Abstract
Importance
Vestibular patients are often complex, require additional clinic visit time, and utilize greater clinical resources for diagnosis. A pre-encounter intake questionnaire may predict the most common disorders allowing for more efficient allocation of resources and use of providers.
Objective
To develop a statistical model for predicting vestibular diagnoses, prior to clinical evaluation, from an intake questionnaire.
Design, Setting and Participants
Retrospective review of 414 new vestibular patient intake questionnaires and medical records with performance of logistic regression analyses and development of predictive models.
Intervention
Use of a vestibular intake questionnaire for triaging of new patients with complaints of dizziness.
Main Outcome Measures
Predictors for the diagnosis of BPPV, Meniere disease, and vestibular migraine.
Results
Ear-related disorders accounted for 48% of all diagnoses while neurologic conditions accounted for 37% of diagnoses. Three disorders – benign paroxysmal positional vertigo (BPPV), Meniere disease and vestibular migraine – comprised 69% of all diagnoses. The diagnosis of BPPV could be predicted from four variables with a sensitivity of 79% and specificity of 65%. The diagnosis of Meniere disease could be predicted from five variables with a sensitivity of 81% and specificity of 85%. The diagnosis of vestibular migraine could be predicted from four variables with a sensitivity of 76% and specificity of 59%.
Conclusions
A pre-encounter history questionnaire can provide useful diagnostic information for common vestibular disorders. This can help direct appointment scheduling to improve clinical efficiency, time to intervention, and use of resources. Further refinement may enable shorter questionnaires or screening algorithms.
Keywords: vestibular, statistical, BPPV, Meniere disease, migraine
INTRODUCTION
Dizziness is among the most common chief complaints presenting to frontline providers.1,2 While dizziness is a symptom with many causes, evaluation by an otolaryngologist is commonly recommended. Vestibular patients are complex, require additional clinic time, and utilize greater resources (e.g., videonystagmography, rotary chair etc.). Many patients fail to have otologic pathology leading to patient and provider frustration and delays in diagnosis.
History plays a critical role in the evaluation of vestibular complaints.3,4 The nature of the dizziness (i.e., vertigo, lightheadedness, imbalance), the temporal pattern of the dizziness (i.e., single episode, recurrent), the duration of attacks (i.e., seconds, hours), and associated symptoms (i.e., hearing loss, headache) can identify otologic versus non-otologic pathology, and even a specific diagnosis.5 Physical examination may help establish a vestibular diagnosis but is often normal. Similarly, vestibular testing may be useful in establishing a diagnosis but requires a narrow differential diagnosis for correct selection and interpretation of tests.3
A questionnaire focusing on key elements of the history may provide adequate information for development of a narrow differential diagnosis prior to the office visit.4,6 Our program began using a vestibular disorders intake questionnaire in September 2012. This 10-page questionnaire was designed as a quality improvement measure to provide more efficient and timely care to patients. The results of the questionnaire have been used to direct appointments (e.g., physician, vestibular therapist, nurse practitioner, neurologist) and to inform choice of testing (e.g. VNG, rotary chair, posturography, VEMPs). Subjectively this appears to have improved clinical efficiency, but places time burden on administrative and clinical staff to manage this system.
We performed data analyses of the triage questionnaire. This study used 414 consecutive patient questionnaires for descriptive analyses and predictive model building. Results of this study may be generalized to practice management for allocating resources and improving efficiency of patient evaluation.
MATERIALS AND METHODS
IRB approval was obtained from our institutional board. This project analyses a clinically utilized intake questionnaire specifically designed to triage new vestibular disorders patients.
Questionnaire
The questionnaire was developed at Mayo clinic and was modified slightly before being implemented in our institution. There were 162 data variables captured from each questionnaire. The questionnaire captures demographic information including medical, family and social history, and current medications. There are sections that focus on:
The nature of the dizziness perception. This includes a series of check-boxes to describe the dizziness, and questions as to the onset, duration and frequency of spells, triggers for spells, and the relationship of spells to motion.
There is a section regarding headache, migraine, and migraine associated symptoms.
There is a section regarding otologic problems including hearing loss, tinnitus, aural pressure, otalgia and otorrhea.
