Abstract
Aims
The relationship between outcomes and time after diagnosis for patients with non-valvular atrial fibrillation (NVAF) is poorly defined, especially beyond the first year.
Methods and results
GARFIELD-AF is an ongoing, global observational study of adults with newly diagnosed NVAF. Two-year outcomes of 17 162 patients prospectively enrolled in GARFIELD-AF were analysed in light of baseline characteristics, risk profiles for stroke/systemic embolism (SE), and antithrombotic therapy. The mean (standard deviation) age was 69.8 (11.4) years, 43.8% were women, and the mean CHA2DS2-VASc score was 3.3 (1.6); 60.8% of patients were prescribed anticoagulant therapy with/without antiplatelet (AP) therapy, 27.4% AP monotherapy, and 11.8% no antithrombotic therapy. At 2-year follow-up, all-cause mortality, stroke/SE, and major bleeding had occurred at a rate (95% confidence interval) of 3.83 (3.62; 4.05), 1.25 (1.13; 1.38), and 0.70 (0.62; 0.81) per 100 person-years, respectively. Rates for all three major events were highest during the first 4 months. Congestive heart failure, acute coronary syndromes, sudden/unwitnessed death, malignancy, respiratory failure, and infection/sepsis accounted for 65% of all known causes of death and strokes for <10%. Anticoagulant treatment was associated with a 35% lower risk of death.
Conclusion
The most frequent of the three major outcome measures was death, whose most common causes are not known to be significantly influenced by anticoagulation. This suggests that a more comprehensive approach to the management of NVAF may be needed to improve outcome. This could include, in addition to anticoagulation, interventions targeting modifiable, cause-specific risk factors for death.
Clinical Trial Registration
http://www.clinicaltrials.gov. Unique identifier: NCT01090362.
Keywords: Atrial fibrillation, Anticoagulation, Stroke prevention, Stroke, Bleeding
See page 2890 for the editorial comment on this article (doi:10.1093/eurheartj/ehw313)
Introduction
Atrial fibrillation (AF), the most frequent of all cardiac arrhythmias, is associated with an increased risk of stroke, systemic embolism (SE), and heart failure. Patients with AF have a two-fold increased risk of death compared with those without AF.1–3 Anticoagulation reduces the risk of stroke/SE and of death at the cost of an increased risk of bleeding. Anticoagulation with vitamin K antagonists (VKAs) or with the newer non-vitamin K antagonist oral anticoagulants (NOACs) is recommended for patients with AF and at least one additional risk factor for stroke, whereas antiplatelet (AP) therapy, either as monotherapy or with concomitant anticoagulation, is indicated in a specific subset of patients.3,4 Currently, there are very limited data on the extended time course of events after diagnosis of non-valvular atrial fibrillation (NVAF) in large multinational populations.
The Global Anticoagulant Registry in the FIELD–Atrial Fibrillation (GARFIELD-AF) is an ongoing, observational, worldwide study of adults with newly diagnosed NVAF, which is governed by the highest academic and ethical standards in the generation, dissemination, and communication of its research findings.5 The registry plans to prospectively recruit ∼52 000 patients (representing all ethnicities and care settings) in five consecutive cohorts from randomly selected centres in 35 countries.
Here, we report 2-year event rates for all-cause mortality, stroke/SE, and major bleeding for the first two cohorts (in 17 162 patients) and the factors that have contributed to these events, namely baseline characteristics and treatment.
Methods
Study design and participants
Men and women aged ≥18 years with NVAF diagnosed according to standard local procedures within the previous 6 weeks, and with at least one additional risk factor for stroke as judged by the investigator, are eligible for inclusion. Risk factors are not prespecified in the protocol nor are they limited to the components of existing risk stratification schemes. The study excludes patients with a transient reversible cause of NVAF and those for whom follow-up is not envisaged or possible.5
Consecutive patients are enrolled prospectively into five sequential cohorts, with the aim of recruiting up to 52 000 patients. Investigator sites have been selected randomly and represent the different care settings in each participating country (office-based practice; hospital departments—neurology, cardiology, geriatrics, internal medicine, and emergency; anticoagulation clinics; and general or family practice).5,6
Ethics statement
Independent ethics committee and hospital-based institutional review board approvals were obtained. The registry is being conducted in accordance with the principles of the Declaration of Helsinki, local regulatory requirements, and the International Conference on Harmonisation—Good Pharmacoepidemiological and Clinical Practice guidelines. Written informed consent is obtained from all study participants. Confidentiality and anonymity of all patients recruited into this registry are maintained.
Procedures and outcome measures
Baseline data collected at inclusion included patient characteristics, medical history, care setting, type of AF, date and method of diagnosis, symptoms, and anticoagulant (AC) treatment (VKAs, factor Xa inhibitors [FXas], and direct thrombin inhibitors [DTIs], as well as AP treatment). Ethnicity was classified by the investigator in agreement with the patient.5
Data on all components of the CHA2DS2-VASc and HAS-BLED risk stratification schemes were collected to assess the risks of stroke and bleeding retrospectively. Vascular disease was defined as peripheral artery disease and/or coronary artery disease (CAD) with a history of acute coronary syndromes (ACS). Hypertension was defined as a documented history of hypertension or blood pressure >140/90 mmHg at rest.
