Abstract
BACKGROUND:
Majority of our computed tomography-pulmonary angiography (CTPA) scans report negative findings. We hypothesized that suboptimal reliance on diagnostic algorithms contributes to apparent overuse of this test.
METHODS:
A retrospective review was performed on 2031 CTPA cases in a large hospital system. Investigators retrospectively calculated pretest probability (PTP). Use of CTPA was considered as inappropriate when it was ordered for patients with low PTP without checking D-dimer (DD) or following negative DD.
RESULTS:
Among the 2031 cases, pulmonary embolism (PE) was found in 7.4% (151 cases). About 1784 patients (88%) were considered “PE unlikely” based on Wells score. Out of those patients, 1084 cases (61%) did not have DD test prior to CTPA. In addition, 78 patients with negative DD underwent unnecessary CTPA; none of them had PE.
CONCLUSIONS:
The suboptimal implementation of PTP assessment tools can result in the overuse of CTPA, contributing to ineffective utilization of hospital resources, increased cost, and potential harm to patients.
Key words: Compliance rate, computed tomography-pulmonary angiography overuse, D-dimer, pulmonary embolism
The estimated annual incidence of pulmonary embolism (PE) is about 70 cases per 100,000.[1,2] This number almost doubled to 112 cases per 100,000 after implementing computed tomographic pulmonary angiography (CTPA) in PE evaluation.[3] PE accounts up to 0.5% of all deaths in the United States (US),[4] with a constant decrease in mortality rate over the past few decades.[1,4,5,6]
With the advent of spiral computed tomography (CT), CTPA has become a method of choice in diagnosing PE, with a sensitivity and a specificity of 83% and 96%, respectively.[7,8] This reliable diagnostic tool is capable of visualizing pulmonary vasculature with a detailed distribution of filling defects caused by pulmonary emboli.[9] In addition, ancillary findings have been detected in several studies and found to be valuable in patient care.[10,11,12,13] On the other hand, CTPA carries some concerning issues which might primarily affect patient safety. This includes ionizing radiation exposure, contrast-induced nephropathy (CIN), contrast-induced anaphylaxis, and dye extravasation.[14] Furthermore, CTPA is an expensive and relatively time-consuming test which may potentially strain facility resources.
The current guidelines recommend assessing clinical pretest probability (PTP) as an initial step in approaching patients with suspected PE.[7,14,15,16] Wells' criteria [Table 1] are by far one of the most well-established scoring systems used in estimating patients' PTP and stratifying individuals into “PE unlikely” and “PE likely” groups.[17] Thus, PE can be safely ruled out in patients who are at a low risk for PE with negative D-dimer (DD) assay.[15,16,17,18,19] Such practice will avert performing unnecessary CTPA and exposing patients to the risk of radiation and kidney injury. Unfortunately, numerous studies have reported a remarkable increase in using CTPA examination with a decrease in its positive yield rate.[9,14,20,21,22,23]
Table 1.
Modified Wells’ criteria
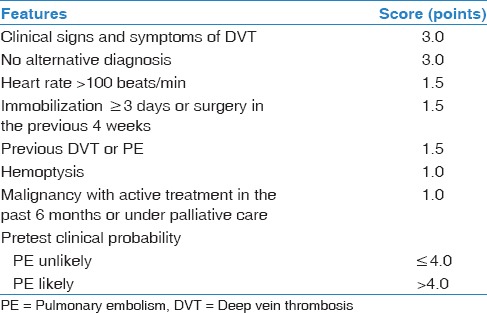
At our tertiary teaching medical center, we noticed an increase in the overall negative testing rate of CTPA in PE workup. Hence, we conducted this study to investigate whether or not CTPA is overused, and assessment of PTP of PE using modified Wells criteria and DD is underutilized.
Methods
Study design and data collection
We conducted our study at the Detroit Medical Center (DMC), which has a total of 1100 beds and an average of 250,000 emergency room visits annually. The Institutional Review Board of Wayne State University, to which DMC is affiliated, has approved the study.
We performed a retrospective chart review on all patients who underwent CTPA for suspected PE during a 6-month period from January 1, 2011 to June 30, 2011 at our institution. The collected data included patient demographic features (coded identifier, age, gender, and race), presenting complaints, comorbidities, physical examination findings, and laboratory results.