There is a section regarding prior tests and results including audiograms, imaging, VEMPs, ENG/VNGs, rotary chair, cardiac holter monitors, tilt table testing, etc.
Predictive Model Development
The development of predictive models for the diagnosis of benign paroxysmal positional vertigo (BPPV), Meniere disease and vestibular migraine incorporated an initial dataset for identifying key variables for further data collection and large dataset for predictive model building. The initial group consisted of 212 consecutive new patient intake questionnaires. All variables and fields were collected from these questionnaires for analysis. We initially tried to develop models utilizing all available variables but this resulted in complex algorithms with unsatisfactory sensitivity and specificity. By repetitively narrowing the dataset, and checking for improvements in sensitivity and specificity, a set of factors with strong correlation with specific diseases was identified. A subsequent 202 consecutive questionnaires were then interrogated for this narrow set of variables. These variables from the combined 414 questionnaires were then analyzed to build the statistical models for diagnosis predictions.
Statistical Analysis
The initial dataset was screened to identify variables using three criteria: (1) significantly (p<0.05) associated with the three diagnoses, (2) sufficient number of observations (at least 5 per cell after cross tabulation with the outcome), and (3) clinically meaningful and relevant. All variables were converted into dichotomous form (i.e. 0=absent; 1=present).
The final dataset had information on 414 individuals of which 381 were ultimately fully evaluable (see below). Logistic regression analyses were performed to build parsimonious predictive models with model variables significant at p<0.05. All two way interactions between significant model variables were investigated for statistical significance. A forward stepwise variable selection procedure was used. The three final parsimonious models included only variables significant at p<0.02 level, which is even stronger than the initially planned significance cutoff of 0.05. The Receiver-Operating Characteristic (ROC) curve, area under ROC curve, sensitivity and specificity at selected cutoffs (i.e., linear predictor (LP) values) were assessed using ten-fold cross-validation.
The statistical analysis was performed using the open source software R 3.1.1 (www.r-project.org). Two tailed Wald tests were used for statistical significance testing.
RESULTS
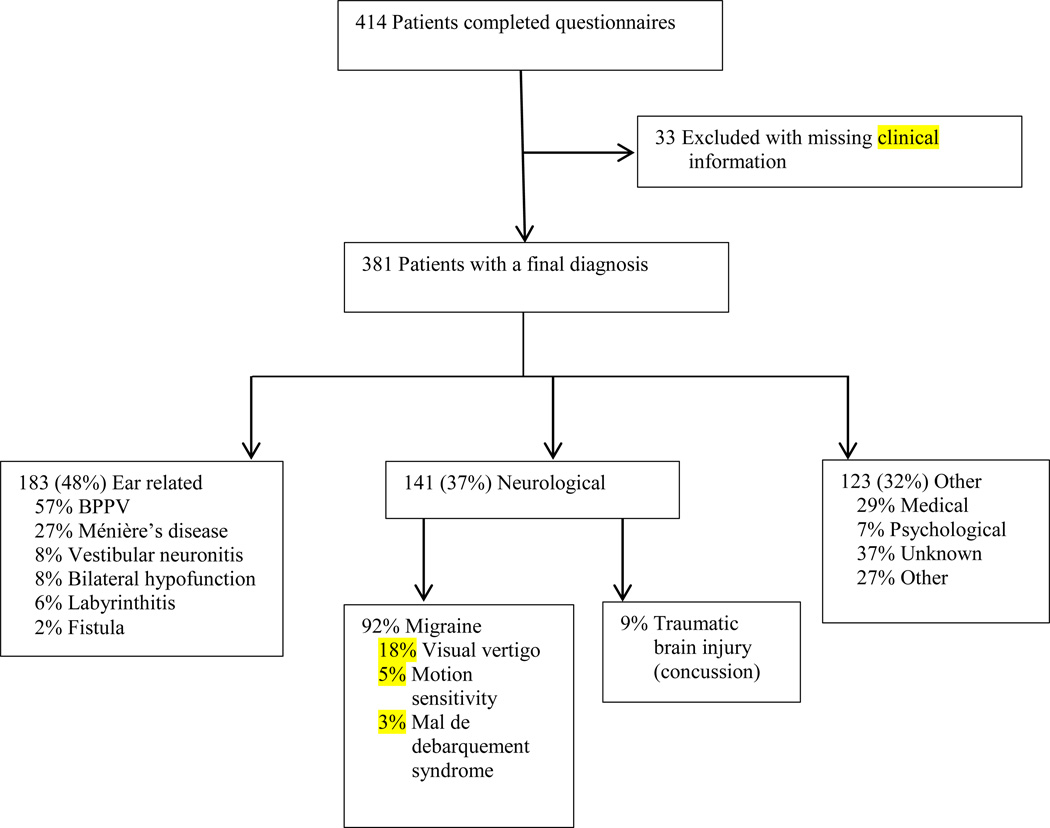
Of the 414 questionnaires analyzed, 381 had provider information necessary to define a final diagnosis (Figure 1). Of these, 183 (48.0%) were ear related, 141 (37.0%) were neurological, 36 (9.4%) considered medical, 8 (2.1%) felt to be primary psychological, 46 (12.1%) remained of unknown etiology and 33 (8.7%) of other causes. Of those deemed ear-related the vast majority were BPPV (56.6%) followed by Meniere disease (26.9%), vestibular neuronitis (8.2%), bilateral hypofunction (8.2%), labyrinthitis (5.5%) and labyrinthine fistula (PLF or SSCD) (2.2%).
