Abstract
AIM:
The aim of this study was to investigate the alterations of pulmonary function tests (PFTs) and their relationship with disease activity in inflammatory bowel diseases (IBDs).
METHODS:
Sixty-four IBD patients (31 Crohn's disease [CD] and 33 ulcerative colitis [UC]) and thirty healthy individuals (controls) were studied with regard to the following parameters of PFTs: Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), their ratio, mid-forced expiratory flow of 25–75% (FEF 25–75), residual volume, total lung capacity, and diffusing capacity of the lung for carbon monoxide (DLCO). The disease activity was calculated using the Crohn's Disease Activity Index for CD and Mayo Clinic Score for UC. Correlation analysis was performed between disease activity and sputum cytology and PFTs.
RESULTS:
Nineteen of the 31 CD patients (61.29%) and 17 of the 33 UC patients (51.52%) but none of the controls showed at least one abnormal PFTs (P < 0.05). Compared with controls, both CD and UC patients exhibited a significant reduction in FEV1 (P < 0.05), FVC (P < 0.05), FEF 25–75 (P < 0.05), and DLCO (P < 0.05). The majority with decreased measurements of PFTs were in the active phase of diseases (P < 0.05). IBD activity scores correlated negatively with some parameters of PFTs and positively with lymphocytosis and eosinophilia of sputum (P < 0.05).
CONCLUSIONS:
Pulmonary function disorders are significantly common in IBD patients. The impairment in active disease is significantly greater than in remission.
Key words: Crohn's disease, inflammatory bowel disease, pulmonary function tests, sputum, ulcerative colitis
Inflammatory bowel diseases (IBDs) are chronic gastrointestinal tract diseases of unknown etiology.[1] More than one-third of the IBD patients are affected by extraintestinal manifestations.[2] Among them, lung involvement is relatively rare and often overlooked. However, a number of such manifestations have been reported in IBD patients, such as inflammatory tracheal stenosis, bronchiolitis obliterans organizing pneumonia, panbronchiolitis, interstitial pneumonitis, and apical fibrosis.[3,4,5]
The true prevalence of pulmonary involvement in IBD remains unknown.[6] A study found that although obvious lung involvement was exceptional, subclinical alterations were identified in at least half of the IBD patients.[7] The subclinical involvement of lung can be detected by lung function abnormalities and/or incremental lymphocyte count in the bronchoalveolar lavage fluid.[8,9]
Lung involvements in IBD may merely consist of a subclinical lung function abnormality while studies on pulmonary function impairments in IBD patients have yielded conflicting results.[10] Some studies show alterations of pulmonary function in IBD patients,[11,12] whereas others do not.[13,14] The relationship between pulmonary impairment and activity of disease also has not been clearly established in IBD patients. Some authors report that pulmonary function test (PFT) abnormalities in the patients of IBD are associated with the activity of disease,[15,16] whereas others find that the activity of bowel disease does not affect the measurements of PFTs.[17,18]
The objectives of this study were to (a) assess PFT abnormalities in IBD patients, (b) to explore whether there was a difference in PFTs between the two IBD entities: Crohn's disease (CD) and ulcerative colitis (UC), and (c) to examine the relationship between abnormal PFTs and cytology of induced sputum, and disease activity in IBD patients.
Methods
Subjects
This prospective study was carried out at our tertiary care hospital, between January 2013 and May 2015. Eighty-six consecutive patients who were more than 18 years old and with the diagnosis of IBD were included in this study. Nine patients declined to participate. Thirteen patients were excluded because of reasons as follows: Abnormal chest computed tomography, acute bronchitis or pneumonia, severe pulmonary bulla, previous bowel surgery, congestive heart failure, connective tissue disease, pregnant women, and lack of compliance in PFTs. Sixty-four patients with IBD (31 CD and 33 UC) were enrolled into the study. All patients had a newly confirmed diagnosis of CD or UC, based on the findings of clinical manifestations and pathology reports. Before they were enrolled, the treatment for CD or UC was not initiated. The control group, consisting of 30 age- and sex-matched participants, was recruited concurrently from healthy volunteers. The general information of the enrollees (age, sex, smoking habit, height, and weight) was gathered.
All the patients and controls agreed to participate in the study and signed an informed consent form. The Ethics Committee of Qianfoshan Hospital Affiliated to Shandong University approved the study.