Collection of follow-up data occurred at 4-month intervals up to 24 months. Outcome measures included clinical events, therapy persistence, and healthcare utilization.5,6 The incidences of stroke/SE, pulmonary embolism, ACS, hospitalization, death (cardiovascular and non-cardiovascular), heart failure (occurrence or worsening), and bleeding (severity and location) were recorded. Submitted data were examined for completeness and accuracy by the coordinating centre (Thrombosis Research Institute [TRI], London, UK), and data queries were sent to study sites.
Data collection/quality control/auditing
GARFIELD-AF data are captured using an electronic case report form (eCRF) designed by Dendrite Clinical Systems Ltd (Henley-on-Thames, UK). Oversight of operations and data management are managed by the sponsor and coordinating centre (TRI), with support from Quintiles (Durham, NC, USA), The University of Birmingham Department of Primary Care Clinical Sciences (Birmingham, UK), Thrombosis Research Group—Brigham and Women's Hospital (Boston, MA, USA), and AIXIAL (Paris, France). The GARFIELD-AF protocol requires that 20% of all eCRFs are monitored against source documentation, that there is an electronic audit trail for all data modifications, and that critical variables are subjected to additional audit.5 Data for the analysis in this report were extracted from the study database on 3 August 2015.
Statistical analysis
Continuous variables are expressed as mean ± SD and categorical variables as frequency and percentage. Use of antithrombotic therapy at baseline was analysed by CHA2DS2-VASc and ‘modified’ HAS-BLED (excluding fluctuations in the international normalized ratio) scores, calculated retrospectively from the data collected. Patients with missing values were not removed from the study.
Occurrence of major clinical outcomes is described using the number of events, the proportion of patients with the event divided by the population at risk at the beginning of the follow-up period, person-time event rate (per 100 person-years), and 95% confidence interval (CI). We estimated person-year rates using a Poisson model, with the number of events as the dependent variable and the log of time as an offset, i.e. a covariate with a known coefficient of 1. Only the first occurrence of each event was taken into account. The 4-monthly event rates were compared with the overall rates using the ratio between the observed and expected numbers of events (applying the overall rate to assess expected rates for that period). The Poisson trend statistic was used to assess the trends over time. Hazard ratios (HRs) were estimated using a proportional hazards Cox model after multiple imputation by the Multiple Imputation by Chained Equations (MICE) algorithm.7,8 We used the MICE algorithm to fill in missing values, creating five complete datasets. Data analysis was performed at the TRI with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata Statistical Software: Release 13 (StataCorp, College Station, TX, USA).
Results
Study population
A total of 17 162 patients with NVAF were prospectively enrolled in the first and second GARFIELD-AF cohorts between March 2010 and June 2013 and are included in this analysis. Patients in these cohorts were recruited from 858 randomly selected study sites representative of routine practice in each of 30 countries. Two-year follow-up was achieved in 97% of patients.
At baseline, mean (SD) age was 69.8 (11.4) years, and 43.8% of patients were female. The mean (SD) CHA2DS2-VASc and HAS-BLED scores were 3.3 (1.6) and 1.5 (0.9), respectively. Other baseline characteristics are shown in Table 1. At diagnosis of AF, 60.8% of patients were prescribed AC therapy (50.0% VKAs and 10.8% NOACs, with or without AP), 27.4% received AP monotherapy, and 11.8% received no AC or AP therapy. The proportion of patients receiving AC therapy (with or without AP) increased with CHA2DS2-VASc score, being lowest for patients with a score of 0 (41.6%) and highest for patients with a score of 5 (67.5%; Cuzick test,9P < 0.001; Figure 1A). The use of AC (with or without AP) decreased with increasing HAS-BLED score, from 76.5 to 49.8% for patients with scores of 0 and ≥4, respectively (Cuzick test, P < 0.001; Figure 1B). Anticoagulant therapy was not prescribed in 36.9% of patients with CHA2DS2-VASc ≥2. Patients not receiving AC therapy tended to be younger (mean [SD] age 68.6 [12.3] vs. 70.6 [10.8] years; P < 0.001), were more likely to have paroxysmal AF [29.6 vs. 22.3%; P < 0.001], and had a lower mean (SD) CHA2DS2-VASc score (3.0 [1.6] vs. 3.4 [1.6]; P < 0.001) but a higher mean (SD) HAS-BLED score (1.7 [0.9] vs. 1.5 [1.0]; P < 0.001) than patients receiving AC therapy.
Table 1.
