Abstract
Aims
The primary safety and efficacy endpoints of the randomized FIRE AND ICE trial have recently demonstrated non-inferiority of cryoballoon vs. radiofrequency current (RFC) catheter ablation in patients with drug-refractory symptomatic paroxysmal atrial fibrillation (AF). The aim of the current study was to assess outcome parameters that are important for the daily clinical management of patients using key secondary analyses. Specifically, reinterventions, rehospitalizations, and quality-of-life were examined in this randomized trial of cryoballoon vs. RFC catheter ablation.
Methods and results
Patients (374 subjects in the cryoballoon group and 376 subjects in the RFC group) were evaluated in the modified intention-to-treat cohort. After the index ablation, log-rank testing over 1000 days of follow-up demonstrated that there were statistically significant differences in favour of cryoballoon ablation with respect to repeat ablations (11.8% cryoballoon vs. 17.6% RFC; P = 0.03), direct-current cardioversions (3.2% cryoballoon vs. 6.4% RFC; P = 0.04), all-cause rehospitalizations (32.6% cryoballoon vs. 41.5% RFC; P = 0.01), and cardiovascular rehospitalizations (23.8% cryoballoon vs. 35.9% RFC; P < 0.01). There were no statistical differences between groups in the quality-of-life surveys (both mental and physical) as measured by the Short Form-12 health survey and the EuroQol five-dimension questionnaire. There was an improvement in both mental and physical quality-of-life in all patients that began at 6 months after the index ablation and was maintained throughout the 30 months of follow-up.
Conclusion
Patients treated with cryoballoon as opposed to RFC ablation had significantly fewer repeat ablations, direct-current cardioversions, all-cause rehospitalizations, and cardiovascular rehospitalizations during follow-up. Both patient groups improved in quality-of-life scores after AF ablation.
Clinical trial registration
ClinicalTrials.gov identifier: NCT01490814.
Keywords: Atrial fibrillation, Catheter ablation, Cryoballoon, Rehospitalization, Radiofrequency, Follow-up
See page 2866 for the editorial comment on this article (doi:10.1093/eurheartj/ehw344)
Introduction
FIRE AND ICE was the first large randomized controlled trial to compare the efficacy and safety of the single-step cryoballoon ablation technique with the point-by-point radiofrequency current (RFC) ablation approach in symptomatic patients with paroxysmal atrial fibrillation (AF).1 The trial confirmed its primary efficacy objective of the non-inferiority of cryoballoon ablation at 1 year with estimated event rates (first documented recurrence of AF, occurrence of atrial flutter or atrial tachycardia, use of antiarrhythmic drugs, or repeat ablation outside a 90-day blanking period) of 34.6% in the cryoballoon and 35.9% in the RFC arm [hazard ratio (HR) = 0.96; 95% confidence interval (CI): 0.76–1.22; P < 0.001 for non-inferiority]. Overall safety was also not significantly different (1 year Kaplan–Meier event rate estimates, 10.2% with cryoballoon and 12.8% with RFC; HR = 0.78; 95% CI: 0.52–1.18; P = 0.24).
While the first FIRE AND ICE report focused on primary outcomes, this current report will focus on secondary endpoints and additional analyses that have important implications on daily clinical practice, including all-cause rehospitalization after the index procedure (which encompasses all repeat ablations for atrial arrhythmias), cardiovascular and non-cardiovascular rehospitalizations, and direct-current cardioversions (DCCVs). Also, the analysis includes an assessment of the quality-of-life surveys for both mental and physical function throughout the 30 months of follow-up.
Methods
Study design and procedure overview
The FIRE AND ICE trial (NCT01490814) was a multicentre, randomized, blinded-outcomes, parallel-group evaluation of cryoballoon and RFC catheter ablation in patients being treated for drug-refractory and symptomatic paroxysmal AF.1,2 A study design manuscript and the primary endpoints have been separately published.1,2 In brief, each treatment centre approved the study design with their local ethics review committee(s), and patient informed consent was obtained prior to enrolment. Patients were randomized to either cryoballoon ablation using the Arctic Front™ family of catheters (Medtronic) or RFC catheter ablation using the ThermoCool® series of catheters (Biosense Webster).
