Abstract
INTRODUCTION:
Pulmonary embolism (PE) is a serious cardiovascular and pulmonary complication worldwide. We aimed to assess the implications of different computed tomography pulmonary angiography (CTPA) parameters in patients with acute PE.
METHODS:
A retrospective observational study to include patients presented with clinical suspicious of PE who underwent CTPA was conducted. Patients' demographics, clinical presentation, risk factors, laboratory investigations, management, and outcome were analyzed. Computed tomography findings included clot burden (Qanadli score [QS]) and right ventricular dysfunction (RVD) parameters.
RESULTS:
A total of 45 patients with radiologically confirmed diagnosis of PE were included in the study; of these patients, 8 (17.8%) died during the hospital course. Patients who died were 13 years older than those who survived, and the mortality rate was significantly higher in patients with cancer. The two groups were comparable for cardiovascular parameters. The mean clot burden (QS) was 19.5 ± 11.3 points and 53% of patients had QS >18 points. Obesity (52.4% vs. 12.5%; P = 0.01), hypertension (54.4% vs. 23.8%; P = 0.03), and median D-dimer levels (7.8 vs. 3.4; P = 0.03) were significantly higher in patients with QS >18. Among right ventricular (RV) dysfunction parameters, only higher RV/left ventricular (LV) ratio (P = 0.001) and bowing of interventricular septum (P = 0.001) were associated with higher QS. A significant positive correlation was found between RV short axis (r = 0.499, P = 0.001), RV/LV ratio (r = 0.592, P = 0.001), and pulmonary artery (PA) diameter (r = 0.301, P = 0.04) with the PA clot burden. Receiver operating characteristic curve for clot burden showed a cutoff value of 17.5 points to accurately predict RV dysfunction.
CONCLUSIONS:
Clot burden >18 is associated with RV dysfunction in patients with acute PE. Echocardiography and RVD parameters showed no correlation with in-hospital deaths. CTPA has clinicoradiological implications for risk stratification in PE patients. As the sample size is small, our findings warrant further larger prospective studies.
Key words: Computed tomography angiogram, pulmonary embolism, right ventricular dysfunction, thromboembolism
Pulmonary embolism (PE) is a serious cardiovascular and pulmonary condition with mortality ranging from 2% to 7% worldwide, even after thromboprophylaxis.[1] It is the third most common cause of cardiovascular death, after myocardial infarction and cerebrovascular stroke.[2] Regardless of the advancement in thromboprophylaxis, diagnosis, and treatment, PE remains an important determinant of in-hospital complications and outcome.[3] In some cases with acute massive PE, severe hemodynamic compromise and systemic hypotension causes sudden death secondary to circulatory collapse and heart failure.[4,5] This occurs due to increased pulmonary vascular resistance with high pulmonary arterial and right ventricle (RV) pressure which overwhelms the RV failure.[6] Risk stratification of patients with acute PE is crucial for early diagnosis and appropriate selection of the treatment.[7]
Echocardiography is a useful diagnostic and prognostic tool for RV dysfunction which is considered as an independent predictor of short-term outcome of PE.[8] Moreover, computed tomography pulmonary angiography (CTPA) has become the modality of choice for the diagnosis of PE as it offers a multiplanar view for the assessment of pulmonary vessels to the subsegmental levels.[9,10] RV dysfunction and extent of pulmonary artery (PA) obstruction at computed tomography (CT) are useful prognostic parameters.[11] Patients with RV dysfunction and PE have a high mortality rate, and hence, RV dysfunction is considered as a poor prognostic marker.[8] Furthermore, the RV/left ventricular (LV) diameter ratio and pulmonary vascular obstruction score are important parameters to evaluate RV dilatation which is associated with significant mortality in PE.[12,13] In addition, the presence of RV dysfunction indicates a high risk of recurrent or fatal PE.
To date, four scoring systems have been proposed by different investigators to determine the presence, location, and degree of obstruction of arterial clots in patients with PE.[14] Among them, Qanadli PE index[13] is easy to calculate, identify complete or partial obstruction due to proximal clot, and thus provides information about the residual pulmonary perfusion. The clot burden score has significant prognostic and therapeutic potential as it facilitates direct visualization of the clot within the PA with a standard reproducible score to quantitate the improvement after therapeutic intervention.[13,14] There are few clinical studies from the Middle East to evaluate the role of CTPA for diagnosis and prognosis of PE.[14,15,16] The aim of this study is to assess the diagnostic implications of different CT parameters, and to correlate the clot burden, cardiovascular measurements, and clinical presentation of patients with acute PE.