Baseline characteristics of all patients
| Variable | Value | % |
|---|---|---|
| Female, n/n (%) | 7518/17 162 | 43.8 |
| Age, mean (SD) (years) | 69.8 (11.4) | n/a |
| Age group, n/n (%) | ||
| <65 years | 5094/17 162 | 29.7 |
| 65–69 years | 2506/17 162 | 14.6 |
| 70–74 years | 3027/17 162 | 17.6 |
| ≥75 years | 6535/17 162 | 38.1 |
| Race, n/n (%) | ||
| Caucasian | 11 078/17 162 | 64.5 |
| Hispanic/Latino | 1260/17 162 | 7.3 |
| Afro-Caribbean | 26/17 162 | 0.2 |
| Asian (not Chinese) | 3004/17 162 | 17.5 |
| Chinese | 977/17 162 | 5.7 |
| Mixed/other | 286/17 162 | 1.7 |
| Unwilling to declare/not recorded | 531/17 162 | 3.1 |
| Body mass index, mean (SD) (kg/m2) | 27.8 (5.4) | n/a |
| Pulse, mean (SD) (b.p.m.) | 89.9 (26.7) | n/a |
| Systolic blood pressure, mean (SD) (mmHg) | 133.9 (19.9) | n/a |
| Diastolic blood pressure, mean (SD) (mmHg) | 80.0 (12.7) | n/a |
| Left ventricular ejection fraction <40%, n/n (%) | 973/9744 | 10.0 |
| Type of AF, n/n (%) | ||
| Permanent | 2243/17 160 | 13.1 |
| Persistent | 2679/17 160 | 15.6 |
| Paroxysmal | 4332/17 160 | 25.2 |
| New (newly diagnosed/new onset) | 7906/17 160 | 46.1 |
| Medical history, n/n (%) | ||
| Congestive heart failure | 3532/17 160 | 20.6 |
| Coronary artery disease | 3416/17 160 | 19.9 |
| Acute coronary syndromes | 1614/17 157 | 9.4 |
| Carotid occlusive disease | 507/17 148 | 3.0 |
| Pulmonary embolism or deep vein thrombosis | 478/17 150 | 2.8 |
| Coronary artery bypass graft | 503/16 654 | 3.0 |
| Stroke/transient ischaemic attack | 2186/17 160 | 12.7 |
| Systemic embolism | 109/17 150 | 0.6 |
| History of bleeding | 497/17 149 | 2.9 |
| History of hypertension | 13 396/17 160 | 78.1 |
| Hypercholesterolaemia | 6875/17 153 | 40.1 |
| Diabetes | 3750/17 160 | 21.9 |
| Cirrhosis | 94/17 148 | 0.5 |
| Chronic kidney disease, n/n (%) | ||
| None or mild (Grades I and II) | 15 399/17 159 | 89.7 |
| Moderate to severe (Grades III to V) | 1760/17 159 | 10.3 |
| Dementia | 264/17 153 | 1.5 |
| Alcohol consumption, n/n (%) | ||
| Abstinent/light | 12 980/14 727 | 88.1 |
| Moderate | 1369/14 727 | 9.3 |
| Heavy | 378/14 727 | 2.6 |
| Current/previous smoker, n/n (%) | 5475/15 621 | 35.0 |
| Antithrombotic treatment, n/n (%) | ||
| Vitamin K antagonists | 6334/16 873 | 37.5 |
| Vitamin K antagonists + antiplatelet | 2103/16 873 | 12.5 |
| Factor Xa inhibitors | 637/16 873 | 3.8 |
| Factor Xa inhibitors + antiplatelet | 287/16 873 | 1.7 |
| Direct thrombin inhibitors | 685/16 873 | 4.1 |
| Direct thrombin inhibitors + antiplatelet | 210/16 873 | 1.2 |
| Antiplatelet only | 4627/16 873 | 27.4 |
| None | 1990/16 873 | 11.8 |
| CHA2DS2-VASc score, mean (SD) | 3.3 (1.6) | n/a |
| CHA2DS2-VASc score categories, n/n (%) | ||
| 0 | 381/16 699 | 2.3 |
| 1 | 1965/16 699 | 11.8 |
| 2 | 3220/16 699 | 19.3 |
| 3 | 3988/16 699 | 23.9 |
| 4 | 3681/16 699 | 22.0 |
| 5 | 2020/16 699 | 12.1 |
| 6–9 | 1444/16 699 | 8.6 |
| HAS-BLED score, mean (SD) | 1.5 (0.9) | n/a |
| HAS-BLED score categories, n/n (%) | ||
| 0 | 1463/10 863 | 13.5 |
| 1 | 4428/10 863 | 40.8 |
| 2 | 3542/10 863 | 32.6 |
| 3 | 1217/10 863 | 11.2 |
| 4 | 189/10 863 | 1.7 |
| 5 | 23/10 863 | 0.2 |
| 6–9 | 1/10 863 | <0.1 |
| Care setting speciality at diagnosis, n/n (%) | ||
| Internal medicine | 3378/17 160 | 19.7 |
| Cardiology | 10 614/17 160 | 61.9 |
| Neurology | 375/17 160 | 2.2 |
| Geriatrics | 78/17 160 | 0.5 |
| Primary care/general practice | 2715/17 160 | 15.8 |
Figure 1.
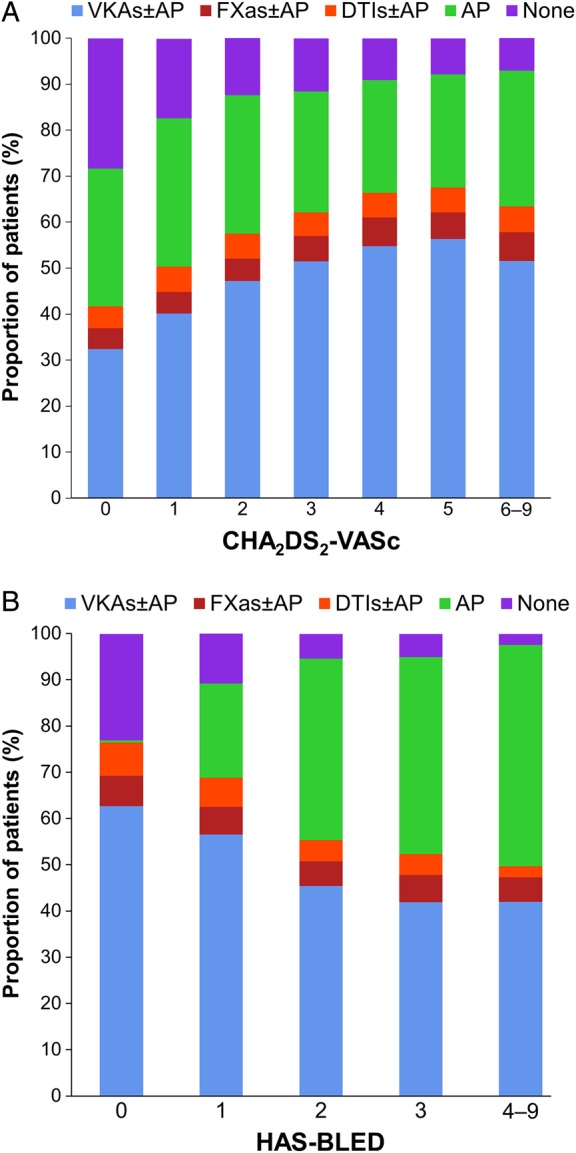
Antithrombotic treatment at baseline stratified by CHA2DS2-VASc score (A) and by HAS-BLED score (B).