Figure 1. Distribution of Diagnoses.
Distribution of diagnoses among subjects completing a vestibular intake questionnaire and having subsequent clinical assessment.
Of the 141 conditions felt to be neurological, there were 118 with a specific diagnosis. These consisted of migraine (92.4%) and traumatic brain injury/post-concussive syndrome (9.3%). There were 33 migraine patients classified further as having visual vertigo (69.7%), severe motion sensitivity (21.2%) and Mal de Debarquement syndrome (9.1%). Non-neurological medical diagnoses (9.4% of total) included orthostasis and cardiogenic causes, and represented 27.8% and 44.4% of this category, respectively.
BPPV
There were 103 patients who had BPPV. All of them were seen and evaluated by a provider to confirm BPPV. Some had resolved by the time of evaluation but a clinically obtained history, rather than just the questionnaire, strongly suggested BPPV as the definitive diagnosis.
As expected, 78% of those with BPPV indicated lying down/rolling in bed was a trigger as compared to 32% of those without BPPV (p<.0001). Similarly, 78% of those with BPPV described their dizziness as vertigo compared to 57% of those with other diagnoses (p=.0002). Reported length of attacks were also significantly different with 48% of BPPV patients indicating they last seconds while only 19% of those without BPPV indicated attacks last seconds.
Those with BPPV were more likely to say that the dizziness was not continuous (p=.0113) and that it occurred when they moved (p=.0386). Those without BPPV were more likely to indicate that automobile rides or loud sounds were triggers than those with BPPV (p=.0023 and p=.0006, respectively). Stress as a trigger was also significantly more prevalent in those without BPPV (p=.0030). Those with BPPV were less likely to exhibit hearing loss than those with other diagnoses, 42% to 61% (p=.0048).
Meniere Disease
There were 49 patients evaluated in clinic with confirmed Meniere disease meeting probable or definite criteria.7,8 Those with Meniere disease, as compared to those without, were more likely to describe their dizziness as vertigo, 86% to 59% (p=.0002). They also strongly indicated duration of attacks as minutes to hours, with 75% choosing this option.
Hearing loss is a hallmark of Meniere disease and there were 96% of Meniere patients who indicated they had documented hearing loss compared to only 49% of those without Meniere disease (p=.0001). Fluctuating hearing also strongly favored Meniere disease patients with 46% noting changes in hearing as opposed to only 6% with other disorders (p=.0001).
Vestibular Migraine
There were 109 patients ultimately felt to have vestibular migraine. Diagnosis was based on clinical impression, which generally follows defined diagnostic criteria for vestibular migraine.9,10 As expected, those with vestibular migraine had a higher likelihood of self-reporting migraine than those with other vestibular conditions, 42% to 22% (p<0.0001). Photophobia with a headache was reported in 80% of those diagnosed with vestibular migraine as compared to 37% of those with other conditions (p<0.0001). Similarly, other migraine symptoms also showed increased prevalence in those with vestibular migraine such as history of headache with nausea and vomiting (p=.0074), unilateral headache (p=.0193), and throbbing headache (p=.0078).
