Abstract
Background
Difficulty modeling complex behavioral phenotypes in rodents (e.g., language) has hindered pathophysiological investigation and treatment development for autism spectrum disorders (ASD). Recent human neuroimaging studies, however, have identified functional biomarkers that can be more directly related to the abnormal neural dynamics of ASD. This study assessed the translational potential of auditory evoked-response endophenotypes of autism in parallel mouse and human studies of autism.
Methods
Whole-cortex magnetoencephalography was recorded in 17 typically developing and 25 autistic children during auditory pure-tone presentation. Superior temporal gyrus activity was analyzed in time and frequency domains. Auditory evoked potentials were recorded in mice prenatally exposed to valproic acid (VPA) and analyzed with analogous methods.
Results
VPA-exposed mice demonstrated selective behavioral alterations related to autism, including reduced social interactions and ultrasonic vocalizations, increased repetitive self-grooming, and prepulse inhibition deficits. Autistic subjects and VPA-exposed mice showed a similar 10% latency delay in the N1/M100 evoked response and a reduction in gamma frequency (30–50 Hz) phase-locking factor (PLF). Electrophysiological measures were associated with mouse behavioral deficits. In mice, gamma PLF was correlated with expression of the autism risk gene neuroligin-3 and neural deficits were modulated by the mGluR5-receptor antagonist MPEP.
Conclusion
Results demonstrate a novel preclinical approach toward mechanistic understanding and treatment development for autism.
Keywords: autism, electrophysiology, endophenotype, magnetoencephalography (MEG), animal model, gamma oscillations
Introduction
Autism spectrum disorders (ASD) are highly heritable neurodevelopmental disorders characterized by reduced social interactions, language impairment, and repetitive or restricted interests and behaviors. Despite strong genetic etiology, diagnosis is based purely on behavioral criteria. However, individuals with ASD display marked phenotypic heterogeneity and frequent medical comorbidities, which have hindered advances in diagnosis and treatment development (1). It is likely that underlying structural or functional neural abnormalities associated with ASD may be more robust targets for diagnostic and therapeutic advancement than behavioral phenotypes (2).
Using magnetoencephalography (MEG), we recently proposed a neural biomarker for autism (3). Specifically, autistic children showed delays in the superior temporal gyrus (STG) 100 ms auditory response (M100), with this latency prolongation providing accurate ASD classification. The present study builds on these findings by investigating gamma frequency synchronization in this same group of subjects. Emerging evidence suggests that neural synchrony is disturbed in autism, particularly in the gamma-band (30–50 Hz) (4). Such oscillations are important for sensory integration and connectivity, which are disturbed in autism, and may relate to deficits in cortical inhibition (5–7). We examine gamma power and phase-locking – the similarly in phase across trials – in ASD and typically developing controls as well as the association between frequency measures and M100 latency.
Difficulty modeling complex human behaviors, like language, in rodents has been a significant obstacle for translational studies of autism. Here, the utility of electrophysiological biomarkers in preclinical investigation was examined. Using an analogous auditory paradigm, latency delays and gamma-band responses were investigated in the valproic acid (VPA) mouse model of autism. Prenatal VPA exposure in humans is associated with a 7–10× increase in relative risk for ASD (8–11). In rats, prenatal VPA exposure is an emerging insult-based model of autism with a well-replicated phenotype including reduced social preference, increased repetitive behaviors, and deficits in sensorimotor integration (12–14). VPA-exposed rodents demonstrate cerebellar and brainstem pathologies, down-regulation of autism risk genes, and altered excitatory:inhibitory balance, all abnormalities consistent with theories of ASD pathophysiology (15–20). Despite these results, few studies have explored prenatal VPA exposure in mice. Replicating human behavioral and electrophysiological deficits in mice would bolster the validity of this model and offer new targets for preclinical therapeutic development.
The goals of this study were several-fold. First, we characterized two functional ASD biomarkers. Next, we performed an extensive behavioral battery in the VPA mouse model and assessed analogs of the human electrophysiological endophenotypes. Finally, we attempted to rescue neural deficits in the mice with the mGluR5 receptor antagonist MPEP, which ameliorates aberrant phenotypes in other mouse models relevant to autism (21).