Pulmonary function tests
Spirometry (Erich Jaeger GmbH, Hoechberg, Germany) was used to determine forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and their ratio (FEV1/FVC), residual volume (RV), total lung capacity (TLC), and mid-forced expiratory flow of 25–75% (FEF 25–75%). Each measurement was repeated three times, and the highest value was accepted. All data were expressed as measurement values and the percentage of predicted values for gender, age, and height.[19] The percentage of predicted values was used for statistical analysis. Diffusing capacity of the lung for carbon monoxide (DLCO) was measured by the single-breath method. The individuals were classified as normal when all the measurements of FVC, FEV1, FEV1/FVC, and DLCO were more than 75% of the predicted values and FEF 25–75% was more than 70%. All operations of PFTs were performed on the same instrument by the same technologist.
Disease activity
The Crohn's Disease Activity Index (CDAI)[20] was used to evaluate disease activity for CD and the Mayo Clinic Score[21] for UC. A score below 150 in CDAI is associated with clinical remission and above 150 with active disease.[22] Patients with Mayo Clinic Score <3 were classified as being in remission, and those with a score ≥3 were classified as having active disease.
Induced sputum and cytology
The method described by Pin et al. was adopted for inducing sputum.[23] The patients inhaled an aerosol of hypertonic saline delivered by an ultrasonic nebulizer (BSW-2A, Shenyang, China) after they were pretreated with 200 µg inhaled salbutamol to inhibit airway constriction. The concentrations of saline were 3%, 4%, and 5%, and the duration of inhalation was 7 min for each concentration. After each period of inhalation, the patients were asked to expectorate sputum into a sterile container. If they had symptoms of chest congestion or dyspnea or a decline of 20% or more in FEV1 relative to the baseline value, the operation must be discontinued immediately and FEV1 should be monitored.
The expectoration was processed as soon as possible within 2 h according to the adapted method of Mohamed-Hussein et al.[16] Qualified sputum was selected and mixed with four times its volume of 0.1% dithiothreitol. The mixture was put into water of 37°C temperature for 10 min and the mucus was filtered out. Then, the suspension was centrifuged at 1000 revolutions/min for 5 min. Finally, the cell count was performed by an experienced technician with a hemocytometer who was blinded to the patients.
Statistical analysis
All quantitative data were expressed as mean ± standard deviation. Data were tested for normal distribution, and Kruskal–Wallis test and Mann–Whitney test were employed to evaluate significances of the differences between the groups. Correlation coefficients were calculated using Spearman's ρ correlation to assess the correlation between disease activity and lung function data, and sputum lymphocytosis and eosinophilia. All the analyses were conducted using SPSS 20.0 (SPSS, Chicago, IL, USA). The statistical significance level was set a priori at 0.05.
Results
Patient description
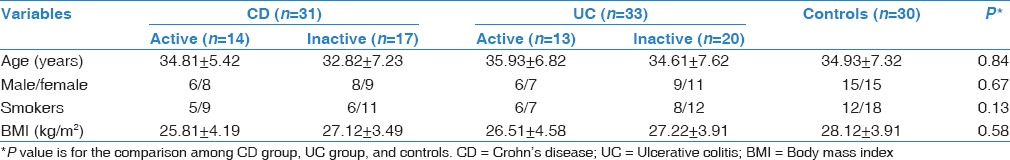
The general information on the three groups (CD, UC, and controls) is shown in Table 1. No significant difference was found among the three groups in age, sex, body mass index (BMI), and smoking habit. There were 14 CD patients (45.16%) and 13 UC patients (39.39%) in the active phase of disease (P > 0.05).
Table 1.