There was a significantly higher response that visual and motion stimuli could trigger dizziness in vestibular migraine patients. Automobile rides (p=.0007), reading (p=.0019), going through aisles/tunnels (p=.0028), and turning when walking (p=.0017) were all more commonly noted as triggers. In addition, stress (p=.0315) and association with menstrual cycle (p=.0132) were slightly more common in those felt to have vestibular migraine.
Predictive Model Building (Table 1)
Table 1.
Variables used in the predictive model building.
| Variables Used in Predictive Models | |||
|---|---|---|---|
| Coefficient | p-value | Comment | |
| BPPV | |||
| Lying down/rolling over | 1.87 | p<0.001 | Hallmark of BPPV |
| Vertigo | 0.92 | p=0.003 | Consistent with BPPV |
| LOS* minutes to hours | −0.98 | p<0.001 | Negative predictor; distinguish from Meniere Disease |
| LOS: days, <week | −1.11 | p=0.018 | Negative predictor; distinguish from Vestibular Migraine |
| Vertigo and LOS: days to weeks | −1.85 | p=0.002 | Negative predictor; distinguish from Vestibular Migraine |
| Meniere Disease | |||
| Vertigo | 1.78 | p<0.001 | Consistent with Meniere Disease |
| Documented hearing loss | 3.22 | p<0.001 | Note this is documented; not subjective |
| LOS: minutes to hours | 1.40 | p<0.001 | Hallmark of Meniere Disease attacks |
| Tinnitus: right | 2.04 | p<0.001 | Unilateral tinnitus |
| Tinnitus: left | 1.52 | p<0.001 | Unilateral tinnitus |
| Vestibular Migraine | |||
| History of Migraine | 0.98 | p=0.003 | Consistent with vestibular migraine diagnostic definition |
| Photophobia | 1.06 | p<0.001 | Consistent with history of migraine |
| LOS: seconds | −0.86 | p=0.012 | Negative predictor; distinguish from BPPV |
| Automobile rides | 0.94 | p=0.003 | Visual vertigo and motion sickness; consistent with vestibular migraine |
| Migraine and automobile rides | −1.24 | p=0.017 | Correction factor due to strength of having both descriptors together |
LOS = length of spell as reported by patient in questionnaire
BPPV
The variables predicting BPPV related to triggers for dizziness, the nature of the dizziness and the timing of spells. In particular, having dizziness described as vertigo and indicating lying down/rolling over as the main trigger were the strongest positive predictors. The other main predictors were related to duration of spells.
The questionnaire had 4 check boxes for duration of spells: 1) seconds to minutes, 2) minutes to hours but less than 24 hours, 3) days, but less than a week, and 4) days, and can be continuously for weeks. A patient with BPPV would be expected to choose category 1, and indeed this was selected by 48% of BPPV sufferers. However, 33% chose minutes to hours and about 10% chose each of the longer durations. As such, duration of seconds to minutes was not a positive predictor on its own. Therefore, the model uses longer duration spells to negatively impact the predictive formula, thus strengthening the relationship between short spells and BPPV. The formula identified for the linear predictor (LP) of BPPV is thus:
In this formula, if the variable is present replace it with a “1” and if not present replace it with a “0”. For example, if the patient indicates dizziness with rolling over, vertigo, and spells lasting days, the formula computes as LP=−2.19+1.87+.92−1.11 which equals −.51. The LP is then transformed into an estimated probability of BPPV with the following formula
For example, LP=−.51 translates into a probability estimate of BPPV equal to 0.375. Cross-validation of this model confirmed good predictive properties with an area under the curve (AUC) of 0.76. At LP≥0.2 the cross-validated sensitivity for BPPV is 0.79 and specificity for BPPV is 0.65.
Meniere Disease
Positive predictors for Meniere disease included classification of the dizziness as vertigo and indicating a length of spell lasting minutes to hours. A strong predictor relating to hearing loss was having a documented history of hearing loss, in contrast to a perception of hearing loss. Further, having unilateral tinnitus, in contrast to bilateral tinnitus or no tinnitus, was a strong predictive variable. Tinnitus in the right ear only was a slightly stronger predictor than tinnitus in the left ear. The resultant formula for the linear predictor of Meniere disease is thus:
Cross-validation of this model confirmed an ROC curve with area under the curve (AUC) of 0.86. At LP≥0.15 the cross-validated sensitivity for Meniere disease is 0.81 and cross-validated specificity for Meniere disease is 0.85.