During the index procedure, the (1:1) randomly selected ablation modality was used in the left atrium to electrically isolate the pulmonary veins (PVs).3–5 In the cryoballoon procedures, pulmonary vein isolation (PVI) was achieved using fluoroscopic guidance to position the cryoballoon catheter. Once PV-to-balloon occlusion was confirmed by retrograde radiopaque contrast agent retention, circumferential ablation was performed by freezing with a ‘single-shot’ delivery of coolant to the balloon. In the RFC procedure, PVI was achieved using a focal ‘point-by-point’ catheter approach, which delivers heat energy to the cardiac tissue. RFC lesion sets encircle the PV antra using electroanatomical mapping for guidance. In both cohorts, a PVI-only strategy was used to ablate AF, and acute index procedure success was documented in both arms with diagnostic testing. All investigators demonstrated the success of PVI by the abolition of conduction of atrial impulses into the PVs. After the index procedure, subjects were followed in this study for up to 33 months.1,2 In both groups, subjects were followed-up for a mean time of 1.54 ± 0.8 years.1
In the publication of the primary endpoints, the data were presented as time-to-first event reported per subject, and a 90-day blanking period was predefined.1 Recurrences of atrial arrhythmias and repeat ablations within the 90-day blanking period did not contribute towards the primary endpoint.1 However, robust clinical data were collected (on reinterventions and rehospitalizations) in this trial both within the 90-day blanking period and after the primary endpoint event to allow for the analyses of the current presented endpoints. The data presented in this current analysis are the total clinical events that were documented in this trial from the index procedure through the study exit for each subject to provide a comprehensive summary of the disease burden to the patients and to the healthcare systems.
Rehospitalization
During the trial, study sites were required to report all patient rehospitalizations after the index ablation procedure.2 All-cause rehospitalizations are inclusive of every recorded event, and cardiovascular rehospitalizations are a subset of all-cause rehospitalizations. Both repeat ablations and DCCVs are specific rehospitalization events that occur within cardiovascular rehospitalizations. In the study, a rehospitalization was defined as a prolonged stay (i.e. of two or more nights) or an in-patient stay of more than one calendar day (i.e. at least one overnight stay) not concurrent with the index ablation procedure. The pre-specified main causes for cardiovascular rehospitalization included (i) unstable or stable angina or atypical chest pain; (ii) syncope; (iii) transient ischaemic attack or stroke; (iv) non-fatal cardiac arrest; (v) ventricular arrhythmia; (vi) cardiac transplantation; (vii) any type of cardiovascular surgery; (viii) implantation of a pacemaker, implantable cardioverter defibrillator, or any other cardiac device; (ix) percutaneous coronary, cerebrovascular, or peripheral intervention; (x) blood-pressure-related rehospitalization; (xi) cardiovascular infection; (xii) major bleeding; (xiii) pulmonary embolism or deep vein thrombosis; and (xiv) other adjudicated cardiovascular events, including atrial arrhythmias. A complete list of cardiovascular rehospitalization events are given in the Supplementary material online (see Supplementary material online, Table S3). Any hospital stay planned prior to the index intervention was not considered a rehospitalization in this analysis.
Quality-of-life
Quality-of-life was assessed using the Medical Outcome Study Short Form-12 (SF-12) questionnaire to evaluate the subject's mental and physical performance at baseline and every 6 months after the index ablation procedure for up to 30 months. Physical and mental health composite scores are calculated using responses to 12 questions with a response range from 0 to 100, where a 0 score indicates the lowest level of health measured by the scale and 100 indicates the highest level of health. Questionnaires were completed without input from study personnel. Additionally in this study, an estimate of generic health status was made using the EuroQol five-dimension (EQ-5D) questionnaire, during the same period of examination. The EQ-5D-3L (used in this study) measures five dimensions of health, including (i) mobility, (ii) self-care, (iii) physical activities, (iv) pain and discomfort, and (v) anxiety and depression. This survey uses a three-tier evaluation indicating no problem, some problem, or extreme problem. The EQ-5D-3L uses a scale that measures up to one, which is the best health imaginable, and all measurements (in this study) were analysed using the utility score based on the US value set.
Statistical analyses
Data analyses were conducted using the modified intention-to-treat (mITT) study populations (consistent with previous presentations of the data).1,2 The mITT evaluation consisted of those patients that were enrolled and randomized (n = 762), with subjects removed due to screening failures (n = 6), withdrawal of consent (n = 4), declining ablation (n = 1), and being mentally unfit for study (n = 1).1 In the mITT examination, 374 subjects comprised the cryoballoon group, and 376 subjects were included in the RFC group.1 This cohort is inclusive of four subjects treated with a non-study RFC catheter (St. Jude Medical), and five subjects treated with a ThermoCool RFC catheter originally randomized to the cryoballoon group.
Repeat ablations, rehospitalizations, and DCCVs are presented in two formats: as dot plots showing the number of events by days since the index procedure and as Kaplan–Meier event-free survival curves. Time-to-first events between the cryoballoon and RFC arms are compared with the log-rank test. Cox proportional-hazards regression model was utilized to assess consistency across subgroups for cardiovascular hospitalization rates. For each subgroup, a Wald test for interaction was performed. A subgroup interaction term was considered significant for P < 0.05. Kaplan–Meier survival curves are presented for the subgroups with significant interaction terms. Hazard ratios for hospitalization rates and subgroup hospitalization rates are estimated with a Cox proportional-hazards model.
Changes in quality-of-life (SF-12 and EQ-5D-3L scores) are presented as mean ± standard deviation from baseline. Box plots are utilized to graphically display the changes in SF-12 scores over the study visits. A linear mixed model (accounting for repeated quality-of-life measurements within a subject across visits) was utilized to compare quality-of-life scores between the groups. t-Tests were utilized to assess quality-of-life changes from baseline within a group.