Our investigators reviewed the documented notes for each patient and retrospectively calculated PTP of PE at the time of ordering CTPA. Subsequently, they reported the initial diagnostic testing (DD versus CTPA) in PE evaluation for each case.
We relied on the independent interpretation of CTPA imaging reported by in-house board-certified chest radiologists to divide the CTPA results into positive or negative for PE. DD assay in our medical center was measured in micrograms per milliliter (μg/mL) using enzyme-linked immunosorbent assay. DD value was considered positive in our laboratory when it exceeds 0.58 µg/mL.
Outcome
The primary outcome of the study was to assess the compliance with evidence-based guidelines in approaching patients with suspected PE. Noncompliance was considered when CTPA was ordered without checking DD or following negative DD in “PE unlikely” group.
The secondary outcome of this review was to estimate the rate of CIN which was defined as an increase in serum creatinine more than 0.5 mg/dL or more than 25% from its baseline level, 48–72 h postcontrast exposure.[24,25] We calculated the prevalence rate of CIN in our sample after excluding the patients with end-stage renal disease (ESRD) who were on dialysis management.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences software version 18. We applied Chi-square and Fisher's exact tests to compare between the categorical variables (e.g., gender, race, elements of Wells' criteria, and CTPA results), whereas we used an unpaired t-test to determine the difference among continuous variables (e.g., age and Wells scores). All P values were two-sided, considering values <0.05 as statistically significant.
Results
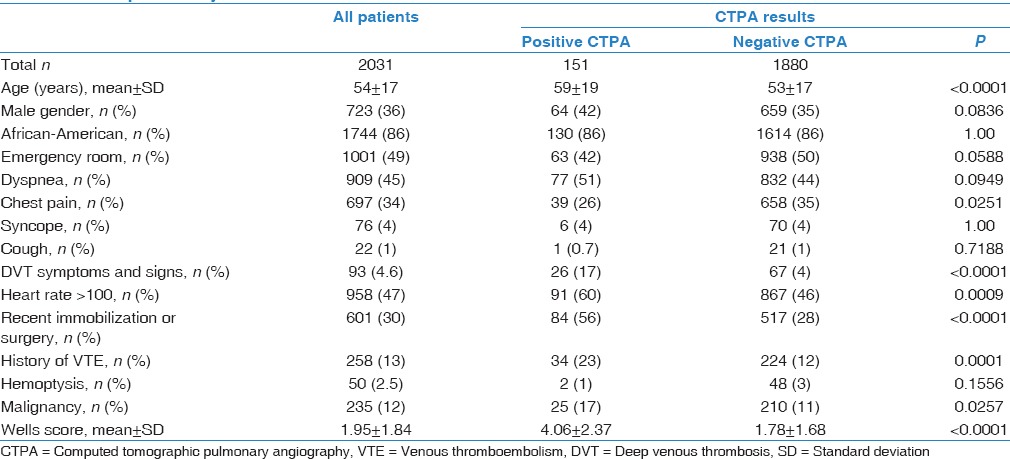
We reported 2136 consecutive CTPA scans performed at the DMC during a period of 6 months. We excluded 96 cases secondary to unavailable proper documentations, and nine cases because of repeating CTPA due to intravenous (IV) contrast extravasation. A total of 2031 cases of CTPA have been retrospectively analyzed. Almost half of them (1001 cases, 49%) were from emergency department (ED), while the rest (1030 cases, 51%) were inpatients. PE was diagnosed in 151 out of 2031 cases (7.4%); 63 were ED patients and 88 were inpatients.
Our sample had a female (64%) and African-American (AA) (86%) predominance, with a mean age of 54 years (range 18–101). The cardinal complaints leading to ordering CTPA were dyspnea (45%) and chest pain (34%). Tachycardia was the most frequent component of Wells' criteria among patients with diagnosed PE (60%). The average of Wells scores was significantly higher in PE-positive cases compared with PE-negative ones; 4.06 versus 1.78, respectively (P < 0.0001) [Table 2].
Table 2.
Patients’ demographics and Wells’ criteria comparison between patients with pulmonary embolism and those without pulmonary embolism
Based on Wells scores, we categorized 1784 patients (88%) into “PE unlikely” group and 247 patients (12%) into “PE likely” group. There was no significant difference in patients' demographics between these two groups [Table 3]. However, PE was remarkably more prevalent in “PE likely” group compared with “PE unlikely” one; 30% versus 4%, respectively (P < 0.0001) [Figure 1].