Vestibular Migraine
The nature of the dizziness was not a predictive variable for vestibular migraine. Vestibular migraine patients noted many forms of dizziness including vertigo (69%), wooziness (60%), imbalance (70%), faint (57%), swimming sensation (34%), pulsion (23%) and other (9%). Only the sensation of fainting approached statistical significance when comparing vestibular migraine to other conditions (p=0.075).
The positive predictors for vestibular migraine related to a history of migraine, migraine aura symptoms, and motion sensitivity, which is frequently found in migraine sufferers.11 Thus, the variables diagnosis of migraine and photophobia with headaches were both significantly related to vestibular migraine in contrast to other conditions. Also, selecting automobile rides as a trigger for attacks of dizziness was a strong positive predictor.
The effect of having a diagnosis of migraine and dizziness with automobile rides together skewed the balance between sensitivity and specificity in the model and required a negative correction factor if both were present. A negative predictor was also the indication that attacks last seconds. The final linear predictor for vestibular migraine is thus:
Cross-validation of this model confirmed good predictive properties with area under the curve (AUC) of 0.65. At LP≥0.25 cross-validated sensitivity for vestibular migraine is 0.76 and cross-validated specificity for vestibular migraine is 0.59. Given the often vague or varied complaints of dizziness in vestibular migraine it is expected the specificity would be lower compared to other disorders.
DISCUSSION
The efficacy of any questionnaire relies on accuracy in completing the form. The vast majority of patients were comprehensive in addressing all fields but some were cursory. In these cases an APNP with training in vestibular disorders called the patient for further detail. Some of these cases seem to reflect patient attitude that the questionnaire is a formality to obtaining a physician appointment rather than a useful diagnostic tool.
Effectiveness of the questionnaire is also dependent upon patient interpretation of the questions. For example, a number of patients with BPPV chose the prompt that dizziness lasts for days to weeks. We interpret this as a failure to distinguish between individual episodes and the period of time during which they have episodes. This confusion has been previously noted.5 This suggests that questionnaires may need follow-up questions to clarify answers or rigorous study to validate each field. Reliability may be improved with an electronic questionnaire using branching logic to ask additional questions if needed to clarify answers.12
It is not clear if the high association of some variables in this study is specific to the form in which they are presented to the patient. For example, the variable lying down/rolling in bed was a strong positive predictor of BPPV but was presented as a check-box within a list of 17 potential triggers. Zhao and colleagues, using a questionnaire that primarily asked yes/no questions, also found that dizziness with lying down was a strong predictor of BPPV.6 Similarly, they found BPPV negatively associated with long attacks, vestibular migraine positively associated with light sensitivity, and Meniere disease positively associated with unilateral hearing loss or tinnitus. While this may suggest good concordance with the present study, they also identified many variables that differed from those in this report. Therefore, the manner of presentation of the question may play a role in the utility of the questionnaire to predict specific conditions.
The strongest model was that predicting the diagnosis of Meniere disease. Similar high sensitivity and specificity has been found with other questionnaires for Meniere disease.13 This may reflect the strong association of hearing loss and tinnitus with Meniere disease.7 These conditions are easily recognized by patients and thus the data collection for these variables may be more accurate. Further, the diagnostic criteria for Meniere disease include these conditions thus increasing the probability of Meniere disease when present.7
In contrast to Meniere disease, the model for predicting vestibular migraine had comparable sensitivity but low specificity. This may reflect the varied clinical nature of vestibular migraine and the weaker diagnostic criteria.10,14 For example, patients with vestibular migraine often describe dizziness as an “off” sensation which may be poorly interrogated by the questionnaire. Migraine is also significantly underdiagnosed15,16 and therefore a key diagnostic criteria for vestibular migraine may be absent from many questionnaires. Further, by written history, subtle distinctions that would allow discriminating vestibular migraine from persistent postural and perceptual dizziness (3PD) can be missed. In fact, this study categorized visual vertigo and motion sensitivity as forms of vestibular migraine, which has been our traditional clinical practice, but which may be better considered as 3PD (aka chronic subjective dizziness).17–19
Limitations of this study include the use of a single center with the reliance on clinical impression, rather than strict diagnostic criteria, to obtain final vestibular diagnoses. A multi-center study with additional clinicians can better reflect the general clinical experience as regards these disorders. Statistically, we performed validation on the same dataset as the model building. The internal cross validation (ten-fold cross validation) partially addressed the validation issue but is not as robust as validation of the predictive models on external, or separately collected, data.