Quality-of-life, cardiovascular rehospitalization, and repeat ablation examinations were predefined in the study protocol and the statistical analysis plan. All-cause hospitalization and DCCV analyses were not predefined; however, the data fields were present in the case report form. No adjustments were made for multiple testing. All analyses were conducted using SAS software, version 9.4 (SAS Institute), and the R statistical package, version 3.2.2 (www.r-project.org).
Results
Rehospitalization
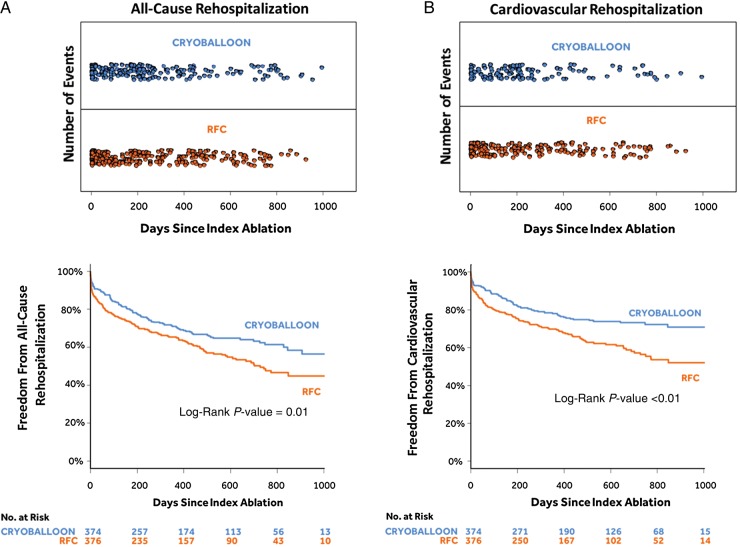
As depicted in Figure 1A, 49 repeat ablations were conducted in 44 subjects (44/374; 11.8%) in the cryoballoon group and 70 repeat ablations were conducted in 66 subjects (66/376; 17.6%) in the RFC group. As shown in Figure 1B, a log-rank test confirmed that the cryoballoon group had a statistically significant lower rate of repeat ablation compared with the RFC group [HR = 0.65 (95% CI: 0.45–0.95); P = 0.03]. As shown in Figure 2A, 210 all-cause rehospitalizations occurred in 122 subjects (122/374; 32.6%) in the cryoballoon group when compared with 267 all-cause rehospitalizations that occurred in 156 subjects (156/376; 41.5%) in the RFC group. A log-rank test confirmed that there was a statistical difference, with the cryoballoon cohort having fewer all-cause rehospitalizations [HR = 0.72 (95% CI: 0.57–0.91); P = 0.01; Figure 2A]. When examining cardiovascular rehospitalizations, Figure 2B demonstrates that 139 cardiovascular rehospitalizations occurred in 89 subjects (89/374; 23.8%) of the cryoballoon group, whereas 203 cardiovascular rehospitalizations occurred in 135 subjects (135/376; 35.9%) of the RFC group. Statistical analysis by log-rank test demonstrated that the cryoballoon group had fewer cardiovascular rehospitalizations compared with the RFC group [HR = 0.61 (95% CI: 0.47–0.80); P < 0.01; Figure 2B]. The entire rehospitalization data set is summarized in Table 1, and the cardiovascular rehospitalizations are further detailed in the Supplementary material online.
Figure 1.
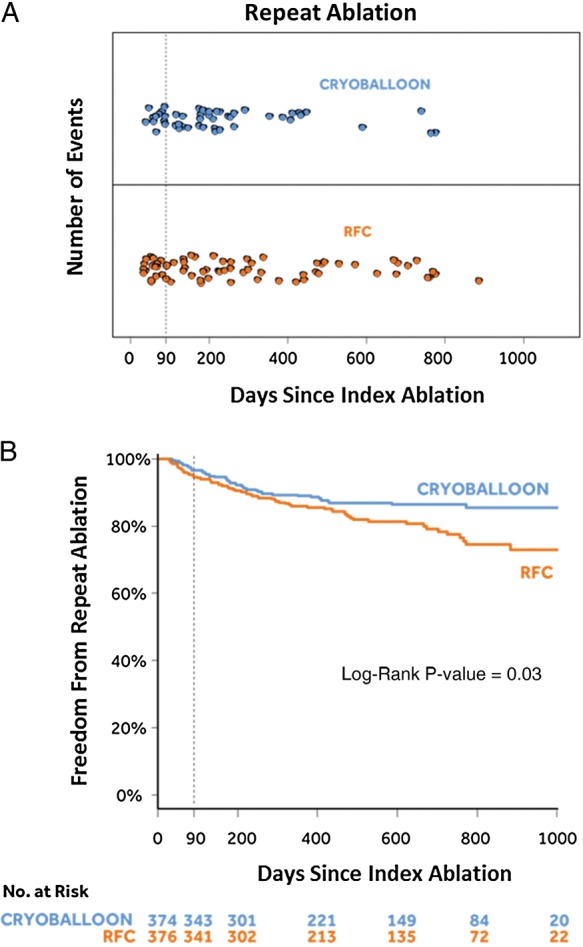
Repeat ablations. (A) Number of repeat ablations by days since the index ablation. (B) Kaplan–Meier event-free survival curves.