Demographic data of the studied groups
Pulmonary function testing
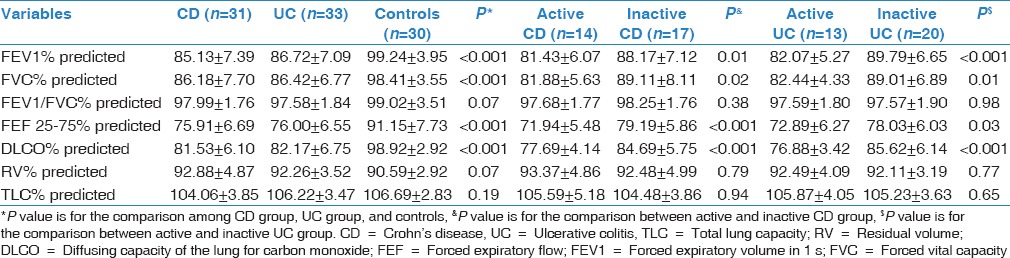
Nineteen CD patients (61.29%) and 17 UC patients (51.52%) showed at least one pathological PFT (P < 0.05). The reduction of measurements in CD and UC patients was significant in FEV1 (P < 0.05), FVC (P < 0.05), FEF 25–75 (P < 0.05), and DLCO (P < 0.05) compared to controls. These could be revealed by predicted FEV1% (−14.11% of predicted value in CD and −12.52% in UC, P < 0.05), predicted FVC (−12.23% in CD and −11.99% in UC, P < 0.05), predicted FEF 25–75% (−15.24% in CD and −14.3% in UC, P < 0.05), and predicted DLCO (−17.39% in CD and 16.75% in UC, P < 0.01). There were no significant differences in FEV1/FEV, RV, and TLC between patients with CD and UC and controls (P > 0.05). No significant differences were found between patients with CD and UC in PFTs (P > 0.05). In contrast to patients with quiescent diseases, those with active diseases had a pronounced abnormality in FEV1 (P < 0.05), FVC (P < 0.05), FEF 25–75 (P < 0.05), and DLCO (P < 0.05). The measurements of patients and controls are shown in Table 2.
Table 2.
Pulmonary function measurements in patients with inflammatory bowel diseases and controls
Sputum cytology
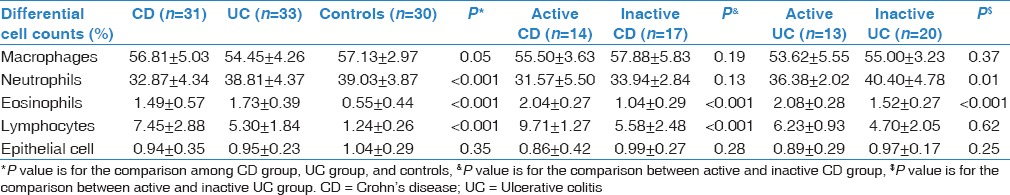
The differential counts of lymphocyte (L) and eosinophil (E) were significantly higher whereas the differential counts of neutrophil (N) were lower in IBDs patients than in controls (P < 0.05). It could be showed by lymphocytes (+6.21% in CD and +4.06% in UC, P < 0.05), eosinophils (+0.94% in CD and + 1.18% in UC, P < 0.05), and neutrophils (−6.16% in CD, P < 0.05). There were no significant differences between patients with CD and UC (P > 0.05). In comparison to inactive IBDs patients, the lymphocytosis and eosinophilia were more significant in active ones (P < 0.05). The measurements of induced sputum are shown in Table 3.
Table 3.
Induced sputum cell counts in inflammatory bowel disease patients
Correlation between pulmonary function tests, sputum cytology, and disease activity
Correlation analysis was performed for IBD patients, and the Spearman's correlation coefficients were calculated. The results were shown in Table 4. A significant negative correlation was found between the activity of disease and predicted FEV1%, predicted FVC%, predicted FEF 25–75%, and predicted DLCO%. In addition, a positive correlation existed significantly between IBD activity and lymphocytosis and eosinophilia of sputum.
Table 4.
Correlation between pulmonary function tests, sputum cytology, and disease activity

Discussion
In the present study, we found that IBD patients and healthy controls displayed a marked disproportion in the prevalence of pulmonary function abnormalities. Thirty-six (19 in CD and 17 in UC) of 64 IBD patients (52.25%) were identified to have abnormal PFTs measurements. There was no difference between UC and CD in routine PFTs. These findings were in accordance with the study of Kuzela et al., who found that more than half of the IBD patients had abnormalities in lung function despite the lack of abnormal radiological findings.[7] Similar results were found by Storch et al. in a review including over 400 patients with IBD.[10] In addition, the abnormalities of PFTs were more prevalent and pronounced in patients with active disease.