Table 3.
Patients’ demographics and positivity rate of computed tomographic pulmonary angiography comparison between “pulmonary embolism unlikely” and “pulmonary embolism likely” groups
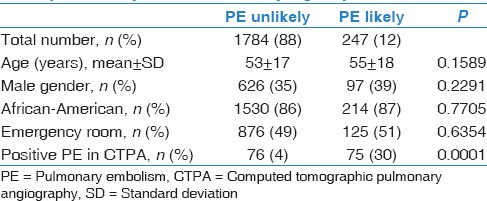
Figure 1.
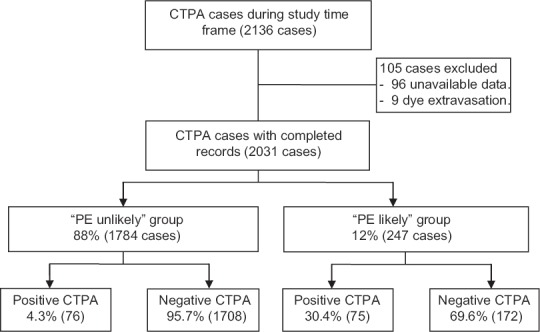
Stratifying the patients who underwent CTPA to “PE unlikely” and “PE likely” groups based on Wells' criteria. CTPA=Computed tomographic pulmonary angiography, PE=Pulmonary embolism
In 1784 cases of “PE unlikely” group, physicians conducted CTPA instead of ordering DD as the first test in 1084 cases (61%); out of those, 1039 CTPA scans (96%) came back negative for PE. Such noncompliance rate was even significantly higher in the inpatient cohort (65%) compared with ED one (57%) (P < 0.001). Yet, both of them showed a remarkable nonadherence rate to the current guidelines. Moreover, we reported 78 cases from “PE unlikely” group where CTPA was performed in spite of negative DD assay. All of those CTPA scans had normal pulmonary vasculature [Figure 2].
Figure 2.
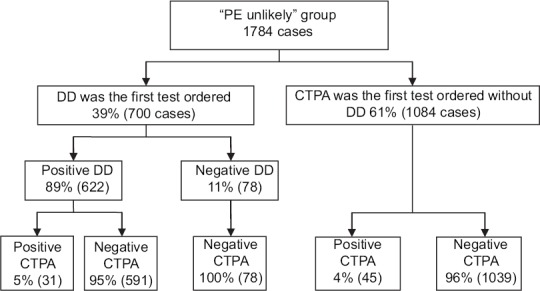
“PE unlikely” group based on modified Wells' criteria (score ≤4). PE=Pulmonary embolism, CTPA=Computed tomographic pulmonary angiography, DD=D-dimer
Finally, DD was unnecessarily ordered in 68 cases from “PE likely” group; definitive diagnostic imaging would have been the appropriate next step in such patients with a high clinical PTP for PE [Figure 3].
Figure 3.
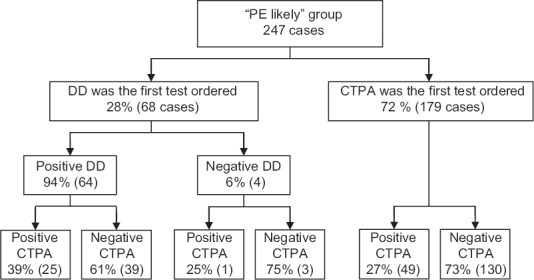
“PE likely” group based on modified Wells' criteria (score >4). PE=Pulmonary embolism, CTPA=Computed tomographic pulmonary angiography, DD=D-dimer
The kidney function was assessed post-CTPA in 1322 patients in our sample. After excluding those with ESRD (80 cases), CIN was found in 94 out of 1242 cases (7%); none of them required continuous renal replacement therapy.