Methods and Materials
Subjects
STG M100 latency findings for these subjects were previously reported (3). In this study, data were further analyzed to examine left- and right-hemisphere STG gamma power and phase-locking. MEG data collection and analysis procedures are briefly described below. Children with ASD (n=25, age 10.20±2.15) and typically developing (TD, n=17, age 10.77±1.98) children participated. Subject inclusion, screening, and diagnostic assessment was performed as previously published (3). Groups did not differ in hearing threshold and all subjects had full-scale IQ>75. All subjects and families were native English speakers and had no known genetic syndromes or neurological or sensory impairments. The study was approved by the Children’s Hospital of Philadelphia Institutional Review Board and all participants’ families gave written informed consent.
MEG Recordings
Recordings were collected using a whole-cortex 275-channel MEG system (VSM MedTech Inc., Coquitlam, BC) as published (see supplement) (3). Briefly, 105 sinusoidal tones at each of 4 frequencies (200, 300, 500, 1000 Hz) were binaurally presented with a 1s interstimulus interval over 7 min. Left and right STG activity was estimated using a standard source model to transform raw MEG surface activity into brain space using a model with multiple sources in BESA 5.2 (MEGIS Software GmbH, Gräfelfing, Germany). M100 latency and amplitude were measured from STG source waveforms after orienting the left and right STG dipoles to capture the maximal variance at the M100 response. Similarly, gamma frequency analyses were performed after orienting the left and right STG dipole to capture maximal variance of 35–45 Hz filtered activity 40–100 ms post-stimulus. Goodness of fit did not differ between groups for either source model (M100-oriented: ASD:92.7±1.6%, TD:93.3±1.0%, F1,43=0.07, p=0.79; Gamma-oriented: ASD:80.2±1.4±, TD:80.1±2.1%, F1,43=0.002, p=0.96).
For all MEG analyses, subjects more than 3 standard deviations from the group mean were excluded (typically 1–2 subjects per variable). For time-series analyses, non-contaminated epochs were averaged, prestimulus baseline activity subtracted, and left and right M100 latency calculated from the source waveform peak between 90–180 ms. Groups did not differ in number of uncontaminated trials (ASD: 381±1.6, TD: 383.8±1.2, F1,43=0.8, p=0.38). Using the gamma-oriented source waveform data, gamma power and phase-locking factor (PLF, e.g. intertrial coherence) values were calculated with Morlet wavelet decomposition using EEGLab, with wavelet cycles increasing from 2 to 10 (22). To boost signal to noise, frequency analyses were performed across all four auditory conditions. Power measures were separated into evoked (phase-locked and time-locked) and induced (time- but not phase-locked) components (23). PLF is expressed as a unitless ratio between 0 and 1, where 1 represents complete phase synchrony at a given frequency and time across trials. For each subject, power and PLF were calculated in a window around the peak gamma response, defined as the region from 40–100 ms poststimulus and from 30–50 Hz. Gamma power and PLF responses were baseline corrected and significance was assessed with group × hemisphere ANOVAs.
Animals
C57BL/6Hsd (B6) mice were obtained at 7–8 weeks of age from Harlan (Indianapolis, IN) and were mated with pregnancy confirmed by the presence of a vaginal plug on embryonic day 0 (E0). On E13, pregnant females (n=18) received a single subcutaneous injection of 600 mg/kg valproic acid (Tocris Bioscience, Ellisville, MO) dissolved in saline. Control females (n=13) received an equal volume of saline (SAL) only. There were 39 pups delivered from VPA-treated dams and 43 pups from SAL-treated females. Day of birth was recorded as P0. Animals were maintained in a standard 12-h light/dark cycle with free access to food and water. Protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Behavior
From P0–P70, mice underwent a battery of behavioral tests conducted in the following order: neonatal vocalizations (P2–P12), developmental milestones (P2–P20), prepulse inhibition of startle (P35), locomotor testing (P42), open field testing (P49), social testing (P56), rotarod (P63), adult vocalizations (70), olfactory testing (P75). See supplement.