Methods
We conducted a retrospective observational study of fifty consecutive patients presented with a clinically suspected PE and underwent CTPA. Patients were identified from the radiology database, and the relevant clinical information was retrieved from the medical records at Hamad General Hospital between May 2011 and February 2015. Clinically suspected patients with PE who were not confirmed by CT imaging were excluded from the study. Images from 16- or 64-slice multidetector CT angiography were reviewed in 45 patients, after excluding patients in whom images were not available on the database or in whom poor image quality precluded accurate assessment. Clinical presentation, preexisting comorbidities, radiologic imaging, detailed notes for in-hospital course, and discharge summaries were reviewed for each patient. Data collection included patient demographics (age, sex, and nationality), clinical presentation and predisposing factors, laboratory investigations (D-dimer testing, coagulation profile), Doppler ultrasound, finding of echocardiography (ejection fraction [%], RV wall hypokinesis and pulmonary arteries dilation) and CTPA, management, hospital length of stay, complications, and outcome. Patients were followed up for routine clinical care as per the standard practice. The plasma D-dimer concentrations were measured at the clinical laboratory, Hamad General Hospital, using a well-validated commercial assay used for routine D-dimer testing. The normal range of plasma D-dimer concentration in our hospital laboratory is 0–0.55 mg/L. Coagulation profile includes protein S deficiency, protein C deficiency, hyperhomocysteine, antithrombin III deficiency, and antiphospholipid syndrome. PE was defined radiologically as the presence of an endoluminal central filling defect partially or completely occluding the pulmonary arteries.[17] The RV dysfunction refers to the presence of RV hypokinesis, explained by a qualitative evaluation of the RV wall motion.[18]
Computed tomography pulmonary angiography
The occurrence of PE was confirmed by CTPA examination. In brief, all patients were examined using Siemens SOMATOM Sensation (Siemens AG, Munich, Germany) 16- or 64-slice machine. For all examinations, kV was set as 100, collimation of 0.6, rotation time of 0.5 s, slice thickness of 5 mm, and pitch of 1.0. Nonionic intravenous contrast (100 ml of Omnipaque 350 mg/ml) was injected at a rate of 4.0–5.0 ml/s. Pulmonary arterial phase bolus tracking through main pulmonary trunk was carried out followed by aortic phase immediately following pulmonary arterial phase. (In pregnant patients, only pulmonary arterial phase was performed.) Moreover, three-dimensional reconstruction was performed, if indicated.
Imaging analysis
Clot burden
Qanadli et al.'s[13] scoring system was used to quantify the vascular obstruction index using CTPA which is based on the percentage of vascular obstruction of the pulmonary arterial tree developed secondary to PE. In brief, the Qanadli index determines the number of blocked segmental arterial branches and then to be adjusted by a factor of one for partial blockage or a factor of two for completely obstructive PE. The maximum attainable score in this system is 40 (pulmonary trunk completely obstructed by thrombus), which represents complete obstruction index (100%). In this study, we used the same scoring system based on the site and degree of occlusion of pulmonary arteries.
Computed tomography signs of right heart dysfunction
CT findings considered for the functional cardiovascular measurements include the ratio of RV to LV diameter (RV/LV ratio), ratio of main PA diameter to ascending aorta (AO) diameter (PA/AO ratio), superior vena cava (SVC) and azygos vein diameter, bowing of interventricular septum, clot burden (Qanadli score [QS]). The other observations such as infarction, pulmonary effusion (right, left, and bilateral), pneumothorax and rib fracture, and pulmonary metastasis were also recorded. RV and LV dimensions were identified based on the maximal distance between the ventricular endocardium and the interventricular septum, perpendicular to the long axis. RV dysfunction was diagnosed if the RV to LV diameter ratio was >1.2.[14]
Inferior vena cava reflux to determine signs of right ventricular dysfunction
Aviram et al.[19] classified reflux of contrast medium into inferior vena cava (IVC) or hepatic veins based on severity into six different categories such as (a) no reflux into IVC, (b) trace of reflux into IVC only, (c) reflux into IVC but not hepatic veins, (d) reflux into IVC with opacification of proximal hepatic veins, (e) reflux into IVC with opacification of hepatic veins down to the mid-portion of the liver, and (f) reflux into IVC with opacification of distal hepatic veins.
The Institutional Review Board (IRB# 15139/15) of the Hamad Medical Corporation has approved and granted exempt status for this retrospective study.
Statistical analysis
Data were reported as percentage, mean (±standard deviation), median, and range, where applicable. Baseline characteristics, echocardiography, and right ventricular dysfunction (RVD) parameters were analyzed according to clot burden (QS ≤18 vs. QS >18) and outcome (survivors vs. nonsurvivors). Student's t-test was used to compare continuous variables, and Pearson's Chi-square test was used for categorical variables. Pearson's correlation coefficient (r) has been calculated between QS and cardiovascular measurements. Receiver operating characteristic (ROC) curve was constructed to establish the best cutoff for QS and RV dysfunction. To look for predictors of mortality, multivariate regression analysis was performed after adjustment for the significant variables on univariate analysis, and data were expressed as odd ratio and 95% confidence interval (CI). Two-tailed P < 0.05 was considered statistically significant. Data analysis was carried out using the Statistical Package for Social Sciences version 18 (SPSS Inc., Chicago, IL, USA).
Results
A total of 45 consecutive patients with radiologically confirmed diagnosis of PE were eligible for enrollment in the present study. Table 1 shows the demographics, comorbidities, and outcome of PE patients. The mean age of the patients was 49 ± 11.4 years, and 29 (64.4%) were males. Thirty (67%) patients developed PE during hospitalization whereas 15 (33%) patients presented to the emergency department with PE. Deep vein thrombosis (DVT) (60.0%), hypertension (37.8%), obesity (average BMI 32.8 ± 8.6; 28.9%), diabetes mellitus (26.7%), and abnormal coagulation (24.4%) were the frequent preexisting comorbidities. Recurrent PE was observed in 6 (14.6%) patients. Overall mortality was 18% during the hospital course.
Table 1.