Clinical outcomes
During the 2-year follow-up, the rates (95% CI) of all-cause mortality, stroke/SE, and major bleeding (first occurrences) were 3.83 (3.62; 4.05), 1.25 (1.13; 1.38), and 0.70 (0.62; 0.81) per 100 person-years, respectively (Table 2). The rates of all three major events were significantly higher during the first 4 months of follow-up (mortality +29%; stroke/SE +35%; major bleeding +56%) compared with the overall event rates (Figure 2 and see Supplementary material online, Table S1). Beyond the first 4 months, the rates of events were lower and modestly declined over the course of follow-up (χ2 test for trend, P < 0.001 for mortality and stroke/SE, P = 0.001 for major bleeding). The early higher risk of death was observed irrespective of AF pattern, but was higher with new (newly diagnosed/new onset) AF than with other patterns of AF (standardized mortality rate 1.41 [95% CI 1.19; 1.66] vs. 1.19 [1.00; 1.41], respectively). The same was also true for major bleeding, with standardized incidence rates of 1.70 (95% CI 1.19; 2.44) vs. 1.43 (1.01; 2.03), respectively, for new AF compared with other AF patterns. No difference in early excess of risk was observed for stroke/SE.
Table 2.
Event rates (per 100 person-years) for selected clinical outcomes at 2 years of follow-upa
| Rate (95% CI) | |
|---|---|
| Death | 3.83 (3.62; 4.05) |
| Cardiovascular death | 1.55 (1.42; 1.70) |
| Non-cardiovascular death | 1.37 (1.25; 1.51) |
| Undetermined cause | 0.91 (0.81; 1.02) |
| Stroke/SE | 1.25 (1.13; 1.38) |
| Major bleeding | 0.70 (0.62; 0.81) |
| Acute coronary syndromes | 0.63 (0.55; 0.73) |
| Congestive heart failureb | 2.41 (2.24; 2.59) |
CI, confidence interval; SE, systemic embolism.
aOnly the first occurrence of each event was taken into account.
bOccurrence of new CHF or worsening of pre-existing CHF.
Figure 2.
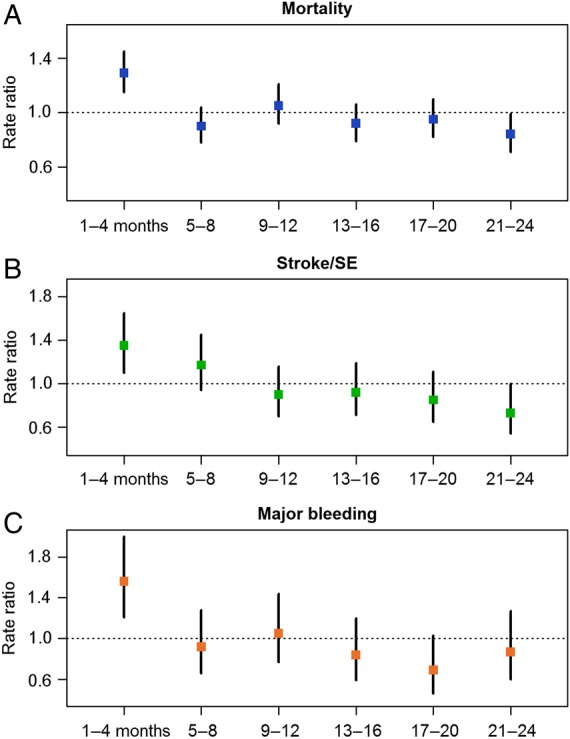
Four-monthly rate ratios* for all-cause mortality (A), stroke/systemic embolism (B), and major bleeding (C). *The ratio between the observed number of events in the period and the expected number of events obtained by applying the overall rate to the period. Only the first occurrence of each event was taken into account.
The rate of death due to cardiovascular causes including fatal bleeds was 1.55 (1.42; 1.70) per 100 person-years (Table 2). The most frequent causes of cardiovascular death were congestive heart failure (CHF), sudden or unwitnessed death, ACS, and ischaemic stroke (Table 3). The rate of non-cardiovascular causes of death was lower (1.37 [1.25; 1.51] per 100 person-years) and mainly due to malignancy, respiratory failure, and infection/sepsis (Table 3). The primary cause of death could not be identified in 280 of 1181 deaths. By multivariate analysis, the baseline variables significantly associated with a higher risk of death were older age, diabetes mellitus, CHF, vascular disease, history of stroke/SE, history of bleeding, chronic kidney disease (CKD), smoking, and non-paroxysmal forms of AF. Anticoagulation was associated with a significantly lower risk of death (Figure 3).
Table 3.