Lung diffusion impairment, as shown by decreased DLCO values, was a common abnormality of PFTs in patients with IBD in our study. Supporting this finding, several studies have confirmed a high incidence of decreased DLCO in IBD patients.[8,12,24,25] The mechanism of reduction in DLCO remains uncertain, and the impaired lung diffusion may suggest the presence of an interstitial pulmonary process, which may indicate that pulmonary mesenchyme would be potentially damaged. Therefore, the DLCO may provide a useful noninvasive indicator of subclinical lung involvement in IBD patients.[26] In addition, a significant reduction of FEV1, FVC, and FEF of 25–75% was detected in this study, suggesting different patterns of pulmonary function impairment in IBD patients. These results accord with various studies, which have revealed a spectrum of abnormalities of PFTs in IBD patients, such as obstructive or restrictive disease and bronchial hyperresponsiveness.[27,28]
An increased percentage of lymphocytes in induced sputum was found in IBD patients. These findings agreed substantially with the results from the previous studies.[29,30] The nature of the lymphocytosis in sputum remains unclear, and it may suggest that immune and inflammatory cells accumulate within the alveolar structures. It was known that in patients with active IBD, CD4 was vigorously activated in the intestinal mucosa.[31] Moreover, both gastrointestinal and respiratory systems have a common mucosal immune system, and lymphocytes activated at one mucosal surface would circulate and localize at another mucosal surface, which would lead to lymphocyte alveolitis.[32]
The effect of disease activity on PFTs and induced sputum cytology continues to be obscure in IBD patients. We found that there were negative and positive correlations between the activity of disease and some parameters of PFTs and sputum lymphocytosis and eosinophilia, respectively. In accordance with our findings, previous studies reported that the abnormalities of PFTs were more evident in IBD patients with active disease and persisted during remission, which may reflect bowel disease activity.[33,34] In IBD patients, the pathological features of lung and intestines are similar when the lung is involved. For example, Kayser et al. performed wedge biopsies of the lungs in two male CD patients who suffered from dyspnea, shortness of breath, and ventilation disturbances. The pathological findings in their study suggested that the alterations of lung tissue were related to CD.[35] Therefore, abnormalities of PFTs, as an indicator of lung involvement, correlated with the activity of disease in IBD patients.
The mechanism of lung involvement in IBD remains unclear. First, both the colonic and respiratory epithelia share an embryonic origin from the primitive foregut and have similarity in the mucosal immune system which causes the same pathogenic changes.[36] Second, the activated inflammatory cells in the bowel tissues are able to produce several circulating cytokines that are inducing damage to lung parenchyma.[37] As a simple noninvasive method, induced sputum was investigated in CD patients and it was found that the lymphocyte count was significantly higher in CD patients, indicating lung involvement.[29] In accordance with this, sputum lymphocytosis was significantly correlated with the reduction of some parameters as well as disease activity in PFTs in our study.
Sulfasalazine and mesalamine are medications used commonly for the treatment of IBD, with side effects being dose-related or idiosyncratic. Interstitial diseases are commonly reported lung diseases related to the usage of these drugs, although bronchiolitis obliterans and eosinophilic pneumonia have also been described.[38,39,40] Conversely, the current therapy may reduce the impact of respiratory abnormalities if colonic and ileal mucosal inflammation is under control. In our study, patients who are receiving sulfasalazine or mesalamine were excluded, and it was unlikely to attribute the abnormalities of PFTs seen in patients to these medications.
Malnutrition is frequently observed in patients with IBD, and various methods including BMI can be used to assess the status of malnutrition in hospitalized patients.[41] The nutritional status has an obvious effect on the entire lung function in IBD patients. In this study, we investigated BMI as the nutritional status index of the patients and controls and found no significant difference. Consequently, it seemed unlikely that the abnormality of PFTs was relevant to nutritional status.
While this study provided interesting and valuable findings, it has weaknesses. One of the limitations of this study is the population of IBD patients in the present study is relatively small. Another potential limitation is the cross-sectional design used in our study is not the best way to investigate group wise differences. A longitudinal study is needed in which the patients are studied during the active and inactive phases of their disease.