Electrophysiology
At 12 weeks, animals underwent stereotaxic implantation of a stainless steel tripolar electrode assembly (Plastics1 Inc, Roanoke, VA) as published (24). Animals were anesthetized with isofluorane, and a low-impedance (<5 kΩ, 1000 Hz) macro-electrode was stereotaxically positioned between auditory cortex and auditory thalamus (1.4 mm posterior, 2.65 mm lateral, 2.75 mm deep relative to bregma) and referenced to frontal sinus. This configuration captures both early and late components of the auditory evoked potential (AEP), including the acoustic brainstem response, mid-latency P20 (e.g., human P50/M50) and N40 (e.g., human N100/M100) as published (25–27).
One week after surgery, AEPs were recorded with Micro1401 hardware and Spike6 software (CED, Cambridge, UK). A total of 3000 6 and 9 kHz pure tones were presented with a 500 ms interstimulus interval at 85 dB. Time series and frequency analyses were performed in EEGLab using analogous methods as in the clinical study. See supplementary methods.
Western Blotting
Western blotting was performed as published, using anti-NLGN3 (rabbit polyclonal, sc-50395, Santa Cruz, 1:200) and anti-β-actin (mouse monoclonal, Abcam ab6276, 1:5000) (28). See supplementary methods.
Pharmacologic Effects
The effect of the mGluR5 antagonist MPEP (6-Methyl-2-(phenylethynyl)pyridine, Sigma) was assessed on AEP and PPI phenotypes. Mice were injected with MPEP (10 mg/kg, i.p.) dissolved in saline 5 min prior to electrophysiological and behavioral testing. This dose was based on previous work demonstrating efficacy in mice (21).
Statistics
Significance for all measures was assessed by repeated measures or one-way ANOVA, followed by Fisher-LSD post-Hoc where appropriate. All exploratory comparisons were Bonferroni corrected for Type I error.
Results
Human MEG
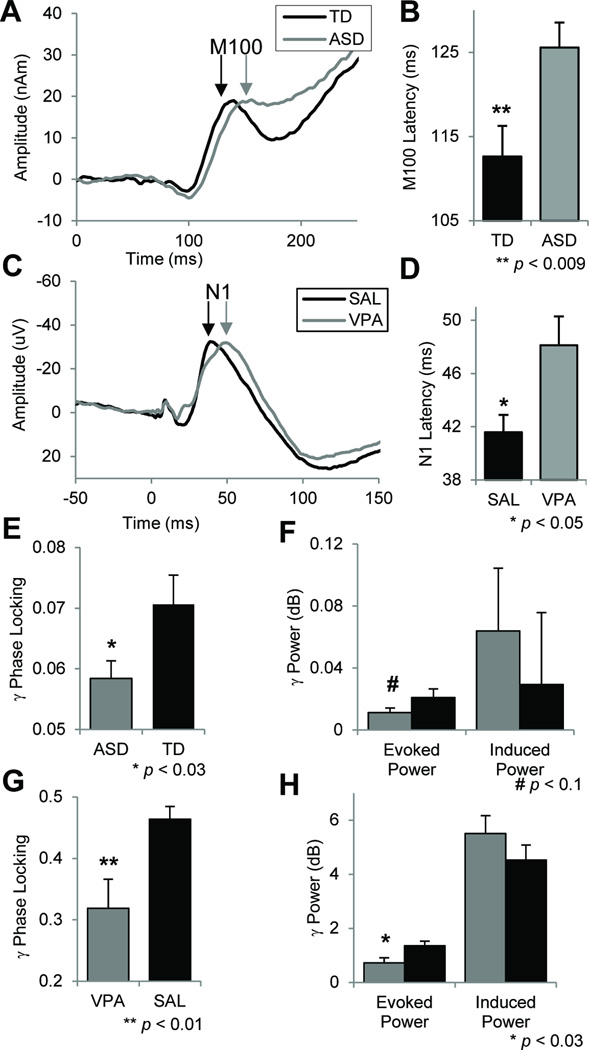
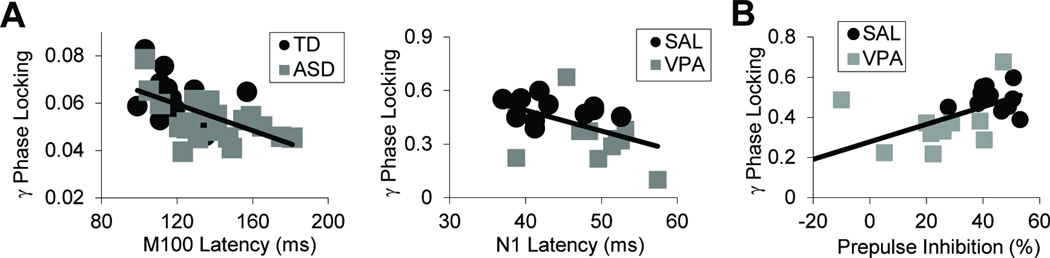
As reported, subjects with ASD showed a ~10% delay in the RH M100 response (Fig 2; F1,38=7.6, p<0.009) (3). Using a cutoff of 116ms, M100 latency classified ASD subjects with a sensitivity of 75% and specificity of 81% (3). Building on these findings, transient gamma-band power and phase-locking were calculated to assess neural oscillatory activity. ASD subjects showed significantly reduced gamma phase-locking across hemispheres (Fig 2; F1,43=5.2, p<0.028), but no significant change in evoked (F1,43=2.8, p=0.10) or induced (F1,43=0.3, p=0.58) gamma-power. Significant zero-order correlations between M100 latency and gamma PLF (R2=0.15, p=0.02, corrected; Fig 