Breakdown of primary outcomes by type of event at 2-year follow-upa
| Event | N | % |
|---|---|---|
| All-cause death | 1181 | |
| Cardiovascular causes | 478 | 40.5 |
| Congestive heart failure | 128 | 10.8 |
| Sudden or unwitnessed death | 89 | 7.5 |
| Acute coronary syndromes | 70 | 5.9 |
| Ischaemic stroke | 60 | 5.1 |
| Otherb | 131 | 11.1 |
| Non-cardiovascular causes | 423 | 35.8 |
| Malignancy | 121 | 10.3 |
| Respiratory failure | 95 | 8.0 |
| Infection/sepsis | 79 | 6.7 |
| Otherc | 128 | 10.8 |
| Undetermined causes | 280 | 23.7 |
| Stroke (not including systemic embolism) | 365 | |
| Primary ischaemic | 260 | 71.2 |
| Secondary haemorrhagic ischaemic | 15 | 4.1 |
| Primary intracerebral haemorrhage | 37 | 10.1 |
| Intracerebral | 20 | 5.5 |
| Intraventricular | 5 | 1.4 |
| Subarachnoid | 3 | 0.8 |
| Undeterminedd | 9 | 2.5 |
| Undeterminede | 68 | 18.6 |
| Bleeding events (not including minor bleeds) | 504 | |
| Severity of bleed | ||
| Non-major, clinically relevant | 288 | 57.1 |
| Major | 216 | 42.9 |
| Fatalf | 24 | 4.8 |
aOnly the first occurrence of each event was taken into account.
bIncludes deaths due to intracranial haemorrhage, atherosclerotic vascular disease, dysrhythmia, pulmonary embolism, and haemorrhagic stroke.
cIncludes deaths due to accidents/trauma, renal disease, and liver disease.
dIncludes patients with unknown type of primary intracerebral haemorrhage and patients with combinations of types of stroke.
eIncludes patients with unknown types of stroke and those with both primary ischaemic and primary intracerebral haemorrhagic strokes.
fAll fatal bleeds are included in major bleeds and are also included in the mortality analysis.
Figure 3.
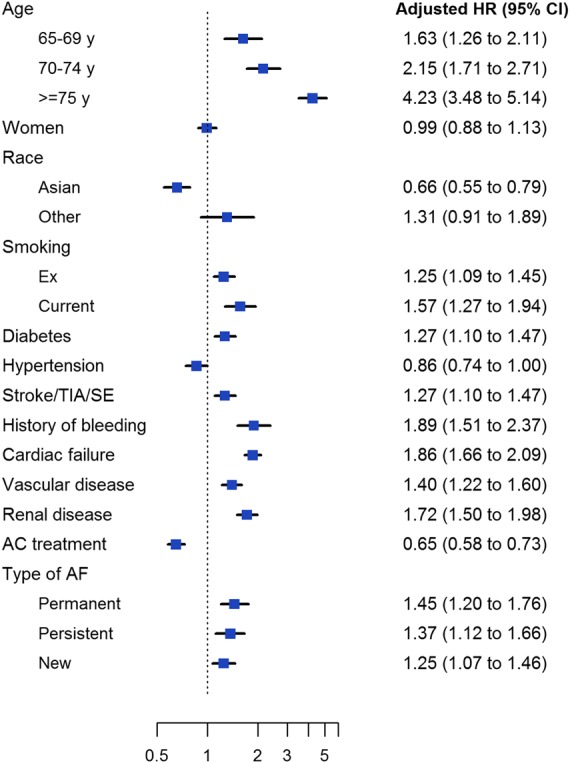
Adjusted hazard ratios for 2-year all-cause mortality according to baseline characteristics and anticoagulant treatment. Anticoagulant treatment includes both vitamin K antagonists and non-vitamin K antagonist oral anticoagulants. Hazard ratios were adjusted for all variables in the model. Reference groups, from top: <65 years, men, Caucasian/Hispanic/Latino, never smoker, no history of disease (for diabetes, hypertension, stroke/TIA/systemic embolism, history of bleeding, cardiovascular failure, vascular disease, and renal disease), no anticoagulant treatment, and paroxysmal AF. TIA, transient ischaemic attack.
The rates of different types of stroke are detailed in Table 3, along with the rates of bleeding events of different severity. Strokes were predominantly ischaemic, and primary haemorrhagic strokes were very uncommon. Sixty patients died from ischaemic stroke, 5 from haemorrhagic stroke, and 89 patients who survived a stroke died over the course of follow-up. The most frequent site for bleeding events was the gastrointestinal tract (occurring in 1.47% of the total population). Bleeds in a critical organ (intra-ocular/retinal, intra-spinal, haemo-pericardium, haemothorax, retroperitoneal) and intracranial bleeding (epidural/subdural haematomas) each occurred in 0.22% of the population.
At 2-year follow-up, the rates of ACS and CHF were 0.63 (95% CI 0.55; 0.73) and 2.41 (2.24; 2.59) per 100 person-years, respectively (Table 2).