Demographics, clinical presentation, comorbidities, and outcome of patients with pulmonary embolism (n=45)
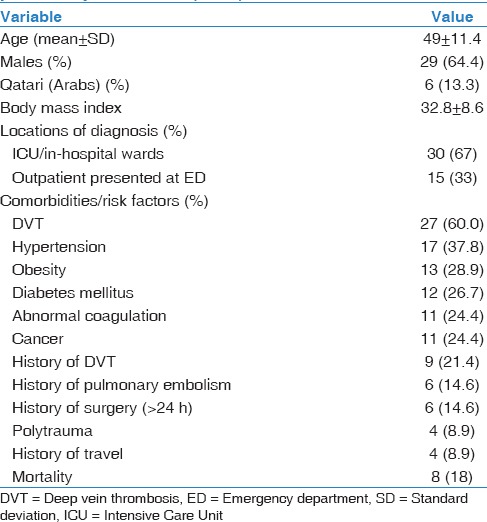
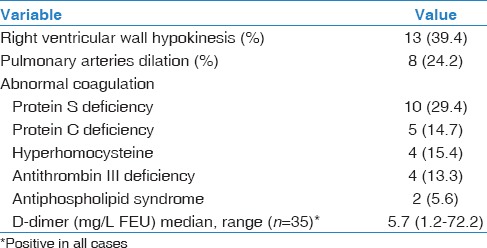
Table 2 shows the coagulation profile and echocardiography findings. Thirty-three patients (73.3%) underwent echocardiography, of which 13 (39.4%) cases had RV dysfunction in terms of RV dilatation and hypokinesia. Pulmonary arteries dilation was found in 8 (24.2%) patients. Protein S deficiency was found in one-third (30%) of the patients, and the median D-dimer value was 5.7 (1.2–72.2) mg/L FEU.
Table 2.
Echocardiography and coagulation profile
Parameters of the right heart dysfunction as identified by the CTPA are shown in Table 3. The mean clot burden (QS) was 19.5 ± 11.3 points and 53% of patients had QS >18 points. One-third (33.3%) of the patients had PA/AO ratio >1.0. The number of patients with RV dysfunction (RV/LV ratio >1.2) was 27 (60.0%). IVC reflux was found to be mild-to-moderate (Grades I–III) in 73.3% of patients and severe (Grades IV–VI) in 26.7% of patients. Interventricular septal abnormality was identified in 29 (64.4%) patients.
Table 3.
Computed tomography pulmonary angiography findings of right ventricular dysfunction
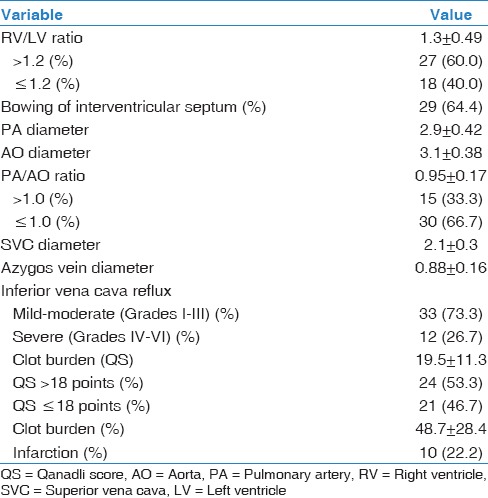
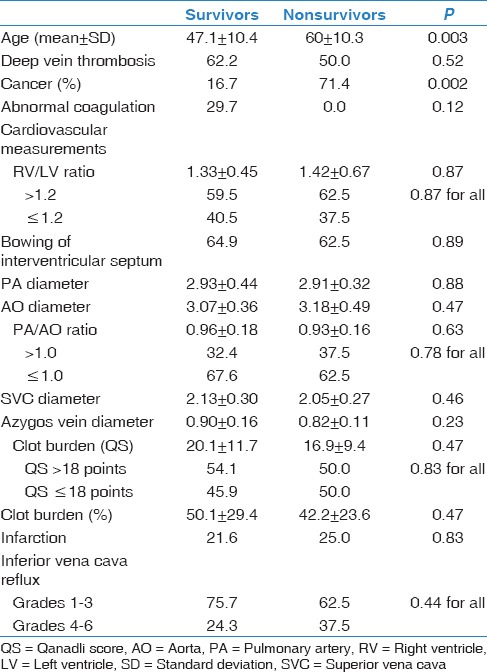
Table 4 demonstrates univariate association between cardiovascular measurements and mortality. The RV/LV ratio, PA/AO ratio, bowing of interventricular septum, IVC reflux, SVC, and azygos vein diameters were comparable in survivor and nonsurvivor groups. In our study, cancer patients were more likely to die due to paraneoplastic embolism than those without malignancy (71.4% vs. 16.7%; P = 0.002).
Table 4.
Univariate association between cardiovascular measurements and mortality
Clinicoradiological characteristics of PE patients according to clot burden (QS ≤18 vs. >18) are shown in Table 5. The two groups were comparable for age, gender, and preexisting comorbidities/risk factors and echocardiography findings. However, obesity (52.4% vs. 12.5%; P = 0.01), hypertension (54.4% vs. 23.8%; P = 0.03), and median D-dimer levels (7.8 vs. 3.4; P = 0.03) were significantly higher in patients with QS >18. Among RV dysfunction parameters, only RV/LV ratio (1.61 ± 0.48 vs. 1.04 ± 0.29; P = 0.001) and bowing of interventricular septum (91.7% vs. 33.3%; P = 0.001) were associated with higher QS. The mortality rate was comparable in patients with higher clot scores (QS >18) (16.7% vs. 19.0%; P = 0.83) as compared to low scores (QS ≤18). A significant positive correlation was found between RV short axis (r = 0.499, P = 0.001), RV/LV ratio (r = 0.592, P = 0.001), and PA diameter (r = 0.301, P = 0.04) with the PA clot burden. Moreover, LV short axis showed a significant negative correlation with clot burden (r = −0.515, P = 0.001) [Table 6]. Figures 1 and 2 demonstrate CTPA findings of two of our PE patients.
