Heart failure (HF) with preserved ejection fraction (HFpEF) has traditionally been viewed as a form of hypertensive heart disease with increased left ventricular (LV) afterload ultimately leading to HFpEF. However, HFpEF patients typically have numerous other comorbidities1. While the severity of LV remodeling and ventricular vascular dysfunction2 and the rate of adverse outcomes3 are greater in HFpEF than in patients with comorbidities alone, some argue that HFpEF is not a distinct syndrome but rather a collection of comorbidities masquerading as HF.
More recently, based on integrative physiologic studies in human HFpEF, the biology of nitric oxide (NO) - cyclic guanosine monophosphate (cGMP) – protein kinase G (PKG) signaling and elegant studies of human HFpEF myocardium, Paulus and Tschope synthesized an alternate HFpEF pathophysiologic paradigm1. This paradigm postulates that multi-morbidity creates a systemic pro-inflammatory milieu, coronary microvascular endothelial inflammation and migration of inflammatory cells to the myocardium with production of pro-inflammatory cytokines, myocardial inflammation and subsequent fibrosis. Further, they propose that microvascular endothelial inflammation promotes oxidative stress with generation of oxidative/nitrosative species which limit myocardial NO-cGMP-PKG signaling by diminishing NO bioavailability and oxidizing downstream NO targets, thus promoting cardiomyocyte hypertrophy. Impaired PKG activity and peroxynitrite stimulated phosphatases are proposed to alter the phosphorylation status of proteins which influence myofiber relaxation and stiffness and thus, further impair LV diastolic function.
This paradigm has sparked great interest in therapies which target impaired myocardial cGMP-PKG signaling in HFpEF. However, the Paulus paradigm has an important correlate in that an established consequence of coronary microvascular endothelial inflammation is impaired coronary microvascular endothelial function and ultimately coronary microvascular remodeling and rarefaction with decreased microvascular density (MVD)4. The potential impact of diffuse microvascular endothelial inflammation on the functional and structural integrity of the microcirculation and the role that microcirculatory dysfunction itself may play in HFpEF pathophysiology are unclear. In this issue of Circulation Heart Failure, Srivaratharajah et al5 add to a small but growing body of literature suggesting a role for microvascular dysfunction in the pathophysiology of HFpEF.
The coronary vasculature in health and disease
Coronary arterial function is highly coordinated6, 7 with flow regulated by shear stress and endothelial function (flow mediated vasodilatation) in the epicardial arteries and prearterioles, myogenic tone in prearterioles and larger arterioles and metabolic vasodilatation in the arterioles during increases in myocardial oxygen consumption. This coordinated network serves to match coronary blood flow with myocardial oxygen requirements.
Impaired microcirculatory function related to endothelial dysfunction is seen in patients with atherosclerotic risk factors6, 7. These comorbidities and coronary atherosclerosis are common in HFpEF2, 8, 9. Ultimately, perivascular fibrosis, microvascular remodeling and microvascular rarefaction can further limit microcirculatory function. Such structural alterations are seen in myocardial diseases including dilated and hypertrophic cardiomyopathies and LV hypertrophy related to hypertension or aortic stenosis6, 7.
Despite documentation of coronary microvascular dysfunction in patients with atherosclerotic risk factors or significant myocardial disease, convincing evidence of a causal relationship between coronary microvascular dysfunction and myocardial ischemia has been difficult to establish. Epicardial coronary artery stenoses occur proximally in the coronary vascular network and cause global or large regional areas of ischemia with stress, increased O2 extraction and thus, increased transcardiac O2 gradients with readily detectable contractile dysfunction and production of ischemic metabolites7, 10. In contrast, microvascular dysfunction occurs in the terminal vascular network and causes increased heterogeneity of flow and oxygen supply which leads to functional shunting, localized hypoxia, reduced O2 extraction and decreases in the transcardiac O2 gradient. The patchy impairment in perfusion may make detection of contractile abnormalities or production of ischemic metabolites using traditional techniques difficult7.