Discussion
Positive yield rate of computed tomography-pulmonary angiography
CTPA confirmed the presence of PE in 7.4% of our patients (2031 cases). Moore et al.[26] performed a meta-analysis of 23 studies and found that positivity rates of CTPA ranged between 13% and 42% with an overall PE prevalence average of 27%. Thereafter, several worldwide reviews reported lower diagnostic yield rates of CTPA studies. Over the past decade, positivity rates of CTPA reported in the US have ranged between 8% and 10% on average,[11,14,21,22,23,27,28,29,30,31,32,33,34,35,36,37] whereas such rates are slightly higher (14–16%) in studies performed outside the US.[10,20,38,39,40,41,42,43,44] Reasons for this low diagnostic yield of CTPA in the US are not clear. However, nonadherence to guidelines and legally protective practice adopted by some physicians are likely to be the culprit.
Our study helps in assessing the clinicians' practice at one of the academic tertiary centers in Detroit metropolitan area. Surprisingly, the rate of positive CTPA in our institution was found to be less than the national average, suggesting a significant overuse of this test.
Compliance rate and avoidable computed tomography-pulmonary angiography
Majority of the physicians in this study (61%) did not check DD level prior to ordering CTPA for “PE unlikely” patients. This percentage reflects a significant nonadherence to evidence-based guidelines for PE workup. The elevated noncompliance rate might be attributed to unfamiliarity with scoring systems to estimate PTP of PE, lack of knowledge, the fear of malpractice litigation, or using CTPA as a diagnostic tool in assessing the thorax looking for an alternative diagnosis.
Similar retrospective studies have been performed in the US to oversee the compliance with the current guidelines. Significant nonadherence rates (46–73%) were reported at different institutions where the physicians did not order DD test as the first step in PE workup for “PE unlikely” patients.[23,28,29,30,31] This rate was found to be lower in a small study performed in New Zealand (23%).[40] Crichlow et al.[31] obtained DD samples from 72 patients who did not get DD assay prior to CTPA from “PE unlikely” group; 16 of them (22%) had negative DD and normal CTPA.
Moreover, there were 78 cases (4%) in our sample where CTPA was ordered in spite of negative DD in patients with low PTP; none of them had PE. According to the current guidelines, those CTPA scans are considered unnecessary and should be avoided. Multiple studies showed that the rates of ordering CTPA in “PE unlikely” patients with negative DD ranged between 5% and 18%.[23,28,29,30,31] Yin et al.[33] reviewed all DD tests performed on 1056 patients with low-intermediate probability of PE and found 111 cases (11%) where CTPA was ordered despite negative DD; all of them were negative for PE.
Our study shows a disturbing pattern of CTPA overuse that could have been easily avoided with the implementation of recommended diagnostic algorithms.
Risks of ordering unnecessary computed tomography-pulmonary angiography
CTPA is the most reliable imaging modality to diagnose PE in the current medical practice.[7] Nevertheless, it is costly and can potentially cause serious problems.
Ionizing radiation exposure is one of the major drawbacks of using CT imaging. Mayo et al.[45] reported that the effective dose of conventional CTPA is 9.0 mSv. However, this dose was measured by Hurwitz et al.[46] and found to be 19.9 ± 1.38 mSv, which is significantly higher than what was previously reported. Sarma et al.[47] estimated the radiation exposure of CTPA to be equivalent to 750 postero-anterior chest radiographs given together at one time. The highest radiation doses during CTPA are absorbed by liver, skin, esophagus, heart, breast, and lungs.[46,48] This kind of radiation exposure has been linked to an increased risk of cancer, especially in younger ages.[46,49,50] Estimated relative risks for breast and lung cancer incidences were 1.002–1.011 and 1.005–1.022, respectively.[46] In addition, Brenner and Elliston[51] estimated the lifetime attributable cancer death risk in 45-year-old adults who underwent a full-body CT test to be around 0.08%. Thus, appropriate use of CTPA is warranted to avoid unnecessary radiation exposure, especially in women and young patients.[52]
IV iodinated contrast media used in CTPA have a variety of potential adverse effects, which might be severe enough to cause fatal consequences.[50] These side effects became less common after developing nonionic low-osmolality contrast materials (1–3%).[53] However, severe adverse reactions are still possible (0.03%) with a mortality rate of 1–3 per 100,000 cases of contrast use.[53] Such reactions include urticaria, nausea, vomiting, bronchospasm, and dyspnea. Life-threatening allergic manifestations, such as angioedema and anaphylactic shock, may occur.[50] Furthermore, extravasation of IV contrast can induce local skin irritation and image failure. This often leads to repeating CTPA and exposing patients to more radiation. The extravasation can occur in 0.1–0.9% of the patients, especially those with vascular diseases.[50] Wang et al.[54] reviewed 69,657 computed tomography scans with IV contrast and reported 475 extravasation events (0.7%). Similarly, in our study, nine cases (0.4%) had repeated CTPA because of image failure secondary to contrast extravasation.