Table 5.
Clinicoradiological characteristics of pulmonary embolism patients according to clot burden (Qanadli score)
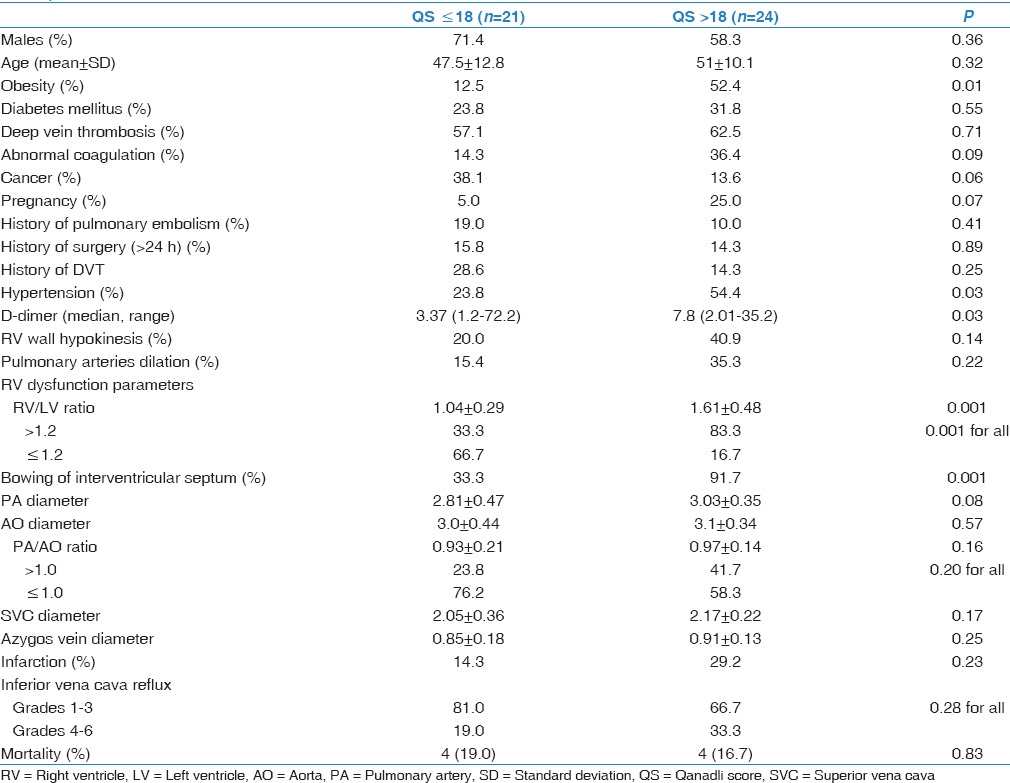
Table 6.
Correlation between Qanadli score and cardiovascular measurements
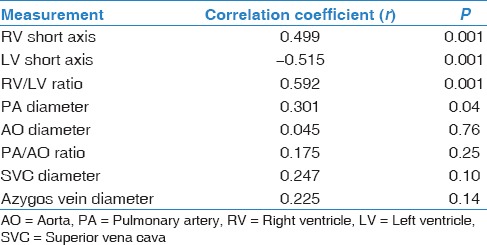
Figure 1.
Computed tomography pulmonary angiography axial (a, c, and d) and coronal (b) images reveal pulmonary embolism involving bilateral main pulmonary arteries with infarct in the left lung. (d) Right ventricular/left ventricular ratio = 2.1
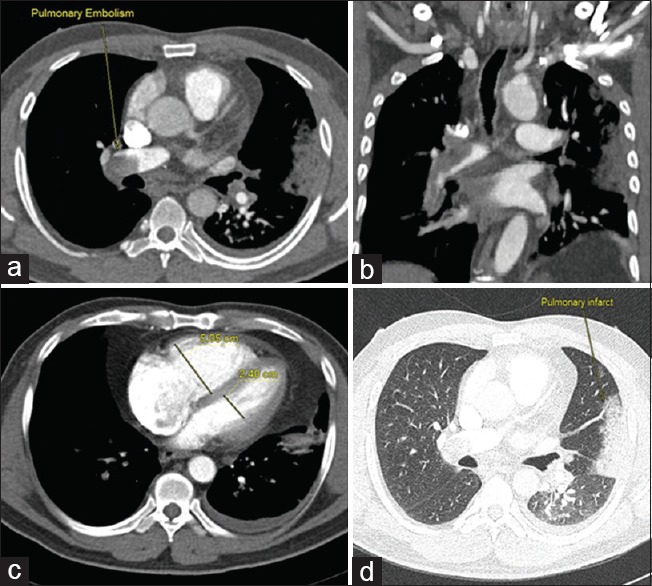
Figure 2.