Figure 2.
All-cause rehospitalizations (A) and cardiovascular rehospitalizations (B). Both panels show the number of rehospitalizations by days since the index ablation in the upper panel and Kaplan–Meier event-free survival curves in the lower panel.
Table 1.
Summary of rehospitalization data
| Total number of events; subjects with events |
Hazard ratio (95% CI) | P-value | ||
|---|---|---|---|---|
| Cryoballoon (n = 374) | RFC (n = 376) | |||
| All-cause rehospitalizations | 210; 122 (32.6%) | 267; 156 (41.5%) | 0.72 (0.57–0.91) | 0.01 |
| Cardiovascular rehospitalizations | 139; 89 (23.8%) | 203; 135 (35.9%) | 0.61 (0.47–0.80) | <0.01 |
| Repeat ablations | 49; 44 (11.8%) | 70; 66 (17.6%) | 0.65 (0.45–0.95) | 0.03 |
| Direct-current cardioversions | 13; 12 (3.2%) | 28; 24 (6.4%) | 0.49 (0.25–0.98) | 0.04 |
Direct-current cardioversions post-ablation
As shown in Figure 3A, 13 DCCVs were administered during follow-up to 12 subjects (12/374; 3.2%) in the cryoballoon group, whereas 28 DCCVs were given to 24 subjects (24/376; 6.4%) in the RFC group. As illustrated in Figure 3B, a log-rank test demonstrated a significant difference between the cryoballoon group and the RFC group with regard to DCCV after the index ablation [HR = 0.49 (95% CI: 0.25–0.98); P = 0.04].
Figure 3.
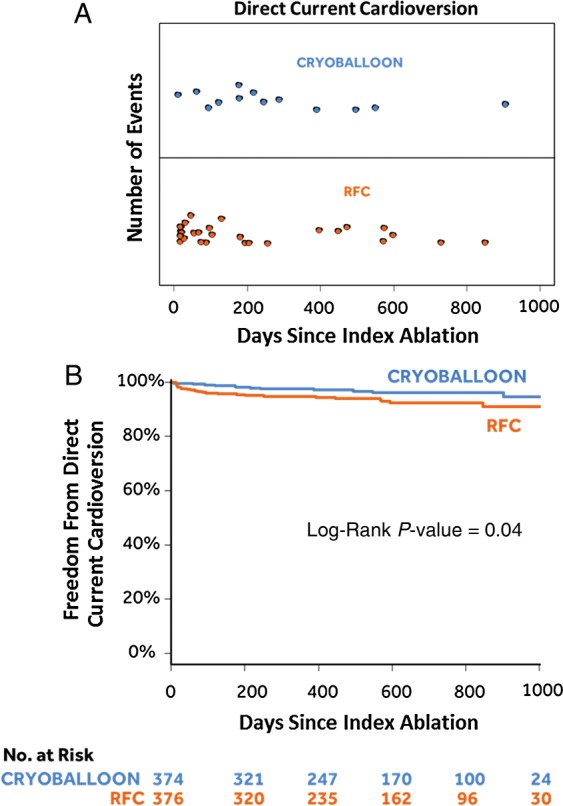
Direct-current cardioversion. (A) Number of direct-current cardioversions by days since the index ablation. (B) Kaplan–Meier event-free survival curves.