Conclusion
There are subclinical abnormalities of PFTs in IBD patients which could be detected even in the remission periods and become pronounced in the active phase of diseases. PFTs might be used as a noninvasive means of diagnosis to determine the activity of disease and might contribute to the early detection of latent pulmonary involvement.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ji XQ, Wang LX, Lu DG. Pulmonary manifestations of inflammatory bowel disease. World J Gastroenterol. 2014;20:13501–11. doi: 10.3748/wjg.v20.i37.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585–95. doi: 10.1038/nrgastro.2013.117. [DOI] [PubMed] [Google Scholar]
- 3.Casey MB, Tazelaar HD, Myers JL, Hunninghake GW, Kakar S, Kalra SX, et al. Noninfectious lung pathology in patients with Crohn's disease. Am J Surg Pathol. 2003;27:213–9. doi: 10.1097/00000478-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Camus P, Colby TV. The lung in inflammatory bowel disease. Eur Respir J. 2000;15:5–10. doi: 10.1183/09031936.00.15100500. [DOI] [PubMed] [Google Scholar]
- 5.Spira A, Grossman R, Balter M. Large airway disease associated with inflammatory bowel disease. Chest. 1998;113:1723–6. doi: 10.1378/chest.113.6.1723. [DOI] [PubMed] [Google Scholar]
- 6.Rogers BH, Clark LM, Kirsner JB. The epidemiologic and demographic characteristics of inflammatory bowel disease: An analysis of a computerized file of 1400 patients. J Chronic Dis. 1971;24:743–73. doi: 10.1016/0021-9681(71)90087-7. [DOI] [PubMed] [Google Scholar]
- 7.Kuzela L, Vavrecka A, Prikazska M, Drugda B, Hronec J, Senkova A, et al. Pulmonary complications in patients with inflammatory bowel disease. Hepatogastroenterology. 1999;46:1714–9. [PubMed] [Google Scholar]
- 8.Heatley RV, Thomas P, Prokipchuk EJ, Gauldie J, Sieniewicz DJ, Bienenstock J. Pulmonary function abnormalities in patients with inflammatory bowel disease. Q J Med. 1982;51:241–50. [PubMed] [Google Scholar]
- 9.Smiéjan JM, Cosnes J, Chollet-Martin S, Soler P, Basset FM, Le Quintrec Y, et al. Sarcoid-like lymphocytosis of the lower respiratory tract in patients with active Crohn's disease. Ann Intern Med. 1986;104:17–21. doi: 10.7326/0003-4819-104-1-17. [DOI] [PubMed] [Google Scholar]
- 10.Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:104–15. doi: 10.1097/00054725-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Neilly JB, Main AN, McSharry C, Murray J, Russell RI, Moran F. Pulmonary abnormalities in Crohn's disease. Respir Med. 1989;83:487–91. doi: 10.1016/s0954-6111(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz A, Yilmaz Demirci N, Hosgün D, Uner E, Erdogan Y, Gökçek A, et al. Pulmonary involvement in inflammatory bowel disease. World J Gastroenterol. 2010;16:4952–7. doi: 10.3748/wjg.v16.i39.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson NM, Mee AS, Jewell DP, Clarke SW. Pulmonary function in inflammatory bowel disease. Digestion. 1978;18:416–8. doi: 10.1159/000198228. [DOI] [PubMed] [Google Scholar]
- 14.Tzanakis N, Bouros D, Samiou M, Panagou P, Mouzas J, Manousos O, et al. Lung function in patients with inflammatory bowel disease. Respir Med. 1998;92:516–22. doi: 10.1016/s0954-6111(98)90301-8. [DOI] [PubMed] [Google Scholar]
- 15.Pasquis P, Colin R, Denis P, Baptiste P, Galmiche JP, Hecketsweiler P. Transient pulmonary impairment during attacks of Crohn's disease. Respiration. 1981;41:56–9. doi: 10.1159/000194359. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed-Hussein AA, Mohamed NA, Ibrahim ME. Changes in pulmonary function in patients with ulcerative colitis. Respir Med. 2007;101:977–82. doi: 10.1016/j.rmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Tunc B, Filik L, Bilgic F, Arda K, Ulker A. Pulmonary function tests, high-resolution computed tomography findings and inflammatory bowel disease. Acta Gastroenterol Belg. 2006;69:255–60. [PubMed] [Google Scholar]
- 18.Sommer H, Schmidt M, Gruber KD. Pulmonary functional disorders in ulcerative colitis and Crohn's disease. Dtsch Med Wochenschr. 1986;111:812–5. doi: 10.1055/s-2008-1068536. [DOI] [PubMed] [Google Scholar]
- 19.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 20.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–44. [PubMed] [Google Scholar]