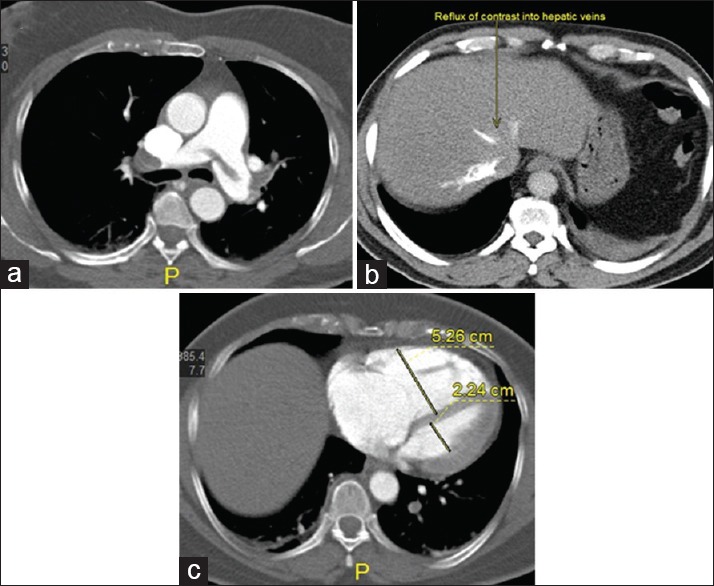
(a-c) Computed tomography pulmonary angiography axial images reveal saddle thrombus extending to bilateral pulmonary arteries with reflux of contrast into inferior vena cava and hepatic veins. (b) Right ventricular/left ventricular ratio = 2.4
ROC curve showed an area under the curve (AUC) for the prediction of RV dysfunction based on PA clot burden to be 0.822 (95% CI: 0.698–0.947, P = 0.001). In our study, the optimal cutoff value of QS for the identification of RV dysfunction was considered based on highest pair of sensitivity (77.8%) and specificity (72.2%). In addition, the AUC was 0.822; 95% CI: 0.698–0.947, P = 0.001, which is considered a good diagnostic value. Hence, the clot burden cutoff level of 17.5 points was found to predict RV dysfunction which is also consistent with Qanadli et al.[13] scoring system.
Multivariate regression analysis showed that after adjustment for age and cancer, neither RV/LV ratio (odds ratio [OR] 1.6; 95% CI: 0.13–20.15, P = 0.71) nor clot scores (OR 0.99; 95% CI: 0.87–1.14, P = 0.92) were independent predictor for mortality.
Discussion
PE is a serious medical condition that could ultimately lead to death within few hours due to RV failure and circulatory collapse. Therefore, RV burden should be detected rapidly to identify patients who could benefit from early aggressive therapy. The noninvasive CTPA can safely and quickly identify the presence and extent of PE.
The present study highlights the diagnostic implications of CTPA parameters and assesses the correlation between clinicoradiological characteristics of 45 patients with PE. In our series, echocardiographic evaluation revealed RV wall hypokinesis and PA dilation in 39.4% and 24.2% of cases, respectively. Earlier studies have suggested that echocardiography might be effective in predicting RV dysfunction and clinical outcome.[20] However, due to its lower sensitivity to diagnose PE, echocardiography is recommended mainly for hemodynamically unstable patients.[15] At present, CTPA is considered as the modality of choice for accurate diagnosis of PE.[6] In comparison to echocardiography, CTPA enables a thorough assessment of embolization in the pulmonary arteries and detects associated underlying pulmonary disorders and other causes of acute chest pain.[21] Moreover, it facilitates risk stratification of patients based on the degree of vascular obstruction (i.e., clot burden) which could be used as a marker for appropriate selection of treatment.[22] Apart from the size of embolus, the clinical outcome of patients with PE also depends on various cardiopulmonary measurements (RV, LV, RV/LV ratio, PA, AO diameter, and PA/AO ratio) identified by CTPA.
In our study, patients with PE presented at a young age, more likely to be males, and two-thirds of them were identified during hospitalization. Our findings are consistent with an earlier study from Saudi Arabia with a similar age at presentation, male predominance, and in-hospital diagnosis of PE.[15] Prior hospitalization could not represent a real limit in our study as only one patient had prolonged immobilization (bedridden) which could associate with venous thromboembolism (VTE). We do not believe that our findings are influenced by other acute diseases as we anticipated all major risk factors in our study. A previous study from our center reported mean age of 50 years in patients with confirmed diagnosis of thromboembolism.[23] In that study, the proportion of PE was 12.2% among 662 thromboembolism cases who were screened over 5 years. However, other investigators reported the diagnosis of PE with an advanced mean age of 63 years.[24]
We observed a higher rate of protein S deficiency (30%) in patients who developed PE at relatively younger age. Similarly, an earlier study showed that low free protein S carried a high risk of developing or recurring of VTE in young patients with a mean age 39 years.[25] In addition, preexisting comorbidities were frequent in our study cohort. Al Otair et al.[15] reported recent surgery, obesity, immobilization, and recurrent DVT to be the frequent risk factors associated with PE.