Moreover, CIN is one of the most serious nonallergic adverse effects to IV contrast agents. On contrary to anaphylactoid reaction, CIN is dependent on the injected dye dose. It is defined as an increase in serum creatinine more than 0.5 mg/dL or more than 25% than its baseline level, within 2–7 days postcontrast exposure.[55] Nash et al.[56] described CIN as the third most frequent cause (11%) of hospital-acquired acute kidney injury cases. In a prospective cohort performed on 174 patients who underwent CTPA, Mitchell et al.[35] reported a CIN incidence rate of 14% of the cases. This percentage was as high as 25% in a retrospective review performed by Reagle et al.[34] on 925 patients who underwent CTPA. In our study, two-thirds of cases (65%) had their kidney function checked after contrast exposure, with a CIN rate of 7%.
Finally, air embolism, seizure, pulmonary edema, and cardiac arrhythmias have also been reported as rare but serious nonanaphylactoid complications of IV contrast.[50]
Therefore, minimizing unnecessary CTPA scans will improve patients' safety and protect them from potential harm due to unnecessary radiation exposure and contrast media injection, while reducing cost and possibly hospital length of stay.
Suggestions
We have made some suggestions to improve our physicians' compliance with the recommended guidelines. Periodic targeted educational sessions for medical staff are perhaps helpful in keeping physicians aware of the available evidence-based literature and updated trials. Furthermore, the electronic medical records (EMRs) system can be employed to guide clinicians to auto-calculate the probability scores for patients with suspected PE. This will facilitate the utilization of diagnostic algorithms and minimize the nonsystematic approach in PE evaluation. Drescher et al.[36] found that implementing the computerized decision support system improved the diagnostic yield of CTPA, although it was poorly applied by physicians. Similarly, Roy et al.[57] reported a significant improvement in decision-making during PE approach by following a computerized handheld decision-support system. Moreover, the widely used current DD cutoff point carries a high false-positive rate (95% in our study) leading to a significant number of negative CTPA [Figure 2]. In our cohort, using age-adjusted DD cutoff levels in patients older than 50 years may have changed the interpretation from a “positive” to a “negative” DD test in 13 cases, corresponding to 3.4% of those patients, and potentially avoided conducting a significant number of CTPA studies.
Study limitations
The retrospective nature of our study resulted in assessing PTP of PE by research personnel instead of decision-making physicians who evaluated the patients. Besides that, our researchers relied on electronic data documented prior to CTPA images to collect the variables which might not be properly reported in physicians' notes. However, the investigators were instructed to search for each element of Wells' criteria among the best well-documented data to assess PTP, before reviewing CTPA results.
In addition, we studied all patients who underwent CTPA during that time frame, but we did not include patients with suspected PE who did not have CTPA. Adding those patients could have provided a better assessment of physicians' compliance with the current guidelines. Nevertheless, we suspect that this group of patients is small and may not have significantly affected our study result.
Although our sample size is large, it is still limited to a single institution in Detroit area. Besides that, AA race was predominant in this study (86%), which can be attributed to the normal ethnical distribution of Detroit metropolitan area. However, we did not find a significant difference in DD values and PE prevalence between AA and non-AA groups.