The goal of initiating a quality improvement project was to alter the clinical practice paradigm for vestibular disorders from a physician centric model. Barriers to this are patient and provider acceptance of a potential non-physician based assessment and treatment encounter, limited evidence demonstrating efficacy and efficiency of such a program, and uncertainty in key areas of the clinical pathway used for guiding decision making. The results of this study can provide evidence for patients and referring providers as to the diagnostic accuracy of pre-encounter questionnaires and the potential improvement in clinical efficiency. Clinical efficiency is becoming a significant metric used to evaluate provider quality. Time to next appointment, enough time spent with the patient, and running a clinic on time are all metrics being used by health systems to measure the quality of services. Structured systems for triaging patients into those requiring a physician evaluation versus ancillary providers have been highly effective. In a primary care setting access to the practice increased by almost 30% and over 80% of patients triaged to a non-physician provider did not need to follow-up with a physician.20 Similarly, using a structured questionnaire as support for medical decision making for viral respiratory infection showed that military medics could reduce the need for physician referrals by 37%.21
The outcomes in this study have been used in our institution to improve access by utilizing ancillary providers. For example, BPPV patients can be seen within 1 week by vestibular therapy without waiting for a physician appointment. A similar triage model involving vestibular disorders has shown high patient satisfaction, likely due to simultaneous evaluation and treatment.22 In our practice, patients with significant headache component and prediction of vestibular migraine are offered neurological consultation as a best first assessment. Freeing the otolaryngologist’s schedule from non-otologic vestibular patients may allow faster access for those predicted to have Meniere disease or other otologic conditions.
Acknowledgments
The authors would like to thank Neil Shepard PhD and Scott Eggers MD at the Mayo Clinic in Rochester MN for providing the vestibular disorders questionnaire and allowing for its description in this manuscript.
Funding: This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Author Contributions: Friedland, Tarima and Erbe had full access to all data in the study and take responsibility for data integrity and accuracy. Study Concept and Design: all authors; Acquisition of data: all authors; Data analysis or interpretation: Friedland and Tarima; Drafting of manuscript: Friedland and Tarima; Critical revision of manuscript: all authors; Statistical analysis: Friedland and Tarima; Administrative, technical, or material support; Friedland, Miles and Erbe; Study supervision: Friedland.
Conflicts of Interest: None reported.
References
- 1.Crespi V. Dizziness and vertigo: an epidemiological survey and patient management in the emergency room. Neurol Sci. 2004 Mar;25(Suppl 1):S24–S25. doi: 10.1007/s10072-004-0212-9. [DOI] [PubMed] [Google Scholar]
- 2.Maarsingh OR, Dros J, Schellevis FG, van Weert HC, Bindels PJ, Horst HE. Dizziness reported by elderly patients in family practice: prevalence, incidence, and clinical characteristics. BMC Fam Pract. 2010;11:2. doi: 10.1186/1471-2296-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakhit M, Heidarian A, Ehsani S, Delphi M, Latifi SM. Clinical assessment of dizzy patients: the necessity and role of diagnostic tests. Glob J Health Sci. 2014 May;6(3):194–199. doi: 10.5539/gjhs.v6n3p194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kentala E, Rauch SD. A practical assessment algorithm for diagnosis of dizziness. Otolaryngol Head Neck Surg. 2003 Jan;128(1):54–59. doi: 10.1067/mhn.2003.47. [DOI] [PubMed] [Google Scholar]