3A, left) suggest the measures are related but not redundant. Across groups, the peak M100 response occurred at 132±3.15 ms (mean±sem) while the peak gamma-band response was at 95.1±7.4 ms. These latency distributions were highly distinct (F1,83=19.6, p=0.00003), indicating a significant separation between M100 and preceding gamma-band responses. Grand average time-frequency plots are shown in Fig S3.
Fig 2.
Auditory evoked-responses from clinical and pre-clinical studies in time (A–D) and frequency domains (E–H). A) Grand-average superior temporal gyrus auditory evoked-responses are shown in autism (ASD) and typically developing (TD) groups. B) ASD subjects show a 10% delay in right hemisphere M100 latency. C) Grand-average auditory evoked-responses are shown for mice treated prenatally to valproic acid (VPA) or saline (SAL). D) The VPA group shows a 16% delay in N1 (e.g., M100) latency. E) Transient gamma-band phase-locking (PLF; e.g., intertrial coherence) is significantly reduced in ASD across hemispheres. F) Groups did not differ in evoked (time- and phase-locked) or induced (time-, not phase-locked) gamma power. G) Gamma PLF is reduced in VPA-exposed mice. H) Evoked power was reduced in the VPA group with no difference in induced power. All figures indicate mean ± s.e.m.
Fig 3.
Regression analyses between electrophysiological and behavioral measures were performed across clinical and preclinical studies. A) Gamma phase-locking was significantly correlated with M100 latency in clinical subjects (left, R2=0.15, p=0.02, corrected) and with N1 latency in the preclinical study (right, R2=0.25, p<0.02, corrected). The 15–25% shared variance suggests these measures are related but not redundant. B) In mice, gamma phase locking significantly predicated deficits in prepulse inhibition (R2=0.39, p<0.01, corrected), a behavioral measure of sensorimotor integration that is disrupted in autism (64).
Mouse Behavior
See supplement for full results (Fig S2). Briefly, VPA-exposed mice showed disruptions in USVs, including reduced pup distress calls (F1,58=13.31, p<0.0006) and a reduction in adult 70-kHz pre-mating vocalizations (F1,17=5.75, p<0.03). The VPA group demonstrated reduced social preference (F1,20=4.99, p<0.04), increased repetitive self-grooming behavior (F1,22 = 5.77, p=0.025), and deficits in prepulse inhibition of startle (F1,21=5.62, p<0.03). There were no group differences in locomotor activity, rotorod motor coordination, olfactory function, or auditory startle curves, arguing against global neurological impairment.