Tools to assess coronary microvascular function
Animal and a few human studies have utilized invasive assessment of coronary blood flow, oxygen extraction, transcardiac O2 and metabolite gradients and intra-coronary infusion of endothelium-specific and non-specific vasodilators to assess the microvasculature6, 7, 10–12. Myocardial blood flow (MBF) indexed to LV mass and corrected for demand (typically using the rate pressure product) can be measured non-invasively with cardiac positron emission tomography (PET) using a variety of tracers13. Alternatively, cardiac magnetic resonance (CMR) phase contrast imaging of the coronary sinus can measure myocardial (coronary sinus) blood flow14. By either method, myocardial flow reserve (MFR) can be assessed as the ratio of maximal hyperemic to resting MBF. Maximal hyperemic MBF is assessed by the administration of intravenous vasodilators, typically adenosine but dipyridimole or ATP are also used. In the absence of epicardial coronary stenosis, adenosine-induced hyperemic MBF response (and MFR) represents global microvascular function and cannot discriminate between endothelial dysfunction, impaired myogenic responsiveness or altered microvascular structure13.
Coronary microvascular dysfunction and HFpEF
Numerous studies have documented impaired systolic and diastolic cardiac reserve in HFpEF and ischemia due to coronary microvascular dysfunction could contribute to impaired reserve function15. Angina is common in HFpEF but equally common in patients with or without epicardial coronary artery disease8. Moreover, energetic reserve deficit (reduced cardiac creatine phosphate/adenosine triphosphate ratio) has been reported in HFpEF without epicardial coronary artery disease16.
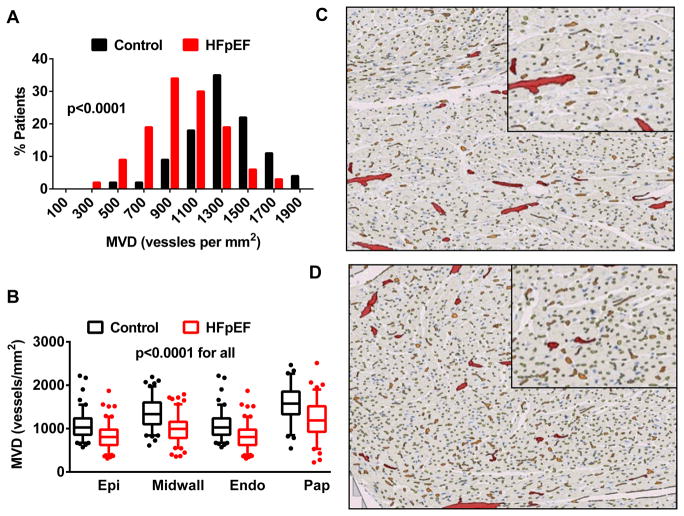
To date, only a few studies have assessed coronary microvascular dysfunction in HFpEF. We studied coronary MVD in autopsy specimens from 124 HFpEF subjects and found that MVD was decreased in HFpEF relative to 104 sex and age appropriate controls who died of non-cardiac causes (Figure 1)9. Further, the severity of microvascular rarefaction was associated with the severity of myocardial fibrosis, consistent with the proposed relationship between microvascular endothelial inflammation, myocardial fibrosis and microvascular rarefaction. Importantly, MVD was reduced independently of epicardial coronary artery atherosclerosis or history of hypertension.
Figure 1.
Microvascular density (MVD) assessed in myocardial autopsy specimens from patients with heart failure and preserved ejection fraction (HFpEF) and similarly aged patients dying of non-cardiovascular causes (Controls). The differential distribution of global average (A) and regional (B) MVD in the two groups and representative examples from HFpEF (C) and Control (D) patients are shown. Abbreviations: epi, epicardial; midwall, mid-myocardial, endo, endocardial; pap, papillary muscle. Reproduced with permission from Mohammed et al9. © 2015 Wolters Kluwer.
van Empel performed assessment of rest and exercise transcardiac O2 gradients, invasive hemodynamics and echocardiography in nine HFpEF patients without coronary disease and hypertensive (n=7) and healthy (n=12) controls11. The increase in the transcardiac O2 gradient with stress was blunted in HFpEF as compared to either control group, consistent with impaired O2 extraction and microcirculatory dysfunction as outlined above7. Further, the severity of stress induced diastolic dysfunction correlated with the severity of impairment in O2 extraction.