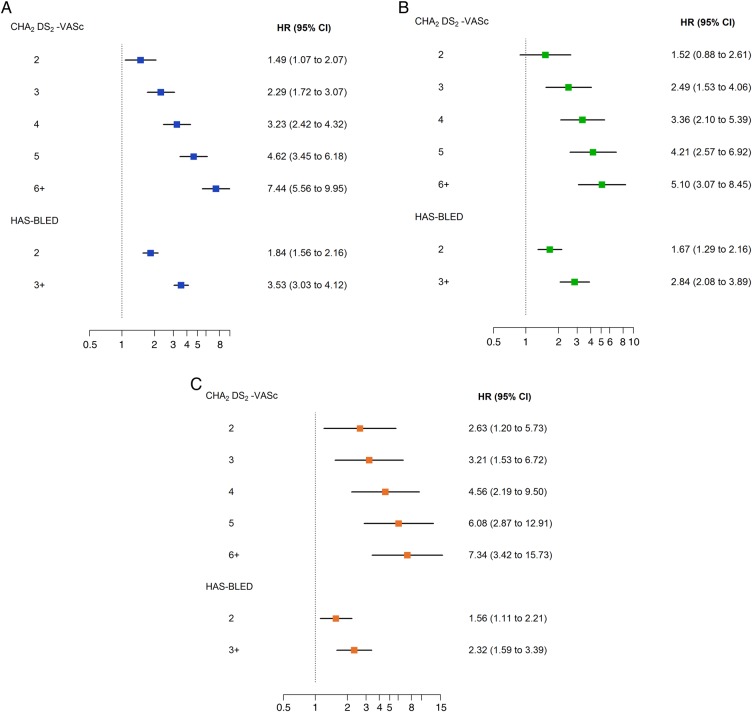
The rates of death, stroke/SE, and major bleeding increased progressively with increasing grades of the CHA2DS2-VASc and HAS-BLED scoring schemes (Cuzick test, P < 0.001; see Supplementary material online, Figure S1) and correspondingly, the HRs for death, stroke/SE, and major bleeding correlated with the CHA2DS2-VASc and HAS-BLED scores (Figure 4).
Figure 4.
Adjusted hazard ratios for 2-year all-cause mortality (A), stroke/systemic embolism (B), and major bleeding (C) according to CHA2DS2-VASc and HAS-BLED scores. Hazard ratios were adjusted for components of CHA2DS2-VASc and HAS-BLED scores. Reference groups, from top: CHA2DS2-VASc score of 0–1, HAS-BLED score of 0–1.
Discussion
Analyses of event rates from the GARFIELD-AF registry have identified that death was the most frequent major adverse clinical event over 2 years of follow-up in patients with NVAF. The rate of death was three-fold higher than the rate of stroke/SE and more than five-fold higher than the rate of major bleeding. The highest frequency of events (for each major outcome measure) occurred during the first 4 months of follow-up. The early risks of death and major bleeding (but not stroke/SE) were higher with new (newly diagnosed/new onset) NVAF than with the other patterns of NVAF. These data suggest that incident NVAF may occur as a complication of a chronic or acute cardiovascular or non-cardiovascular underlying disease that impairs early evolution.10–13 In addition, fluctuations in AC control, which are commonly observed after the initiation of VKA therapy, may explain, at least in part, the early excess of events, as suboptimal VKA control was shown to result in higher rates of stroke/SE, bleeding, and death.14 Furthermore, higher rates of ischaemic events have been observed at the initiation of warfarin in VKA-naive patients, possibly linked to a transient hypercoagulable state due to differential depletion of certain vitamin K-dependent clotting factors.15 Marked hypercoagulability when NVAF first becomes clinically apparent may also play a role. The decline in the rates of events after 4 months (Figure 2 and see Supplementary material online, Table S1) may be related to better AC control and/or to a change in the risk profile of patients over time, and also to the fact that the sickest patients suffered their first event early on after diagnosis of NVAF.
A further important finding relates to the causes of death. As in most12,16,17 but not all reports,18,19 cardiovascular and non-cardiovascular deaths occurred at quite similar rates, but ischaemic and haemorrhagic strokes were clearly not the main drivers of mortality risk, since they accounted for fewer than 10% of all known causes of death.19,20 Such a low rate of stroke-related death may be because >60% of the population was anticoagulated. In contrast, the most frequent causes of death, namely CHF, sudden or unwitnessed death, ACS, malignancy, respiratory failure, and infection/sepsis, which accounted for 65% of all known causes of death (Table 3), are not, or only modestly, affected by AC therapy. Nevertheless, AC therapy was associated with a 35% lower risk of death. Evidently, AC therapy has a favourable impact on the risk of stroke-related death, but part of the reduction in mortality risk is most probably the consequence of prevention of thromboembolism other than stroke/SE, associated with venous thromboembolism in CHF and malignancy. In addition, occurrence and/or complications of ACS can potentially be prevented by AC treatment, as supported by trial data of anticoagulation after myocardial infarction or ACS.21 Conversely, patients without AC treatment (almost 40% of the total population) had a worse outcome compared with anticoagulated patients, despite the fact that they were younger and had a lower risk of stroke.
Newly diagnosed NVAF, in this context, could represent a marker of early death, as a consequence of worsening baseline disease, since survival from several comorbidities, such as CHF, ACS, and respiratory failure, is affected by the occurrence of AF.22–26 Conversely, comorbidities/risk factors may affect the course of NVAF over time, through gradual remodelling of heart chambers triggered by uncontrolled hypertension, progression of CAD, worsening of heart failure, and also ageing that may precipitate the evolution of persistent or paroxysmal NVAF towards permanent NVAF.22,24,26–28 Concomitant with these observations is the strikingly high rate of worsening of heart failure (and to a lesser extent, the rate of new ACS) recorded in these patients (Table 2). Both conditions are risk factors and causes of death, but they are also precipitating factors for the occurrence and progression of AF, and furthermore, they are worsened by the occurrence of AF.22–26,29
The CHA2DS2-VASc score was shown to be an equally good predictor of the risks of all three outcome measures (Figure 4). Most of the variables strongly associated with the risk of death, namely older age, CHF, history of bleeding, CKD, diabetes mellitus, smoking, and pattern of AF, were also associated with the risk of stroke/SE (data not shown). This seems to indicate that the overall prognosis of NVAF in terms of death, stroke/SE and, to some extent, bleeding is tightly linked to the same risk factors/comorbidities.