Cardiovascular rehospitalization subgroup analyses
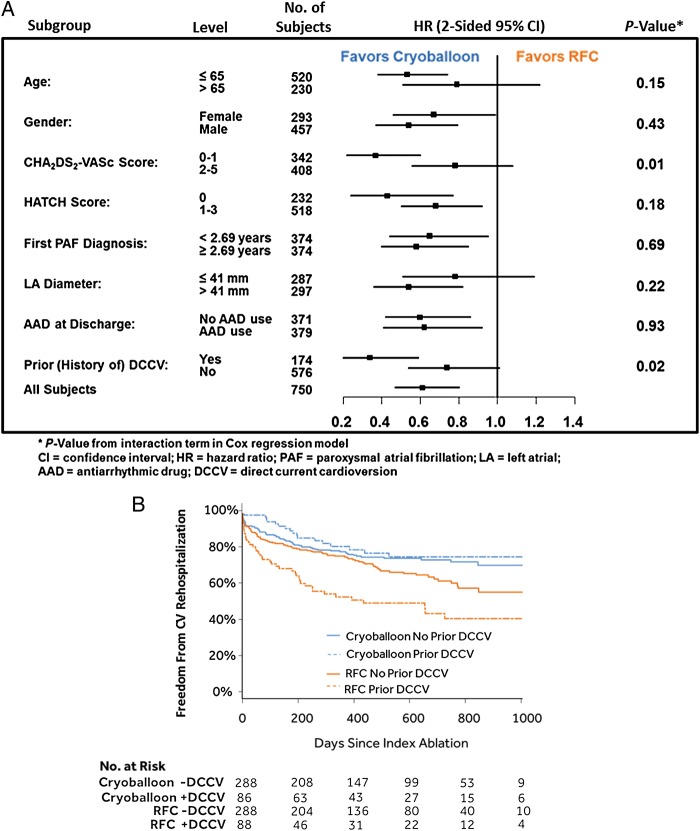
Subgroup analyses of cardiovascular hospitalization rates are displayed in Figure 4A. For all subgroups, cardiovascular rehospitalization rates were consistently lower in the cryoballoon group (all HRs <1). Two subgroups had significant interaction terms, prior (history of) DCCV (P = 0.02) and CHA2DS2-VASc (P = 0.01), indicating the treatment effect as measured by cardiovascular rehospitalization rate was different within the subgroups. In the prior DCCV subgroup, cardiovascular rehospitalization rates were 20.9% (18/86) in the cryoballoon group vs. 48.9% (43/88) in the RFC group [HR = 0.34 (95% CI: 0.20–0.59); P < 0.01]. In the no prior DCCV subgroup, cardiovascular rehospitalization rates were 24.7% (71/288) in the cryoballoon group vs. 31.9% (92/288) in the RFC group [HR = 0.74 (95% CI: 0.54–1.01); P = 0.05]. The cryoballoon group had significantly lower cardiovascular rehospitalization rates in each subgroup, even more so in the prior DCCV subgroup (Figure 4B). In the CHA2DS2-VASc (0–1 subgroup), cardiovascular rehospitalization rates were 13.3% (22/166) in the cryoballoon group vs. 31.8% (56/176) in the RFC group [HR = 0.36 (95% CI: 0.22–0.60); P < 0.01]. In the CHA2DS2-VASc (2–5 subgroup), cardiovascular rehospitalization rates were 32.2% (67/208) in the cryoballoon group vs. 39.5% (79/200) in the RFC group [HR = 0.78 (95% CI: 0.56–1.07); P = 0.13]. The cryoballoon group had lower cardiovascular rehospitalization rates in each subgroup but more significant in the CHA2DS2-VASc 0–1 subgroup.
Figure 4.
Cox proportional-hazards regression model. (A) Subgroup analyses of cardiovascular rehospitalization. (B) Kaplan–Meier event-free survival curves by history of direct-current cardioversion subgroups.
Quality-of-life
As demonstrated in Figure 5, there was an improvement in the quality-of-life parameters (both mental and physical) when evaluating patients with the SF-12 questionnaire throughout the 30 months of follow-up. There was no statistical difference between the ablation modalities. The improvements in both mental and physical scores were first observed at 6 months of follow-up in both ablation groups, and they were maintained throughout the study out to the 30-month follow-up visit. Table 2 summarizes the SF-12 and EQ-5D-3L results at 6 and 12 months, with a test of significance conducted between baseline and 6 months results.
Figure 5.
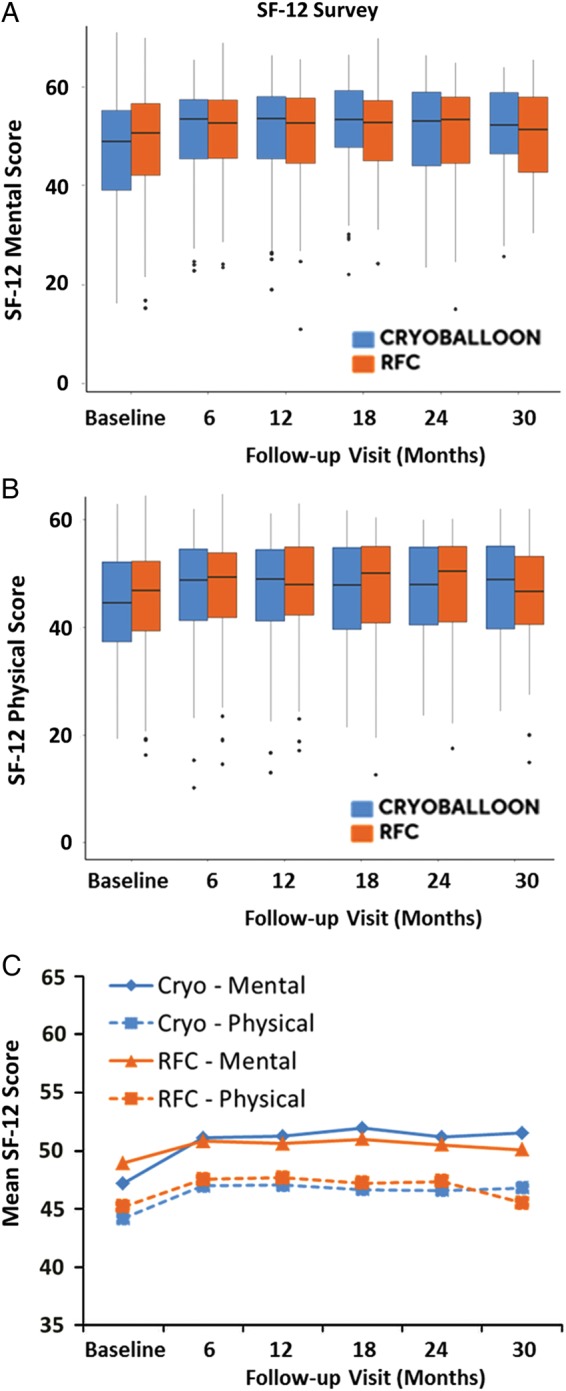
Graphs of quality-of-life (Short Form-12 physical and mental) scores. (A) Subject mental performance from baseline throughout 30 months of follow-up. (B) Physical performance of subjects throughout 30 months of follow-up. (C) The mean Short Form-12 score reported across both mental and physical scores through the 30 months of follow-up.