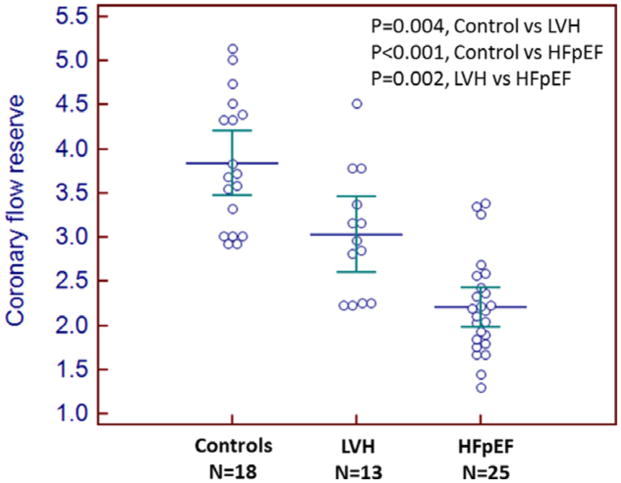
Very recently, Kato et al reported a study which prospectively enrolled 25 HFpEF patients (European Society of Cardiology criteria), 13 non-HF controls with hypertensive LV hypertrophy and 18 healthy non-HF controls17. All participants underwent BNP measurement, echo, CMR and computed tomographic imaging to exclude epicardial coronary artery disease. MFR was assessed using CMR and ATP infusion. MFR was lower in HFpEF than either control population (Figure 2). HFpEF patients (109 g/m2) had higher LV mass index than normal controls (60 g/m2) but hypertensive controls (132 g/m2) had significantly higher LV mass than HFpEF patients, suggesting that hypertension and LV hypertrophy related microvascular dysfunction does not solely account for the reduction in MFR observed in HFpEF. HFpEF patients with lower MFR had higher BNP levels.
Figure 2.
Comparison of coronary flow reserve between heart failure and preserved ejection fraction (HFpEF) patients, non-HF hypertensive patients with ventricular hypertrophy (LVH) and healthy (Controls) patients. Reproduced from Kato et al17 with permission. © 2016 The Authors.
Srivaratharajah et al now confirm these previous findings using a convenience sample of patients referred to cardiac PET for evaluation of clinically suspected coronary artery disease due to symptoms (chest pain, dyspnea or arrhythmia), high risk factor profile or positive stress tests.5 Over 1000 PET-referred patients had a normal ejection fraction and no PET-evidence of epicardial coronary artery disease. Of these, 78 were retrospectively identified to have a previous diagnosis of HFpEF by chart review and were compared to 112 normotensive and 186 hypertensive controls. MFR was assessed by Rb-82 PET and dipyridamole infusion. MFR was lower in HFpEF patients than either control and among HFpEF patients, MFR was lower in HFpEF patients with more severe symptoms. Further, MFR was lower in HFpEF patients after adjusting for pertinent covariates. Only a small number of HFpEF patients had epicardial coronary artery stenosis excluded by angiography and MFR was similar in those patients as compared to the other HFpEF patients. While HFpEF patients had a previous clinical diagnosis of HF, the majority (54%) were asymptomatic as assessed by NYHA class I symptoms. Presumably, NYHA status was collected routinely as part of the clinical PET referral process as it was available in all patients regardless of HF history and thus, may or may not accurately assess HF severity. If accurate, the finding of reduced MFR in these patients with mild or early HFpEF is notable. Echocardiograms were available in 50% of HFpEF patients and about 25% of controls and the severity of reduction in MFR correlated with severity of resting diastolic dysfunction.
The systemic nature of the comorbidity related pro-inflammatory milieu suggests that the skeletal muscle vascular beds would also be affected18. Impaired skeletal muscle oxygen extraction and utilization contribute to exercise intolerance in HFpEF18, 19. Reduced skeletal muscle MVD has been demonstrated in HFpEF18 and correlates with the severity of impairment in peak O2 consumption.
Is coronary microvascular dysfunction sufficient to cause HFpEF?
While the studies above in typical HFpEF patients document an association between coronary microvascular dysfunction and the presence of HFpEF, microvascular dysfunction is not specific to HFpEF6, 7 and associations do not prove causality. However, there is evidence to suggest that microvascular inflammation and dysfunction can cause diastolic dysfunction and HFpEF in the absence of hypertensive remodeling or other cardiac abnormalities.