Study limitations
Most study patients were Caucasians and, to a lesser extent, Asians. Hispanic/Latino and Afro-Caribbean ethnicities were less represented in this analysis of the first two cohorts because recruitment did not start at the same time in all countries involved in GARFIELD-AF.
Study strengths
Several surveys, registries, and regional or national healthcare databases have reported outcomes for patients with NVAF but most studies had a limited duration of follow-up.30–37 Prior studies vary in terms of inclusion criteria, duration of follow-up, and care settings, and in the characterization of outcome events. In contrast, the design of the GARFIELD-AF registry is unique; it has a global reach and extended follow-up and incorporates patients with newly diagnosed NVAF from all care settings, making it representative of real-life management of NVAF worldwide. In addition, GARFIELD-AF audit and quality assurances exceed the standards of most large-scale registries and even some randomized trials.5
Conclusions
Death was the most frequent adverse outcome in NVAF. The highest event rates for death, stroke/SE, and major bleeding occurred during the first 4 months of follow-up, gradually diminishing over time. Stroke-related mortality was not the most frequent cause of death, suggesting that a more comprehensive approach to the management of patients with NVAF may be needed to improve outcome. This could include interventions targeting other modifiable, cause-specific risk factors for death (such as CHF, CAD, ACS, diabetes, and hypertension) in addition to anticoagulation.20,23 CHA2DS2-VASc and HAS-BLED scores were both predictive of all three major outcome measures, suggesting that a single integrated risk score derived from a large cohort of patients like the GARFIELD-AF registry, encompassing all three major outcome measures, may have an added value compared with existing risk scores.
Authors' contributions
J.-P.B., A.J.C., D.A.F., S.Z.G., S.G., S.H., W.H., G.K., L.G.M., F.M., A.G.G.T., F.W.A.V., and A.K.K. contributed to the study design. D.A.F., S.Z.G., F.C. and H.t.C. contributed to data acquisition. G.A. analysed the data. All authors contributed to data interpretation. J.-P.B. drafted the report. All authors critically reviewed the report and approved the final manuscript. A.K.K. and G.K. handled funding and supervised the registry.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by an unrestricted research grant from Bayer Pharma AG, Berlin, Germany, to TRI, London, UK, which sponsors the GARFIELD-AF registry.
Conflict of interest: J.-P.B. reports personal fees from Aspen outside the submitted work. G.A. reports grants from Bayer Pharma AG during the conduct of the study. A.J.C. is an advisor to Bayer, Boehringer Ingelheim, Pfizer/BMS, and Daiichi-Sankyo. F.C. reports consulting and speaker fees from Bayer, speaker fees from BMS and Boehringer Ingelheim. D.A.F. reports personal fees from Bayer outside the submitted work. K.A.A.F. reports grants and personal fees from Bayer, Johnson and Johnson, personal fees from Lilly, grants and personal fees from AstraZeneca, personal fees from Sanofi/Regeneron outside the submitted work. S.Z.G. reports grants from BiO2 Medical, grants from Boehringer Ingelheim, grants from Bristol Meyers Squibb, grants from BTG EKOS, grants from Daiichi-Sankyo, grants from National Heart Lung and Blood Institute of the National Institutes of Health, grants from Janssen, grants from Thrombosis Research Group, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Bristol Meyers Squibb, personal fees from Daiichi-Sankyo, personal fees from Janssen, personal fees from Portola outside the submitted work. S.G. reports personal fees from Bayer, grants from Sanofi, grants from Pfizer, personal fees from Daiichi-Sankyo, personal fees from AstraZeneca during the conduct of the study, and grants from Bayer outside the submitted work. S.H. reports personal fees from Aspen, personal fees from Bayer Healthcare, personal fees from BMS, personal fees from Daiichi-Sankyo, personal fees from Pfizer, personal fees from Sanofi outside the submitted work. W.H. reports personal fees from Bayer during the conduct of the study. G.K. reports grants from Bayer during the conduct of the study. L.G.M. reports grants and personal fees from Bayer Healthcare during the conduct of the study, and grants from Boehringer Ingelheim, grants and personal fees from Pfizer, personal fees from Daiichi-Sankyo outside the submitted work. F.M. reports grants and other from Bayer Pharma AG during the conduct of the study, and other from Bayer Pharma AG outside the submitted work. H.t.C. reports personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from GSK, personal fees from Leo, personal fees from Roche, personal fees from Stago, personal fees from Philips, grants from Bayer, grants from Boehringer Ingelheim, grants from AstraZeneca outside the submitted work, and he is Chair of the board for the Dutch Federation of Anticoagulation Clinics. A.G.G.T. reports consultant fees from Bayer Pharma AG during the conduct of the study. F.W.A.V. reports personal fees from Boehringer Ingelheim, personal fees from Bayer Healthcare, personal fees from BMS/Pfizer, personal fees from Daiichi-Sankyo during the conduct of the study, and personal fees from AstraZeneca outside the submitted work. A.K.K. reports grants and personal fees from Bayer Healthcare, personal fees from Boehringer Ingelheim Pharma, personal fees from Daiichi-Sankyo Europe, personal fees from Sanofi SA, personal fees from Aspen Pharmacare, personal fees from Pfizer, personal fees from Armetheon Inc. outside the submitted work.