Mouse Electrophysiology
For time-domain analyses (Fig 2), amplitudes and latencies of P1 and N1 (e.g., N100/M100) were measured. VPA-exposed mice showed a significant 16% delay in N1 latency to 6 kHz tones (group × peak × stimulus interaction: F1,20=9.1, p=0.007; post-hoc p<0.05, Fisher-LSD). Mirroring human results, there were no amplitude or other latency differences (group effect: F1,20=1.67, p=0.2; group × freq interaction: F1,20=0.76, p=0.4, group × peak interaction: F1,20=0.23, p=0.64, group × peak × freq interaction: F1,20=1.57, p=0.23). In the frequency domain (Fig 2), VPA-treated mice demonstrated significantly reduced gamma phase locking (F1,20=7.0, p=0.01), reduced evoked gamma-power (F1,20=6.1, p=0.02), but no difference in induced gamma power (F1,20=1.3, p=0.27), similar to the clinical results. As in the human subjects, N1 latency and gamma PLF were significantly correlated (Fig 3A; R2=0.25, p<0.02, corrected). The 25% shared variance suggests that these are related but non-overlapping measures.
Mouse Molecular Biology
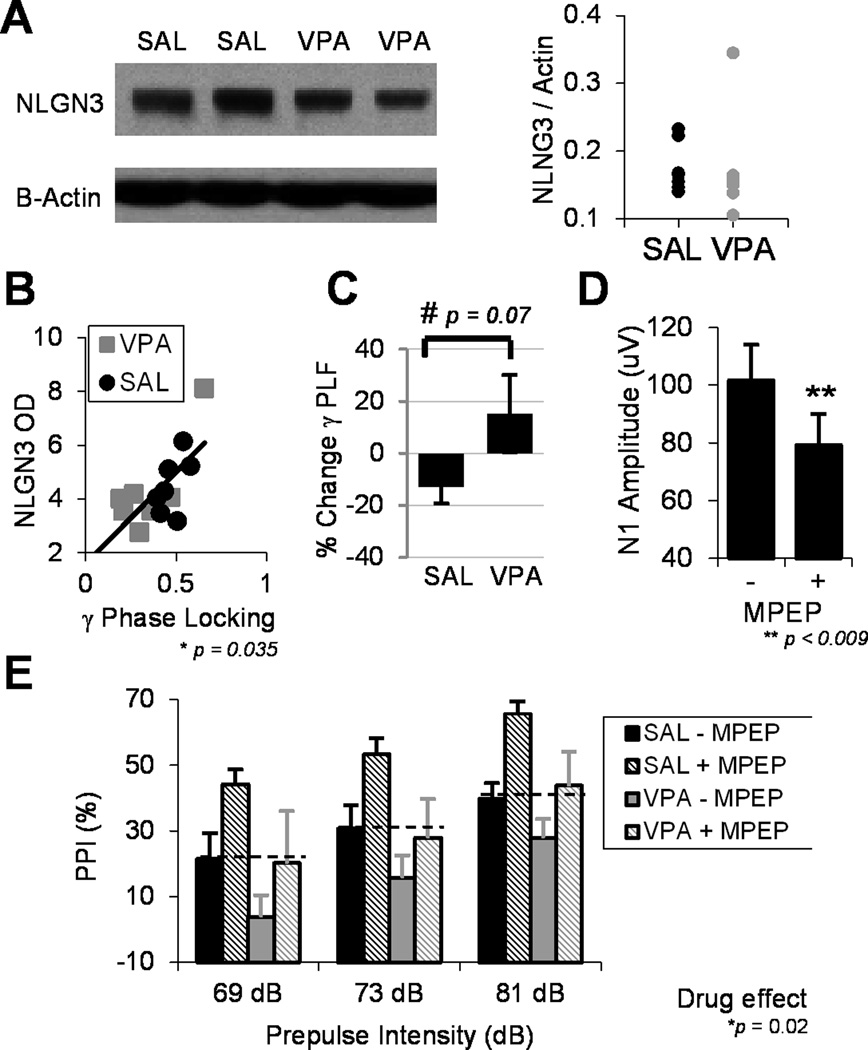
Expression of the autism risk gene neuroligin-3 (NLGN3), an X-linked synaptic cell-adhesion molecule, was measured given previous studies demonstrating that prenatal VPA exposure leads to reduced NLGN3 mRNA levels (21). Group differences in NLGN3 optical density were not observed (F1,11=3.17, p=0.10). β-actin expression was identical between groups. It is possible that our study is underpowered to detect a group difference or that analyses in brain sub-regions would yield significant differences.