Table 2.
Summary of Short Form-12 and EuroQol five-dimension three-level data out to 6 and 12 months
| Survey | Groupa | Baseline | 6 months |
12 months |
|||||
|---|---|---|---|---|---|---|---|---|---|
| n | 6 months | Difference | P-valueb | n | 12 months | Difference | |||
| SF-12 mental | Cryoballoon | 47.1 ± 10.3 | 258 | 51.1 ± 8.9 | 4.0 ± 9.8 | <0.01 | 236 | 51.2 ± 9.4 | 3.7 ± 10.6 |
| RFC | 48.9 ± 9.8 | 267 | 50.8 ± 8.8 | 1.9 ± 9.9 | <0.01 | 230 | 50.7 ± 9.2 | 1.6 ± 10.8 | |
| SF-12 physical | Cryoballoon | 43.7 ± 9.1 | 258 | 47.0 ± 9.1 | 3.2 ± 8.2 | <0.01 | 236 | 47.0 ± 9.2 | 3.0 ± 8.7 |
| RFC | 44.5 ± 9.5 | 267 | 47.6 ± 8.6 | 3.1 ± 8.6 | <0.01 | 230 | 47.8 ± 8.4 | 3.3 ± 8.7 | |
| EQ-5D-3L | Cryoballoon | 0.85 ± 0.14 | 274 | 0.89 ± 0.13 | 0.03 ± 0.14 | <0.01 | 257 | 0.88 ± 0.13 | 0.03 ± 0.14 |
| RFC | 0.87 ± 0.12 | 287 | 0.88 ± 0.14 | 0.02 ± 0.14 | 0.03 | 254 | 0.88 ± 0.13 | 0.01 ± 0.14 | |
aA linear mixed model was utilized to compare groups across all study visits. No differences were observed between arms in quality-of-life metrics.
b t-Test, change from baseline to 6 months.
Trial cohorts
Throughout this analysis, the mITT cohort was utilized; however, it is noted that the study can be presented using an ‘as-treated’ cohort. Specifically, nine patients may be re-assigned to evaluate the data set by the ablation modality used in the index procedure. In an as-treated cohort, four subjects treated with a non-study RFC catheter (St. Jude Medical) were removed from the data set, and five subjects treated with a ThermoCool RFC catheter (but randomized to the cryoballoon group) were removed from the cryoballoon group and re-allocated to the RFC group. An examination of the as-treated cohorts is given in the Supplementary material online. The results of the mITT and as-treated analyses were almost identical, and only post-ablation DCCVs changed in statistical significance when examining the as-treated cohort.
Discussion
The FIRE AND ICE trial showed non-inferiority in efficacy and safety of cryoballoon vs. RFC ablation in symptomatic patients with paroxysmal AF.1 However, the analyses of the secondary endpoints as shown in this article demonstrated significant differences during follow-up in favour of cryoballoon ablation, which are clinically relevant and of importance from a patient disease burden and health economical point of view.6,7 Mainly, the cryoballoon ablation group had significantly fewer reinterventions (repeat ablations and DCCVs), all-cause rehospitalizations, and cardiovascular rehospitalizations. Only the quality-of-life measurements were not statistically different between groups. But both trial groups showed improvements in the mental and physical aspects of the quality-of-life assessment at 6 months after the index ablation, which were maintained throughout the 30 months of follow-up.