Conclusions
The suboptimal implementation of clinical probability assessment tools can result in the overuse of CTPA, contributing to ineffective utilization of hospital resources, increased cost, and potential harm to patients. We believe that this practice is widespread and not limited to our institution. Further multicenter studies are needed to confirm whether this disturbing pattern is widespread. Implementing validated guidelines will optimize the use of CTPA, while helping with better allocation of resources and cost containment. Subsequent prospective trials are important to assess the efficacy of the suggested EMR-guided algorithms and targeted staff education sessions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Morris T, Fedullo P. Pulmonary thromboembolism. In: Broaddus CV, Mason RJ, Ernst JD, editors. Murray & Nadel's Textbook of Respiratory Medicine. 6th ed. Philadelphia, PA: Elsevier Saunders; 2015. pp. 1001–16. Ch. 57. [Google Scholar]
- 2.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 3.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Arch Intern Med. 2011;171:831–7. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: An analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–7. doi: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 5.Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-year mortality after venous thromboembolism: A population-based cohort study. Circulation. 2014;130:829–36. doi: 10.1161/CIRCULATIONAHA.114.009107. [DOI] [PubMed] [Google Scholar]
- 6.Lilienfeld DE. Decreasing mortality from pulmonary embolism in the United States, 1979-1996. Int J Epidemiol. 2000;29:465–9. [PubMed] [Google Scholar]
- 7.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033. [Google Scholar]
- 8.Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–27. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 9.Prologo JD, Gilkeson RC, Diaz M, Asaad J. CT pulmonary angiography: A comparative analysis of the utilization patterns in emergency department and hospitalized patients between 1998 and 2003. AJR Am J Roentgenol. 2004;183:1093–6. doi: 10.2214/ajr.183.4.1831093. [DOI] [PubMed] [Google Scholar]
- 10.Cereser L, Bagatto D, Girometti R, Como G, Zuiani C, Bazzocchi M. Chest multidetector computed tomography (MDCT) in patients with suspected acute pulmonary embolism: Diagnostic yield and proportion of other clinically relevant findings. Radiol Med. 2011;116:219–29. doi: 10.1007/s11547-010-0612-2. [DOI] [PubMed] [Google Scholar]
- 11.Kino A, Boiselle PM, Raptopoulos V, Hatabu H. Lung cancer detected in patients presenting to the emergency department studies for suspected pulmonary embolism on computed tomography pulmonary angiography. Eur J Radiol. 2006;58:119–23. doi: 10.1016/j.ejrad.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Richman PB, Courtney DM, Friese J, Matthews J, Field A, Petri R, et al. Chest CT angiography (CTA) to rule-out pulmonary embolism (PE) frequently reveals clinically significant ancillary findings: A multi-center study of 1025 emergency department patients. Academic Emergency Medicine. 2003;10:564. [Google Scholar]
- 13.Montgomery AB, Gilkeson RC, Glauser J, Applegate KE. The role of spiral CT using pulmonary embolus protocol: A comparison of emergency department and hospitalized populations. Emerg Radiol. 2000;7:25–30. [Google Scholar]
- 14.Mamlouk MD, vanSonnenberg E, Gosalia R, Drachman D, Gridley D, Zamora JG, et al. Pulmonary embolism at CT angiography: Implications for appropriateness, cost, and radiation exposure in 2003 patients. Radiology. 2010;256:625–32. doi: 10.1148/radiol.10091624. [DOI] [PubMed] [Google Scholar]
- 15.Stein PD, Woodard PK, Weg JG, Wakefield TW, Tapson VF, Sostman HD, et al. Diagnostic pathways in acute pulmonary embolism: Recommendations of the PIOPED II investigators. Am J Med. 2006;119:1048–55. doi: 10.1016/j.amjmed.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 16.Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266–74. doi: 10.1056/NEJMra0907731. [DOI] [PubMed] [Google Scholar]
- 17.Wells PS, Ginsberg JS, Anderson DR, Kearon C, Gent M, Turpie AG, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997–1005. doi: 10.7326/0003-4819-129-12-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 18.Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: Management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 19.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: Increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–20. [PubMed] [Google Scholar]