- 21.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 22.Naber AH, de Jong DJ. Assessment of disease activity in inflammatory bowel disease; relevance for clinical trials. Neth J Med. 2003;61:105–10. [PubMed] [Google Scholar]
- 23.Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, et al. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992;47:25–9. doi: 10.1136/thx.47.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munck A, Murciano D, Pariente R, Cezard JP, Navarro J. Latent pulmonary function abnormalities in children with Crohn's disease. Eur Respir J. 1995;8:377–80. doi: 10.1183/09031936.95.08030377. [DOI] [PubMed] [Google Scholar]
- 25.Songür N, Songür Y, Tüzün M, Dogan I, Tüzün D, Ensari A, et al. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol. 2003;37:292–8. doi: 10.1097/00004836-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Marvisi M, Bassi E, Bonassi R, Civardi G, Delsignore R. DLCO correlates with intestinal inflammation in ulcerative colitis, but albuminuria does not. Minerva Gastroenterol Dietol. 2007;53:321–7. [PubMed] [Google Scholar]
- 27.Eade OE, Smith CL, Alexander JR, Whorwell PJ. Pulmonary function in patients with inflammatory bowel disease. Am J Gastroenterol. 1980;73:154–6. [PubMed] [Google Scholar]
- 28.Tzanakis N, Samiou M, Bouros D, Mouzas J, Kouroumalis E, Siafakas NM. Small airways function in patients with inflammatory bowel disease. Am J Respir Crit Care Med. 1998;157:382–6. doi: 10.1164/ajrccm.157.2.97-04075. [DOI] [PubMed] [Google Scholar]
- 29.Wallaert B, Colombel JF, Tonnel AB, Bonniere P, Cortot A, Paris JC, et al. Evidence of lymphocyte alveolitis in Crohn's disease. Chest. 1985;87:363–7. doi: 10.1378/chest.87.3.363. [DOI] [PubMed] [Google Scholar]
- 30.Fireman Z, Osipov A, Kivity S, Kopelman Y, Sternberg A, Lazarov E, et al. The use of induced sputum in the assessment of pulmonary involvement in Crohn's disease. Am J Gastroenterol. 2000;95:730–4. doi: 10.1111/j.1572-0241.2000.01843.x. [DOI] [PubMed] [Google Scholar]
- 31.Müller S, Lory J, Corazza N, Griffiths GM, Z’graggen K, Mazzucchelli L, et al. Activated CD4 and CD8 cytotoxic cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am J Pathol. 1998;152:261–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Welsh L, Haller W, King LE, Soto-Martinez M, Oliver M, Catto-Smith A, et al. Pulmonary function abnormalities in children with active Crohn's disease. Am J Respir Crit Care Med. 2012;186:1060–1. doi: 10.1164/ajrccm.186.10.1060. [DOI] [PubMed] [Google Scholar]
- 33.Marvisi M, Borrello PD, Brianti M, Fornarsari G, Marani G, Guariglia A. Changes in the carbon monoxide diffusing capacity of the lung in ulcerative colitis. Eur Respir J. 2000;16:965–8. doi: 10.1183/09031936.00.16596500. [DOI] [PubMed] [Google Scholar]
- 34.Herrlinger KR, Noftz MK, Dalhoff K, Ludwig D, Stange EF, Fellermann K. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol. 2002;97:377–81. doi: 10.1111/j.1572-0241.2002.05473.x. [DOI] [PubMed] [Google Scholar]
- 35.Kayser K, Probst F, Gabius HJ, Müller KM. Are there characteristic alterations in lung tissue associated with Crohn's disease? Pathol Res Pract. 1990;186:485–90. doi: 10.1016/s0344-0338(11)80468-1. [DOI] [PubMed] [Google Scholar]
- 36.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–32. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 37.Williams H, Walker D, Orchard TR. Extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep. 2008;10:597–605. doi: 10.1007/s11894-008-0108-6. [DOI] [PubMed] [Google Scholar]
- 38.Bitton A, Peppercorn MA, Hanrahan JP, Upton MP. Mesalamine-induced lung toxicity. Am J Gastroenterol. 1996;91:1039–40. [PubMed] [Google Scholar]
- 39.Haralambou G, Teirstein AS, Gil J, Present DH. Bronchiolitis obliterans in a patient with ulcerative colitis receiving mesalamine. Mt Sinai J Med. 2001;68:384–8. [PubMed] [Google Scholar]
- 40.Saltzman K, Rossoff LJ, Gouda H, Tongia S. Mesalamine-induced unilateral eosinophilic pneumonia. AJR Am J Roentgenol. 2001;177:257. doi: 10.2214/ajr.177.1.1770257. [DOI] [PubMed] [Google Scholar]
- 41.Sökülmez P, Demirbag AE, Arslan P, Disibeyaz S. Effects of enteral nutritional support on malnourished patients with inflammatory bowel disease by subjective global assessment. Turk J Gastroenterol. 2014;25:493–507. doi: 10.5152/tjg.2014.4955. [DOI] [PubMed] [Google Scholar]