Regression analyses then assessed associations between electrophysiological measures, NLGN3 expression, and adult behaviors related to auditory processing and communicative function. NLGN3 protein levels strongly predicted gamma phase-locking across both SAL and VPA groups (R2=0.46, p<0.04, corrected). In addition, gamma PLF significantly predicted average PPI responses (R2=0.39, p<0.01, corrected). There was no relationship between NLGN3 and N1 latency. There was a non-significant but qualitative relationship between N1 latency and pre-mating vocalizations (R2=0.34, p=0.10, corrected), suggesting a potential link between receptive auditory processing and expressive communicative functioning in mice.
Pharmacological Effects
The predictive utility of electrophysiological biomarkers as targets for therapeutic development was explored. The effect of the mGluR5 antagonist MPEP was assessed on AEP and PPI measures. Across both groups, MPEP significantly reduced cortical excitability as measured by the N1 amplitude (F1,14=9.23, p<0.009). However, there was no effect of drug on N1 latency (F1,14=1.64, p=0.22). In the frequency domain, there was a trend toward a significant group by drug interaction (F1,15=3.77, p=0.07), in which MPEP reduced gamma phase-locking in the SAL group while increasing gamma PLF in the VPA group. This suggests that restoring the balance of excitation to inhibition in the VPA group may promote gamma synchrony, while altering this balance in wild-type mice may disrupt gamma synchrony.
The effect of MPEP was also assessed on PPI and startle phenotypes. There was no drug effect on startle amplitude (drug effect: F1,22=2.17, p=0.16; group × drug interaction F1,22=0.37, p=0.55), indicating that MPEP does not disrupt gross sensory or motor function. There was a main effect of drug on prepulse inhibition (F1,21=6.48, p<0.02), in which MPEP significantly increased PPI across both groups. Of note, there was no difference in PPI between the VPA+MPEP group and the SAL-MPEP group (p=0.85, Fisher-LSD), demonstrating that MPEP normalizes deficits in sensorimotor integration caused by VPA exposure.
Discussion
This study characterizes two electrophysiological endophenotypes of autism in parallel human and mouse studies. Autistic subjects show delayed M100 STG responses and reduced gamma-band phase-locking. In mice, prenatal VPA exposure causes selective behavioral alterations in domains affected in autism, including social and communicative functioning. The VPA model mirrors evoked-response latency delays and gamma-band deficits identified in the clinical population. In mice, electrophysiological measures predict the severity of behavioral deficits and correlate with expression of the autism risk gene, NLGN3. Finally, the mGluR5 antagonist MPEP reverses PPI deficits and may modulate gamma-band dysfunction, suggesting that mGluR5 receptors may be appropriate therapeutic targets in autism.
Electrophysiological Biomarkers of Autism
Several studies suggests that early auditory encoding processes are impaired in ASD (29). Here, autistic subjects showed delays in the STG M100 response, in accordance with previous reports of delayed M100/N100 latencies in ASD to simple and complex auditory stimuli (30–32). Delayed auditory evoked-responses have been demonstrated in unaffected relatives of autistic subjects, suggesting that such deficits may be heritable (33). Taken together, these findings suggest that delayed AEPs are a replicable endophenotype of ASD. While an underlying neural mechanism for this latency delay is unknown, recent studies have demonstrated a link between white-matter integrity and electrophysiological response timing (34, 35). Given reports of widespread white-matter alterations in ASD, we speculate that latency delays may be related to poorly myelinated acoustic radiations and associated auditory processing pathways (36). The contribution of such a neural deficit to core symptoms of ASD, if any, is also unclear. However, recent work suggests that deficits in the integrity of auditory processing are detrimental to speech and language acquisition, a core ASD impairment (37).