Previous studies mainly in patients undergoing RFC ablation have shown that hospitalization rates are nearly 40% (within the first year) following AF ablation, with ∼10% occurring within the first 30 days post-ablation.6,7 Atrial firillation-related readmission rates were as high as 22% at the 12-month follow-up.6,7 Also, ∼10% of all patients underwent a repeat ablation, exposing patients to the same potential of procedural complications as during the index ablation.4,6,7 In FIRE AND ICE, very similar rates were found across the entire study for repeat ablation, all-cause rehospitalization, and cardiovascular rehospitalization. However, when comparing both ablation groups, there was a decrease in rehospitalization rates for the cryoballoon group when examining relative reductions, including a 33% reduction in repeat ablations, a 50% reduction in DCCVs, a 21% reduction in all-cause rehospitalizations, and a 34% reduction in cardiovascular rehospitalizations. By comparison, the FreezeAF study (a smaller single-centre study of cryoballoon vs. RFC evaluating mostly first generation catheters; NCT00774566) did not detect a statistical difference in redo procedures (19.9% with cryoballoon vs. 19.5% with RFC; P = 0.933).8 However, the redo procedure criteria in FreezeAF were different. Notably, redo procedures were allowed only after 6 months, and the study follow-up concluded at 12 months. Consequently, the FreezeAF study gave only a snapshot of the redo procedures that occurred within a duration of 6 months. Our discrepancy (with the FreezeAF study) potentially demonstrates that the cryoballoon reductions in reinterventions may be more observable on a larger scale (across healthcare systems or larger clinical studies); it may reflect the low usage of advanced generation ablation catheters in the FreezeAF study; and/or it may reflect the small window (time duration) of redo ablations observed in the FreezeAF study.
The significant reductions in reinterventions such as repeat ablations and DCCVs indicate that the primary endpoint (i.e. time-to-first recurrence of atrial tachycardia or AF, re-initiation of antiarrhythmic drug therapy, or repeat ablation) does not fully reflect the benefit of catheter ablation in patients with AF and the technique used to achieve PVI.4 Another important result (namely, the reduction in healthcare burden) is not reflected by the time-to-first event but by the total number of reinterventions and rehospitalizations.9–11 In our analysis, cryoballoon ablation reduced all reintervention and rehospitalization endpoints to a significantly greater amount than RFC ablation, indicating a larger effect of cryoablation on healthcare burden reduction than compared with RFC ablation.
The extent of reduction in reinterventions and rehospitalizations is not only statistically significant but also clinically relevant.6,7 Our presented data are the main events that define the patients' perception regarding the procedural success of an AF ablation procedure. Potentially, these data are as important as the primary endpoint outcomes of a traditional clinical trial (time-to-recurrence/failure). The lower number of reinterventions and rehospitalizations also has a health economic effect, particularly in times of limited resources in most healthcare systems.6,7 A relative reduction in repeat ablations, cardioversions, and rehospitalizations ranging from 21 to 50% as shown in this study is particularly meaningful if the overall procedural costs are taken into account. Therefore, this result should be considered when physicians need to decide which ablation modality (cryoballoon or RFC) should be selected for PVI in patients with AF.
Lastly, in this study, there was an improvement in mental and physical quality-of-life that was apparent at 6 months following the index ablation and maintained throughout the 30 months of follow-up. This improvement was observed across both ablation modalities, and there was no statistical difference between ablation groups. The marked improvement at 6 months is consistent with the alleviation of atrial arrhythmia symptoms.4 However, the SF-12 and EQ-5D-3L surveys can be imprecise tools when evaluating patient performance by ablation modalities because of the typically shorter follow-up periods when comparing between groups (<5–10 years).12 Additionally (specific for the EQ-5D-3L), the three-level scoring system allows for wide usage because of the ease of scoring, but the survey suffers from the lack of specificity that is found in more detailed questionnaires that do not have a ceiling effect.13,14 Importantly, longer term follow-up is needed or disease-specific questionnaires must be used when comparing between ablation modalities.
Limitations
The data presented in this study were collected during the FIRE AND ICE trial which was a non-inferiority study with a traditional 90-day blanking period - an appropriate and common clinical trial practice during the time of the subjects' enrolment into the study.1,2,4 It is reasonable that this could have influenced some operators in some of the clinical judgement(s) (for instance, repeat ablations and DCCVs during the 90-day blanking period were performed without penalty in the data analyses of the primary endpoints), but these 90-day events were included in this current study examination of reinterventions and rehospitalizations for evaluation completeness. Also, the data examined in this study were secondary endpoints, and some analyses were not predefined but have been included due to the clinical implications to the patients.
Conclusions
When comparing cryoballoon and RFC catheter ablation in symptomatic patients with paroxysmal AF, this study demonstrated statistically significant and clinically relevant advantages for patients treated with cryoballoon ablation in terms of repeat ablations, DCCVs, all-cause rehospitalizations, and cardiovascular rehospitalizations. Both patient groups improved in quality-of-life scores after AF ablation. The endpoint events captured in this current analysis are important to the patient's perception of a successful AF ablation procedure and to the burden of disease on the healthcare systems.
Supplementary material
Supplementary material is available at European Heart Journal online.