PA obstructive index is important as it facilitates direct visualization of the clot and allows accurate diagnosis of PE.[26] There are several scoring systems reported to evaluate the pulmonary vascular tree and assessment of clot burden score.[16] The present study quantified clot burden scoring according to QS as it is more objective, easy to calculate, distinguish between partial and complete obstruction, and has less interobserver variability.[13,19] Serial imaging studies could be used to assess the degree of vascular obstruction which helps a clinician determine real-time risk stratification and monitoring of treatment in an objective way. Previous studies have identified the clot burden score to be a predictor of RV dysfunction and poor outcomes. Qanadli et al.[13] found that an obstruction score >40% correlates well with RV dilatation. It has been observed that PE patients with 40% or higher obstruction index had 11-fold increased risk of mortality.[11] Another study reported significant correlation between RV/LV ratio, IVC reflux, QS, and the short-term PE-related mortality.[14] Bazeed et al.[26] observed a significant difference in clot burden scoring among PE patients who died than those who survived. On the other hand, other investigators reported no significant association of obstruction score with PE-related mortality.[14] Consistent with these reports, our findings revealed no correlation of clot burden with in-hospital mortality. This variability could be explained by the fact that pulmonary obstruction could be related to other factors such as mechanical obstruction, vasoactive agents, reflex vasoconstriction, and systemic hypoxemia occurred during PE.[14] However, obstructive index can be accurately used as an indicator of the severity of PE and for treatment response.[27]
The assessment of cardiorespiratory status secondary to an acute PE should not only rely on the degree of pulmonary obstruction but also consider the signs of RV strain (RV/LV diameter ratio >1) as a potential indicator for the RV dysfunctions, which occurs due to sudden increase in the afterload caused by mechanical obstruction and vasoconstriction.[28] It has been suggested that the severity of PE could be assessed more accurately by RV failure rather than the degree of obstruction.[29] Earlier studies have suggested threshold values for RV/LV ratio ranged from 1 to 1.5 as indicative of RV strain[12,30] and can be used as a predictor of PE mortality.[14] In our series, we used a threshold of RV/LV ratio of 1.2 for the diagnosis of RV dysfunction. However, we did not observe a significant correlation between RV dysfunction and PE-related mortality. Our findings are in accordance with previous studies which suggest that RV/LV ratio and clot burden are not associated with PE-related mortality.[31,32] The reason for these controversies could be partially explained by the variability in measurements of RV/LV ratio.
In our study, obesity, hypertension, and D-dimer were significantly associated with severity of obstruction (QS >18). Similar to our findings, Attia et al.[16] reported a significant association between obesity and higher mean obstructive index in patients with acute PE. In our study, patients with right heart dilatation (RV/LV ratio >1.2) were more likely to have larger clot volume as compared to those without RV dilatation. Similarly, the larger clot volume (QS >18) was observed in patients with interventricular septal defect in comparison to patients with normal interventricular septal morphology. The higher clot volume is likely to obstruct the pulmonary circulation, causing RV pressure overload and dilatation. Therefore, estimation of the degree of vascular obstruction (clot burden) would help in the PE risk stratification in clinical practice and also enable treatment monitoring. Moreover, we observed a significant positive correlation between RV/LV ratio and PA diameter with clot burden (QS). Our findings coincide with earlier studies which also observed a good correlation between RV dysfunction parameters (RV/LV ratio and PA diameter) and the severity of obstruction.[16,22]
In the present study, ROC curve analysis determined the cutoff point for the obstructive index (QS) to be 17.5 points (clot burden of 43.7%) which could predict RV dysfunction with reasonable sensitivity (77.8%) and specificity (72.2%). In line with findings of our study, other investigators observed the best cutoff point for clot burden which ranges between 40% and 49% with high sensitivity and specificity, and values ≥40% could potentially identify more than 90% of patients with RV dysfunction.[16] On the other hand, PE patients with a CT burden of <40% are unlikely to develop acute RV dysfunction.[13,22,33] Therefore, the findings of the present study highlighted the clinicoradiological implications of CTPA for the accurate diagnosis and risk stratification of patients with PE.
We observed no significant difference between mortality and clot burden which is consistent with an earlier study by Rodrigues et al.[22] Another important factor was reflux of the dye in the IVC which is an indirect sign of tricuspid valve insufficiency.[29] Kang et al.[34] found that contrast reflux is a bad prognostic factor. Our study did not observe any prognostic role of IVC reflux, and similar results were shown by Collomb et al.[35]
The interventricular septum may shift toward the LV due to increased right heart pressure with severe pulmonary obstruction.[36] The prognostic significance of interventricular septum abnormality is debatable. Araoz et al.[31] reported that patients with ventricular septal defects are more likely to be admitted to the ICU. On the other hand, Van der Meer et al.[11] did not find any relation to mortality from acute PE. It has been revealed that the mean diameter of the azygos vein and SVC was higher in nonsurvivors.[21] A recent study by Furlan et al.[17] showed no significant association between the diameters of the SVC, the azygos vein diameters, and short-term mortality which corroborates our findings. Recently, Meinel et al.[37] concluded in a meta-analysis that increased RV/LV diameter ratio measured on transverse CT images was the strongest risk with a 2.5-fold higher risk for all-cause mortality and 5-fold higher risk for PE-related mortality. Furthermore, the degree of thrombus load and central thrombus location were not predictive for all-cause mortality although both were associated with adverse clinical outcome. In the present study, cancer patients were more likely to die due to paraneoplastic embolism than those without malignancy. Consistent with our findings, a large prospective study based on RIETE registry revealed a higher 3-month mortality rate in the patients with cancer-related VTE as compared to those without cancer (4%).[38] After adjustment for age and history of cancer, the present study showed that RV/LV ratio and clot scores were not independent predictors for mortality.