Supplementary Material
Acknowledgements
We thank the physicians, nurses, and patients involved in the GARFIELD-AF registry, Martin van Eickels (Bayer HealthCare Pharmaceuticals, Berlin, Germany) for his contribution to the study design, and Karen Chiswell and Karen S. Pieper (Duke Clinical Research Institute, Durham, NC, USA) for their statistical expertise. Editorial support was provided by Claire Aukim-Hastie, Emily Chu, Rae Hobbs, and Jane Tricker (TRI). SAS programming support was provided by Jagan Allu (TRI).
References
- 1. Allender S, Scarborough P, Peto V, Rayner M, Leal J, Luengo-Fernandez R, Gray A. European cardiovascular disease statistics, 2008 edition. Brussels: European Heart Network; 2008. [Google Scholar]
- 2. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 4. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 5. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GY, Mantovani LG, Verheugt FW, Jamal W, Misselwitz F, Rushton-Smith S, Turpie AG. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J 2012;163:13–19 e1. [DOI] [PubMed] [Google Scholar]
- 6. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GY, Mantovani LG, Turpie AG, van Eickels M, Misselwitz F, Rushton-Smith S, Kayani G, Wilkinson P, Verheugt FW. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE 2013;8:e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 8. Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 2001;85:85–95. [Google Scholar]
- 9. Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 10. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 11. Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andersson T, Magnuson A, Bryngelsson IL, Frobert O, Henriksson KM, Edvardsson N, Poci D. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J 2013;34:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49:986–992. [DOI] [PubMed] [Google Scholar]
- 14. Gallego P, Roldan V, Marin F, Galvez J, Valdes M, Vicente V, Lip GY. SAMe-TT2R2 score, time in therapeutic range, and outcomes in anticoagulated patients with atrial fibrillation. Am J Med 2014;127:1083–1088. [DOI] [PubMed] [Google Scholar]
- 15. Azoulay L, Dell'Aniello S, Simon TA, Renoux C, Suissa S. Initiation of warfarin in patients with atrial fibrillation: early effects on ischaemic strokes. Eur Heart J 2014;35:1881–1887. [DOI] [PubMed] [Google Scholar]
- 16. Friberg J, Scharling H, Gadsboll N, Truelsen T, Jensen GB. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am J Cardiol 2004;94:889–894. [DOI] [PubMed] [Google Scholar]
- 17. Steinberg JS, Sadaniantz A, Kron J, Krahn A, Denny DM, Daubert J, Campbell WB, Havranek E, Murray K, Olshansky B, O'Neill G, Sami M, Schmidt S, Storm R, Zabalgoitia M, Miller J, Chandler M, Nasco EM, Greene HL. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004;109:1973–1980. [DOI] [PubMed] [Google Scholar]
- 18. Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE, Albert CM. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA 2011;305:2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fauchier L, Samson A, Chaize G, Gaudin AF, Vainchtock A, Bailly C, Cotte FE. Cause of death in patients with atrial fibrillation admitted to French hospitals in 2012: a nationwide database study. Open Heart 2015;2:e000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, Yusuf S. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation 2013;128:2192–2201. [DOI] [PubMed] [Google Scholar]
- 21. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 22. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 23. Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S, Schotten U, Wegscheider K, Boriani G, Brandes A, Ezekowitz M, Diener H, Haegeli L, Heidbuchel H, Lane D, Mont L, Willems S, Dorian P, Aunes-Jansson M, Blomstrom-Lundqvist C, Borentain M, Breitenstein S, Brueckmann M, Cater N, Clemens A, Dobrev D, Dubner S, Edvardsson NG, Friberg L, Goette A, Gulizia M, Hatala R, Horwood J, Szumowski L, Kappenberger L, Kautzner J, Leute A, Lobban T, Meyer R, Millerhagen J, Morgan J, Muenzel F, Nabauer M, Baertels C, Oeff M, Paar D, Polifka J, Ravens U, Rosin L, Stegink W, Steinbeck G, Vardas P, Vincent A, Walter M, Breithardt G, Camm AJ. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options – a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012;14:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation 2012;126:e143–e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail 2011;4:740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–2925. [DOI] [PubMed] [Google Scholar]
- 27. Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, Packer DL, Hammill SC, Shen WK, Gersh BJ. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation 2007;115:3050–3056. [DOI] [PubMed] [Google Scholar]
- 28. Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation 2009;119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation 2011;123:1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 31. Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation 2001;103:2365–2370. [DOI] [PubMed] [Google Scholar]
- 32. Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events – European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014;16:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Ioachim PM, Tica O, Boriani G, Cimaglia P, Diemberger I, Hellum CF, Mortensen B, Maggioni AP. ‘Real-world’ antithrombotic treatment in atrial fibrillation: the EORP-AF pilot survey. Am J Med 2014;127:519–529 e1. [DOI] [PubMed] [Google Scholar]
- 34. Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, Lopez-Sendon J, Vardas PE, Aliot E, Santini M, Crijns HJ. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 2006;27:3018–3026. [DOI] [PubMed] [Google Scholar]
- 35. Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 37. Wieloch M, Sjalander A, Frykman V, Rosenqvist M, Eriksson N, Svensson PJ. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur Heart J 2011;32:2282–2289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.