Author's contributions
M.S., F.J.K., and H.W.L. performed statistical analyses. K.-H.K. handled funding and supervision. K.-H.K., A.F., A.E., A.M., F.O., K.R.J.C., T.A., J.-P.A., C.T., C.S., M.K., and J.B. acquired the data. K.-H.K., A.F., J.B., J.P.A., K.R.J.C., C.T., F.O., C.S., and M.K. conceived and designed the research. K.-H.K., M.S., and H.W.L. drafted the manuscript. K.-H.K., M.S., J.B., and A.F. made critical revisions of the manuscript for key intellectual content.
Funding
The FIRE AND ICE trial was funded by Medtronic.
Conflict of interest: K.-H.K. reports personal fees from Medtronic and Biosense Webster during the conduct of the study and personal fees from St. Jude Medical outside the submitted work. A.F. reports personal fees from Medtronic, both during the conduct of the study and outside the submitted work. A.M. reports personal fees from Medtronic outside the submitted work. K.R.J.C. reports grant support and personal fees from Medtronic during the conduct of the study and personal fees from Biosense Webster outside the submitted work. H.W.L. is an employee of Medtronic. F.J.K. is an employee of Medtronic. T.A. reports grants support and personal fees from Medtronic during the conduct of the study. J.-P.A. reports personal fees from St. Jude Medical and Biosense Webster outside the submitted work. M.K. reports personal fees from Medtronic outside the submitted work. C.S. reports personal fees and grants from Medtronic and Biosense Webster outside the submitted work.
Supplementary Material
Acknowledgement
The authors would like to thank Ralf Meyer, PhD, for study support.
References
- 1. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KR, Elvan A, Arentz T, Bestehorn K, Pocock SJ, Albeneque JP, Tondo C, for the FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–2245. [DOI] [PubMed] [Google Scholar]
- 2. Fürnkranz A, Brugada J, Albenque JP, Tondo C, Bestehorn K, Wegscheider K, Ouyang F, Kuck KH. Rationale and Design of FIRE AND ICE: a multicenter randomized trial comparing efficacy and safety of pulmonary vein isolation using a cryoballoon versus radiofrequency ablation with 3D-reconstruction. J Cardiovasc Electrophysiol 2014;25:1314–1320. [DOI] [PubMed] [Google Scholar]
- 3. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 4. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, Di Marco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haïssaguerre M, Hindricks G, Iseka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kimagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nadamanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Paponne C, Prystowsky E, Raviele E, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 5. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albengue JP, Nardi S, Menardi E, Novak P, Sanders P, for the STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 6. Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol 2012;59:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Opolski G, Januszkiewicz Ł, Szczerba E, Osinska B, Rutkowski D, Kalarus Z, Kazmierczak J. Readmissions and repeat procedures after catheter ablation for atrial fibrillation. Cardiol J 2015;22:630–636. [DOI] [PubMed] [Google Scholar]
- 8. Luik A, Radzewitz A, Kieser M, Walter M, Bramlage P, Hörmann P, Schmidt K, Horn N, Brinkmeier-Theofanopoulou M, Kunzman K, Riexinger T, Schymik G, Merkel M, Schmitt C. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: the prospective, randomized, controlled, noninferiority FreezeAF study. Circulation 2015;132:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pürerfellner H, Aichinger J, Martinek M, Nesser HJ, Ziegler P, Koehler J, Warman E, Hettrick D. Quantification of atrial tachyarrhythmia burden with an implantable pacemaker before and after pulmonary vein isolation. Pacing Clin Electrophysiol 2004;27:1277–1283. [DOI] [PubMed] [Google Scholar]
- 10. Hindricks G, Piorkowski C, Tanner H, Kobza R, Gerds-Li JH, Carbucicchio C, Kottkamp H. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation 2005;112:307–313. [DOI] [PubMed] [Google Scholar]
- 11. Steven D, Rostock T, Lutomsky B, Klemm H, Servatius H, Drewitz I, Friedrichs K, Ventura R, Meinertz T, Willems S. What is the real atrial fibrillation burden after catheter ablation of atrial fibrillation? A prospective rhythm analysis in pacemaker patients with continuous atrial monitoring. Eur Heart J 2008;29:1037–1042. [DOI] [PubMed] [Google Scholar]
- 12. Neyt M, Van Brabandt H, Devos C. The cost-utility of catheter ablation of atrial fibrillation: a systematic review and critical appraisal of economic evaluations. BMC Cardiovasc Disord 2013;13:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bharmal M, Thomas J. Comparing the EQ-5D and the SF-6D descriptive systems to assess their ceiling effects in the US general population. Value Health 2006;9:262–271. [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Han Y, Zhao FL, Zhou J, Chen Z, Sun H. Validation and comparison of EuroQoL-5 dimension (EQ-5D) and Short Form-6 dimension (SF-6D) among stable angina patients. Health Qual Life Outcomes 2014;12:e2–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.