One of the limitations of our study is the retrospective design and underpowered due to small number of cases which needs further validation based on larger sample size. We did not consider the preexisting structural heart defects or pulmonary disease which could affect the cardiopulmonary measurements on CT angiography. Finally, the clot burden score does not consider clots situated within the small pulmonary arteries at the periphery as well as unresolved previous episodes of PE in cases of recurrence. Although multivariate logistic regression analysis could not identify the independent predictors of outcomes, this could be limited by the sample size which is insufficient to draw findings of statistical significance to be used for the risk stratification. The total number of PE cases in this study underestimates and does not reflect the real number in our institution as we retrospectively analyzed only cases with detailed CTPA data.
Conclusion
Clot burden >18 is associated with RV dysfunction in patients with acute PE. Echocardiography and RVD parameters showed no correlation with in-hospital deaths. CTPA has clinicoradiological implications for risk stratification in PE patients. As the sample size is small, our findings warrant further larger prospective studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank all the staff of Radiology Department at Hamad General Hospital, for their contribution and support. This study was approved by the Medical Research Center at HMC, Doha, Qatar (IRB# 15139/15).
References
- 1.Subramaniam RM, Mandrekar J, Chang C, Blair D, Gilbert K, Peller PJ, et al. Pulmonary embolism outcome: A prospective evaluation of CT pulmonary angiographic clot burden score and ECG score. AJR Am J Roentgenol. 2008;190:1599–604. doi: 10.2214/AJR.07.2858. [DOI] [PubMed] [Google Scholar]
- 2.Pulido T, Aranda A, Zevallos MA, Bautista E, Martínez-Guerra ML, Santos LE, et al. Pulmonary embolism as a cause of death in patients with heart disease: An autopsy study. Chest. 2006;129:1282–7. doi: 10.1378/chest.129.5.1282. [DOI] [PubMed] [Google Scholar]
- 3.Bahloul M, Chaari A, Kallel H, Abid L, Hamida CB, Dammak H, et al. Pulmonary embolism in intensive care unit: Predictive factors, clinical manifestations and outcome. Ann Thorac Med. 2010;5:97–103. doi: 10.4103/1817-1737.62473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013;18:129–38. [PMC free article] [PubMed] [Google Scholar]
- 5.Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: Results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165–71. doi: 10.1016/s0735-1097(97)00319-7. [DOI] [PubMed] [Google Scholar]
- 6.Wood KE. Major pulmonary embolism: Review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 7.Saar JA, Maack C. Diagnosis and management of acute pulmonary embolism. ESC guidelines 2014. Herz. 2015;40:1048–54. doi: 10.1007/s00059-015-4378-0. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: Right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997;134:479–87. doi: 10.1016/s0002-8703(97)70085-1. [DOI] [PubMed] [Google Scholar]
- 9.Schoepf UJ, Costello P. CT angiography for diagnosis of pulmonary embolism: State of the art. Radiology. 2004;230:329–37. doi: 10.1148/radiol.2302021489. [DOI] [PubMed] [Google Scholar]
- 10.Musset D, Parent F, Meyer G, Maître S, Girard P, Leroyer C, et al. Diagnostic strategy for patients with suspected pulmonary embolism: A prospective multicentre outcome study. Lancet. 2002;360:1914–20. doi: 10.1016/S0140-6736(02)11914-3. [DOI] [PubMed] [Google Scholar]
- 11.van der Meer RW, Pattynama PM, van Strijen MJ, van den Berg-Huijsmans AA, Hartmann IJ, Putter H, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: Prediction of clinical outcomeduring 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005;235:798–803. doi: 10.1148/radiol.2353040593. [Epub 2005 Apr 21]. [DOI] [PubMed] [Google Scholar]
- 12.Reid JH, Murchison JT. Acute right ventricular dilatation: A new helical CT sign of massive pulmonary embolism. Clin Radiol. 1998;53:694–8. doi: 10.1016/s0009-9260(98)80297-3. [DOI] [PubMed] [Google Scholar]
- 13.Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, et al. New CT index to quantify arterial obstruction in pulmonary embolism: Comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176:1415–20. doi: 10.2214/ajr.176.6.1761415. [DOI] [PubMed] [Google Scholar]
- 14.Hefeda MM, Elmasry MM. Prediction of short term outcome of pulmonary embolism: Parameters at 16 multi-detector CT pulmonary angiography. Egypt J Radiol Nucl Med. 2014;45:1089–98. [Google Scholar]
- 15.Al Otair HA, Al-Boukai AA, Ibrahim GF, Al Shaikh MK, Mayet AY, Al-Hajjaj MS. Outcome of pulmonary embolism and clinico-radiological predictors of mortality: Experience from a university hospital in Saudi Arabia. Ann Thorac Med. 2014;9:18–22. doi: 10.4103/1817-1737.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attia MN, Seifeldein GS, Hasan AA, Hasan A. Evaluation of acute pulmonary embolism by sixty-four slice multidetector CT angiography: Correlation between obstruction index, right ventricular dysfunction and clinical presentation. Egypt J Radiol Nucl Med. 2015;46:25–32. [Google Scholar]