Tschope et al studied young patients (mean age 43) with a clinical diagnosis of HFpEF, invasively confirmed diastolic dysfunction and none of the usual comorbidities associated with HFpEF12. Cardiotropic viral genomes were assessed in endomyocardial biopsy samples. There was a high prevalence of parvovirus B19 infection, sometimes in association with human herpes virus 6. Both these viruses specifically infect vascular endothelial cells and are not known to infect cardiomyocytes, suggesting microvascular endothelial inflammation related to viral infection is sufficient to cause diastolic dysfunction and clinical HFpEF. Indeed, virus positive patients had a high rate of endothelial dysfunction assessed by intracoronary Doppler flow-velocity catheters and acetylcholine infusion; although global microvascular function and MVD were not assessed.
Cardiomyocytes are resistant to ionizing radiation and lower dose cardiac radiation exposure selectively induces microvascular endothelial damage with subsequent microvascular inflammation, microvascular rarefaction, myocardial inflammation, oxidative stress and fibrosis in animal models20. Further study of animal models of cardiac radiation exposure and older women undergoing breast cancer radiotherapy may provide insight to a specific link between coronary microvascular dysfunction and HFpEF.
Conclusions
Further studies carefully assessing the degree of microvascular impairment across the spectrum of HFpEF clinical phenotypes and severities are needed. The functional significance of microvascular impairment in terms of its impact on myocardial oxygen extraction, energetic reserve and myocardial reserve function needs to be determined and the role of functional versus structural microvascular abnormalities defined. Such studies are needed to determine if, in which patients and how microvascular function could be specifically targeted for therapeutic intervention in HFpEF.
Footnotes
Disclosures
None.
References
- 1.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: A community-based study. Circ Heart Fail. 2012;5:710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell RT, Jhund PS, Castagno D, Hawkins NM, Petrie MC, McMurray JJ. What have we learned about patients with heart failure and preserved ejection fraction from dig-pef, charm-preserved, and i-preserve? J Am Coll Cardiol. 2012;60:2349–2356. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 4.Goligorsky MS. Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis. 2010;6:1–10. doi: 10.4161/org.6.1.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail. 2016;9:e002562. doi: 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 6.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 7.Pries AR, Reglin B. Coronary microcirculatory pathophysiology: Can we afford it to remain a black box? Eur Heart J. doi: 10.1093/eurheartj/ehv760. http://dx.doi.org/10.1093/eurheartj/ehv760 ehv760 First published online: 2 February 2016. [DOI] [PMC free article] [PubMed]
- 8.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss HR. Effect of coronary artery occlusion on regional arterial and venous o2 saturation, o2 extraction, blood flow, and o2 consumption in the dog heart. Circ Res. 1980;47:400–407. doi: 10.1161/01.res.47.3.400. [DOI] [PubMed] [Google Scholar]
- 11.van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3:e001293. doi: 10.1161/JAHA.114.001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschope C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck PL, Pauschinger M, Poller WC, Kuhl U, Kandolf R, Schultheiss HP. High prevalence of cardiac parvovirus b19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation. 2005;111:879–886. doi: 10.1161/01.CIR.0000155615.68924.B3. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann PA, Camici PG. Myocardial blood flow measurement by pet: Technical aspects and clinical applications. J Nucl Med. 2005;46:75–88. [PubMed] [Google Scholar]
- 14.Schwitter J, DeMarco T, Kneifel S, von Schulthess GK, Jorg MC, Arheden H, Ruhm S, Stumpe K, Buck A, Parmley WW, Luscher TF, Higgins CB. Magnetic resonance-based assessment of global coronary flow and flow reserve and its relation to left ventricular functional parameters: A comparison with positron emission tomography. Circulation. 2000;101:2696–2702. doi: 10.1161/01.cir.101.23.2696. [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 16.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Kimura K, Umemura S. Impairment of coronary flow reserve evaluated by phase contrast cine-magnetic resonance imaging in patients with heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5:e002649. doi: 10.1161/JAHA.115.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitzman DW, Upadhya B, Vasu S. What the dead can teach the living: Systemic nature of heart failure with preserved ejection fraction. Circulation. 2015;131:522–524. doi: 10.1161/CIRCULATIONAHA.114.014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–294. doi: 10.1161/CIRCHEARTFAILURE.114.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart FA, Seemann I, Hoving S, Russell NS. Understanding radiation-induced cardiovascular damage and strategies for intervention. Clin Oncol (R Coll Radiol) 2013;25:617–624. doi: 10.1016/j.clon.2013.06.012. [DOI] [PubMed] [Google Scholar]