- 20.David S, Beddy P, Babar J, Devaraj A. Evolution of CT pulmonary angiography: Referral patterns and diagnostic yield in 2009 compared with 2006. Acta Radiol. 2012;53:39–43. doi: 10.1258/ar.2011.110186. [DOI] [PubMed] [Google Scholar]
- 21.Donohoo JH, Mayo-Smith WW, Pezzullo JA, Egglin TK. Utilization patterns and diagnostic yield of 3421 consecutive multidetector row computed tomography pulmonary angiograms in a busy emergency department. J Comput Assist Tomogr. 2008;32:421–5. doi: 10.1097/RCT.0b013e31812e6af3. [DOI] [PubMed] [Google Scholar]
- 22.Weir ID, Drescher F, Cousin D, Fraser ET, Lee R, Berman L, et al. Trends in use and yield of chest computed tomography with angiography for diagnosis of pulmonary embolism in a Connecticut hospital emergency department. Conn Med. 2010;74:5–9. [PubMed] [Google Scholar]
- 23.Costantino MM, Randall G, Gosselin M, Brandt M, Spinning K, Vegas CD. CT angiography in the evaluation of acute pulmonary embolus. AJR Am J Roentgenol. 2008;191:471–4. doi: 10.2214/AJR.07.2552. [DOI] [PubMed] [Google Scholar]
- 24.Gleeson TG, Bulugahapitiya S. Contrast-induced nephropathy. AJR Am J Roentgenol. 2004;183:1673–89. doi: 10.2214/ajr.183.6.01831673. [DOI] [PubMed] [Google Scholar]
- 25.Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: A clinical and evidence-based approach. Circulation. 2006;113:1799–806. doi: 10.1161/CIRCULATIONAHA.105.595090. [DOI] [PubMed] [Google Scholar]
- 26.Moores LK, Jackson WL, Jr, Shorr AF, Jackson JL. Meta-analysis: Outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography. Ann Intern Med. 2004;141:866–74. doi: 10.7326/0003-4819-141-11-200412070-00011. [DOI] [PubMed] [Google Scholar]
- 27.Stojanovska J, Carlos RC, Kocher KE, Nagaraju A, Guy K, Kelly AM, et al. CT pulmonary angiography: Using decision rules in the emergency department. J Am Coll Radiol. 2015;12:1023–9. doi: 10.1016/j.jacr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Perelas A, Dimou A, Saenz A, Rhee JH, Teerapuncharoen K, Rowden A, et al. CT pulmonary angiography utilization in the emergency department: Diagnostic yield and adherence to current guidelines. Am J Med Qual. 2015;30:571–7. doi: 10.1177/1062860614543302. [DOI] [PubMed] [Google Scholar]
- 29.Shujaat A, Shapiro JM, Eden E. Utilization of CT pulmonary angiography in suspected pulmonary embolism in a major urban emergency department. Pulm Med. 2013;2013:915213. doi: 10.1155/2013/915213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams DM, Stevens SM, Woller SC, Evans RS, Lloyd JF, Snow GL, et al. Adherence to PIOPED II investigators' recommendations for computed tomography pulmonary angiography. Am J Med. 2013;126:36–42. doi: 10.1016/j.amjmed.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Crichlow A, Cuker A, Mills AM. Overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department. Acad Emerg Med. 2012;19:1219–26. doi: 10.1111/acem.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahriar Z, Stephan R, Shweta M, Arun S, Mathew T, Brijal P, et al. Could the number of CT angiograms be reduced in emergency department patients suspected of pulmonary embolism? World J Emerg Med. 2012;3:172–6. doi: 10.5847/wjem.j.issn.1920-8642.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin F, Wilson T, Della Fave A, Larsen M, Yoon J, Nugusie B, et al. Inappropriate use of D-dimer assay and pulmonary CT angiography in the evaluation of suspected acute pulmonary embolism. Am J Med Qual. 2012;27:74–9. doi: 10.1177/1062860611407907. [DOI] [PubMed] [Google Scholar]
- 34.Reagle Z, Tringali S, Gill N, Peterson MW. Diagnostic yield and renal complications after computed tomography pulmonary angiograms performed in a community-based academic hospital. J Community Hosp Intern Med Perspect. 2012;2 doi: 10.3402/jchimp.v2i2.17722. doi: 10.3402/jchimp.v2i2.17722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell AM, Jones AE, Tumlin JA, Kline JA. Prospective study of the incidence of contrast-induced nephropathy among patients evaluated for pulmonary embolism by contrast-enhanced computed tomography. Acad Emerg Med. 2012;19:618–25. doi: 10.1111/j.1553-2712.2012.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drescher FS, Chandrika S, Weir ID, Weintraub JT, Berman L, Lee R, et al. Effectiveness and acceptability of a computerized decision support system using modified Wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57:613–21. doi: 10.1016/j.annemergmed.