- 5.Newman-Toker DE. Symptoms and signs of neuro-otologic disorders. Continuum (Minneap Minn) 2012 Oct;18(5 Neuro-otology):1016–1040. doi: 10.1212/01.CON.0000421618.33654.8a. [DOI] [PubMed] [Google Scholar]
- 6.Zhao JG, Piccirillo JF, Spitznagel EL, Jr, Kallogjeri D, Goebel JA. Predictive capability of historical data for diagnosis of dizziness. Otol Neurotol. 2011 Feb;32(2):284–290. doi: 10.1097/MAO.0b013e318204aad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere's disease. J Vestib Res. 2015 Jan 1;25(1):1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- 8.Thorp MA, Shehab ZP, Bance ML, Rutka JA Hearing A-HCo, Equilibrium. The AAO-HNS Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease: have they been applied in the published literature of the last decade? Clin Otolaryngol Allied Sci. 2003 Jun;28(3):173–176. doi: 10.1046/j.1365-2273.2003.00687.x. [DOI] [PubMed] [Google Scholar]
- 9.Furman JM, Balaban CD. Vestibular migraine. Ann N Y Acad Sci. 2015 Apr;1343(1):90–96. doi: 10.1111/nyas.12645. [DOI] [PubMed] [Google Scholar]
- 10.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(4):167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- 11.Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res. 2014;24(5–6):387–395. doi: 10.3233/VES-140525. [DOI] [PubMed] [Google Scholar]
- 12.Goldbloom RB, Kim RK, Hodder-Malloy C, et al. Design and reliability of pediatric HealthQuiz: preliminary report of a comprehensive, computerized, self-administered child health assessment. Clin Pediatr (Phila) 1999 Nov;38(11):645–654. doi: 10.1177/000992289903801103. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Escamez JA, Lopez-Nevot A, Gamiz MJ, Moreno PM. Effectiveness of a structured questionnaire for diagnosis of Meniere's disease in the first visit. Acta Otorhinolaryngol Belg. 2000;54(4):451–458. [PubMed] [Google Scholar]
- 14.Radtke A, Neuhauser H, von Brevern M, Hottenrott T, Lempert T. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia. 2011 Jun;31(8):906–913. doi: 10.1177/0333102411405228. [DOI] [PubMed] [Google Scholar]
- 15.Arroyo-Quiroz C, Kurth T, Cantu-Brito C, Lopez-Ridaura R, Romieu I, Lajous M. Lifetime prevalence and underdiagnosis of migraine in a population sample of Mexican women. Cephalalgia. 2014 Nov;34(13):1088–1092. doi: 10.1177/0333102414529196. [DOI] [PubMed] [Google Scholar]
- 16.Diamond ML. The role of concomitant headache types and non-headache co-morbidities in the underdiagnosis of migraine. Neurology. 2002 May 14;58(9 Suppl 6):S3–S9. doi: 10.1212/wnl.58.9_suppl_6.s3. [DOI] [PubMed] [Google Scholar]
- 17.Bittar RS, von Sohsten Lins EM. Clinical characteristics of patients with persistent postural and perceptual dizziness. Braz J Otorhinolaryngol. 2014 Sep 6; doi: 10.1016/j.bjorl.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am. 2009 Feb;42(1):71–77. ix. doi: 10.1016/j.otc.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Staab JP. Chronic subjective dizziness. Continuum (Minneap Minn) 2012 Oct;18(5 Neuro-otology):1118–1141. doi: 10.1212/01.CON.0000421622.56525.58. [DOI] [PubMed] [Google Scholar]
- 20.Thorn J, Maun A, Bornhoft L, et al. Increased access rate to a primary health-care centre by introducing a structured patient sorting system developed to make the most efficient use of the personnel: a pilot study. Health Serv Manage Res. 2010 Nov;23(4):166–171. doi: 10.1258/hsmr.2010.010005. [DOI] [PubMed] [Google Scholar]
- 21.Golan D, Zagetzki M, Vinker S. Acute respiratory infections: can a non-physician practitioner triage and treat patients by using an algorithm? Experience in a military primary care clinic. Isr Med Assoc J. 2005 Sep;7(9):578–582. [PubMed] [Google Scholar]
- 22.Kasbekar AV, Mullin N, Morrow C, Youssef AM, Kay T, Lesser TH. Development of a physiotherapy-led balance clinic: the Aintree model. J Laryngol Otol. 2014 Nov;128(11):966–971. doi: 10.1017/S0022215114002060. [DOI] [PubMed] [Google Scholar]