1989 Oct;62(4):864–881. doi: 10.1152/jn.1989.62.4.864. [DOI] [PubMed] [Google Scholar]
- Zarbin M. A., Wamsley J. K., Palacios J. M., Kuhar M. J. Autoradiographic localization of high affinity GABA, benzodiazepine, dopaminergic, adrenergic and muscarinic cholinergic receptors in the rat, monkey and human retina. Brain Res. 1986 May 21;374(1):75–92. doi: 10.1016/0006-8993(86)90396-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Y., Grandy D. K., Thambi L., Kushner J. A., Van Tol H. H., Cone R., Pribnow D., Salon J., Bunzow J. R., Civelli O. Cloning and expression of human and rat D1 dopamine receptors. Nature. 1990 Sep 6;347(6288):76–80. doi: 10.1038/347076a0. [DOI] [PubMed] [Google Scholar]