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. AJR Am J Roentgenol. 2010;194:392–7. doi: 10.2214/AJR.09.2499. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy N, Jayathissa S, Healy P. Investigation of suspected pulmonary embolism at Hutt Valley hospital with CT pulmonary angiography: Current practice and opportunities for improvement. Adv Med. 2015;2015:357576. doi: 10.1155/2015/357576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa AF, Basseri H, Sheikh A, Stiell I, Dennie C. The yield of CT pulmonary angiograms to exclude acute pulmonary embolism. Emerg Radiol. 2014;21:133–41. doi: 10.1007/s10140-013-1169-x. [DOI] [PubMed] [Google Scholar]
- 40.Kim B, Hills M, Beckert L. The use of computed tomography of pulmonary angiogram in a district hospital. ISRN Vasc Med. 2013;2013:582413. [Google Scholar]
- 41.Haap MM, Gatidis S, Horger M, Riessen R, Lehnert H, Haas CS. Computed tomography angiography in patients with suspected pulmonary embolism-too often considered? Am J Emerg Med. 2012;30:325–30. doi: 10.1016/j.ajem.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Leng O, Sitaraaman HB. Application of age-adjusted D-dimer threshold for exclusion thromboembolism (PTE) in older patients: A retrospective study. Acute Med. 2012;11:129–32. [PubMed] [Google Scholar]
- 43.Chin P, Hurrell M, McGregor D, Beckert L. The role of CT pulmonary angiography in patients with suspected pulmonary embolism admitted to general medicine. N Z Med J. 2006;119:U2052. [PubMed] [Google Scholar]
- 44.Engelke C, Rummeny EJ, Marten K. Pulmonary embolism at multi-detector row CT of chest: One-year survival of treated and untreated patients. Radiology. 2006;239:563–75. doi: 10.1148/radiol.2392050118. [DOI] [PubMed] [Google Scholar]
- 45.Mayo JR, Aldrich J, Muller NL. Fleischner Society. Radiation exposure at chest CT: A statement of the Fleischner Society. Radiology. 2003;228:15–21. doi: 10.1148/radiol.2281020874. [DOI] [PubMed] [Google Scholar]
- 46.Hurwitz LM, Reiman RE, Yoshizumi TT, Goodman PC, Toncheva G, Nguyen G, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: Implications for cancer induction. Radiology. 2007;245:742–50. doi: 10.1148/radiol.2453062046. [DOI] [PubMed] [Google Scholar]
- 47.Sarma A, Heilbrun ME, Conner KE, Stevens SM, Woller SC, Elliott CG. Radiation and chest CT scan examinations: What do we know? Chest. 2012;142:750–60. doi: 10.1378/chest.11-2863. [DOI] [PubMed] [Google Scholar]
- 48.Parker MS, Hui FK, Camacho MA, Chung JK, Broga DW, Sethi NN. Female breast radiation exposure during CT pulmonary angiography. AJR Am J Roentgenol. 2005;185:1228–33. doi: 10.2214/AJR.04.0770. [DOI] [PubMed] [Google Scholar]
- 49.Brenner DJ, Hall EJ. Computed tomography – An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 50.Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol. 2008;36:69–74. doi: 10.2967/jnmt.107.047621. [DOI] [PubMed] [Google Scholar]
- 51.Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology. 2004;232:735–8. doi: 10.1148/radiol.2323031095. [DOI] [PubMed] [Google Scholar]
- 52.Remy-Jardin M, Pistolesi M, Goodman LR, Gefter WB, Gottschalk A, Mayo JR, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: A statement from the Fleischner Society. Radiology. 2007;245:315–29. doi: 10.1148/radiol.2452070397. [DOI] [PubMed] [Google Scholar]
- 53.Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5:28–31. doi: 10.1007/s11882-005-0051-7. [DOI] [PubMed] [Google Scholar]
- 54.Wang CL, Cohan RH, Ellis JH, Adusumilli S, Dunnick NR. Frequency, management, and outcome of extravasation of nonionic iodinated contrast medium in 69,657 intravenous injections. Radiology. 2007;243:80–7. doi: 10.1148/radiol.2431060554. [DOI] [PubMed] [Google Scholar]
- 55.Kidney Disease Improving Global Outcomes (KDIGO). Contrast-induced AKI: Definition, epidemiology, and prognosis. Kidney Int Suppl. 2012;2:72–4. [Google Scholar]
- 56.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–6. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 57.Roy PM, Durieux P, Gillaizeau F, Legall C, Armand-Perroux A, Martino L, et al. A computerized handheld decision-support system to improve pulmonary embolism diagnosis: A randomized trial. Ann Intern Med. 2009;151:677–86. doi: 10.7326/0003-4819-151-10-200911170-00003. [DOI] [PubMed] [Google Scholar]