- 17.Furlan A, Aghayev A, Chang CC, Patil A, Jeon KN, Park B, et al. Short-term mortality in acute pulmonary embolism: Clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265:283–93. doi: 10.1148/radiol.12110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Praveen Kumar BS, Rajasekhar D, Vanajakshamma V. Study of clinical, radiological and echocardiographic features and correlation of Qanadli CT index with RV dysfunction and outcomes in pulmonary embolism. Indian Heart J. 2014;66:629–34. doi: 10.1016/j.ihj.2014.10.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aviram G, Rogowski O, Gotler Y, Bendler A, Steinvil A, Goldin Y, et al. Real-time risk stratification of patients with acute pulmonary embolism by grading the reflux of contrast into the inferior vena cava on computerized tomographic pulmonary angiography. J Thromb Haemost. 2008;6:1488–93. doi: 10.1111/j.1538-7836.2008.03079.x. [DOI] [PubMed] [Google Scholar]
- 20.Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101:2817–22. doi: 10.1161/01.cir.101.24.2817. [DOI] [PubMed] [Google Scholar]
- 21.Ghaye B, Ghuysen A, Bruyere PJ, D'Orio V, Dondelinger RF. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 2006;26:23–39. doi: 10.1148/rg.261055062. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues B, Correia H, Figueiredo A, Delgado A, Moreira D, Ferreira Dos Santos L, et al. Clot burden score in the evaluation of right ventricular dysfunction in acute pulmonary embolism: Quantifying the cause and clarifying the consequences. Rev Port Cardiol. 2012;31:687–95. doi: 10.1016/j.repc.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Al-Thani H, El-Menyar A, Asim M, Kiliyanni AS. Clinical presentation, management, and outcomes of deep vein thrombosis based on Doppler ultrasonography examination. Angiology. 2016;67:587–95. doi: 10.1177/0003319715604265. [DOI] [PubMed] [Google Scholar]
- 24.Ceylan N, Tasbakan S, Bayraktaroglu S, Cok G, Simsek T, Duman S, et al. Predictors of clinical outcome in acute pulmonary embolism: Correlation of CT pulmonary angiography with clinical, echocardiography and laboratory findings. Acad Radiol. 2011;18:47–53. doi: 10.1016/j.acra.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Lijfering WM, Mulder R, ten Kate MK, Veeger NJ, Mulder AB, van der Meer J. Clinical relevance of decreased free protein S levels: Results from a retrospective family cohort study involving 1143 relatives. Blood. 2009;113:1225–30. doi: 10.1182/blood-2008-08-174128. [DOI] [PubMed] [Google Scholar]
- 26.Bazeed MF, Saad A, Sultan A, Ghanem MA, Khalil DM. Prediction of pulmonary embolism outcome and severity by computed tomography. Acta Radiol. 2010;51:271–6. doi: 10.3109/02841850903524413. [DOI] [PubMed] [Google Scholar]
- 27.Vedovati MC, Becattini C, Agnelli G, Kamphuisen PW, Masotti L, Pruszczyk P, et al. Multidetector CT scan for acute pulmonary embolism: Embolic burden and clinical outcome. Chest. 2012;142:1417–24. doi: 10.1378/chest.11-2739. [DOI] [PubMed] [Google Scholar]
- 28.Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: A meta-analysis. Crit Care. 2011;15:R103. doi: 10.1186/cc10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RL, Das S, Anandarangam T, Leibowitz DW, Alderson PO, Thomashow B, et al. Association between right ventricular function and perfusion abnormalities in hemodynamically stable patients with acute pulmonary embolism. Chest. 1998;113:665–70. doi: 10.1378/chest.113.3.665. [DOI] [PubMed] [Google Scholar]
- 30.Moroni AL, Bosson JL, Hohn N, Carpentier F, Pernod G, Ferretti GR. Non-severe pulmonary embolism: Prognostic CT findings. Eur J Radiol. 2011;79:452–8. doi: 10.1016/j.ejrad.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Araoz PA, Gotway MB, Harrington JR, Harmsen WS, Mandrekar JN. Pulmonary embolism: Prognostic CT findings. Radiology. 2007;242:889–97. doi: 10.1148/radiol.2423051441. [DOI] [PubMed] [Google Scholar]
- 32.Stein PD, Beemath A, Matta F, Goodman LR, Weg JG, Hales CA, et al. Enlarged right ventricle without shock in acute pulmonary embolism: Prognosis. Am J Med. 2008;121:34–42. doi: 10.1016/j.amjmed.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, et al. Severity of acute pulmonary embolism: Evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol. 2003;13:29–35. doi: 10.1007/s00330-002-1515-y. [DOI] [PubMed] [Google Scholar]
- 34.Kang DK, Thilo C, Schoepf UJ, Barraza JM, Jr, Nance JW, Jr, Bastarrika G, et al. CT signs of right ventricular dysfunction: Prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011;4:841–9. doi: 10.1016/j.jcmg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Collomb D, Paramelle PJ, Calaque O, Bosson JL, Vanzetto G, Barnoud D, et al. Severity assessment of acute pulmonary embolism: Evaluation using helical CT. Eur Radiol. 2003;13:1508–14. doi: 10.1007/s00330-002-1804-5. [DOI] [PubMed] [Google Scholar]
- 36.Oliver TB, Reid JH, Murchison JT. Interventricular septal shift due to massive pulmonary embolism shown by CT pulmonary angiography: An old sign revisited. Thorax. 1998;53:1092–4. doi: 10.1136/thx.53.12.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meinel FG, Nance JW, Jr, Schoepf UJ, Hoffmann VS, Thierfelder KM, Costello P, et al. Predictive value of computed tomography in acute pulmonary embolism: Systematic review and meta-analysis. Am J Med. 2015;128:747–59.e2. doi: 10.1016/j.amjmed.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Gussoni G, Frasson S, La Regina M, Di Micco P, Monreal M. RIETE Investigators. Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thromb Res. 2013;131:24–30. doi: 10.1016/j.thromres.2012.10.007. [DOI] [PubMed] [Google Scholar]