Emerging evidence suggests that neural synchrony may be impaired in autism, particularly in the gamma-band, which may contribute to clinical deficits (4). Gamma oscillations have been widely studied in regard to cognitive correlates, including attention and perceptual ‘feature binding’, as well as neural mechanisms involving the tightly coupled interaction between pyramidal neurons and fast-spiking interneurons (5, 6, 38). Previous studies have reported gamma-band abnormalities in ASD, during visual and auditory tasks (39–43). However, other groups have failed to detect gamma-band deficits in ASD, suggesting that there may be relevant variables in diagnostic specificity or other aspects of study design that contribute to these divergent results (44). Here, we demonstrate reduced gamma phase-locking in autism. The PLF is a measure of local neural synchronization (phase coherence) across trials. As such, it is independent of oscillatory amplitude and therefore is a direct measure of synchronization. We found no group differences in induced gamma power. Surprisingly, differences in evoked gamma power, which tends to reflect phase-locking, were non-significant (p=0.1). However, these results are qualitatively very similar to our preclinical findings and those from other clinical studies, which have identified more significant group differences in gamma phase-locking than evoked power (42, 45). This difference could reflect increased noise in the extracranial MEG recordings relative to intracranial electrodes in mice. Our findings indicate that local auditory cortical networks do not synchronize appropriately to external stimuli in autism, which may contribute to or reflect deficits in auditory perception or attention. In accordance with our results, Wilson et al. reported reduced auditory steady-state gamma responses in autism and Rojas et al. demonstrated reduced auditory gamma PLF in adults with ASD (42, 43). The latter study also showed gamma PLF deficits in relatives of autistic individuals, suggesting that this endophenotype may be heritable. These findings could be explained by a loss of cortical excitatory:inhibitory balance, a theory consistent with post-mortem studies of autism (46).
Finally, we assessed the correlation between the two electrophysiological measures. That both clinical and preclinical studies observed significant correlations of 15– 25% shared variance suggests that these measures are related but not redundant. It is important to note, however, that neither biomarker is specific to autism, as latency delays have been reported in dyslexia and auditory neuropathy, while similar gamma-band deficits have been demonstrated in schizophrenia (47–49)
The VPA Mouse Model of Autism
While environmental contributions are likely multifactorial, two teratogens that have been strongly linked to autism are thalidomide and valproic acid (9, 50–52). The anticonvulsant valproic acid has been associated with adverse neurodevelopmental outcomes, including lower IQ, poor language functioning, and a 7–10× relative-risk for ASD (9, 49–51). Prenatal exposure to VPA in rats is an insult-based model of autism that is increasingly being explored (see (53) for review), with a lasting, well-replicated phenotype that mimics the human disorder (8, 9, 54). VPA exposed rodents show synaptic and molecular changes consistent with autism, including hyper-excitability, serotonergic dysregulation, and reduced expression of neuroligin-3 (13, 15, 16, 53). Despite this, like any rodent model of a complex human behavioral disorder, the VPA insult model has a number of shortcomings. For example, there are questions about generalizability, given that most autistic individuals were not exposed to the drug in utero. In addition, this model does not directly reflect the highly genetic etiology of autism. However, the VPA model is among the most explored insult-based models from a synaptic to behavioral level, allowing us to interpret our results in the context of this larger literature.
In the present study, VPA-exposed mice showed reduced social preference, increased repetitive behaviors, and PPI deficits. Pertinent negative findings include no group differences in locomotor activity, rotorod motor coordination, adult olfactory function, or acoustic startle, suggesting that the drug does not cause global brain dysfunction. In neonates and adults, VPA-exposed mice demonstrated deficits in communicative function, as measured by the density and latency to initiate ultrasonic vocalizations as well as their spectral content. In particular, VPA exposed males failed to emit characteristic 70 kHz “pre-mating” vocalizations when paired with a receptive female. Similar USV findings have been reported in different mouse models of ASD and other speech and language disorders (55). As such, these mice demonstrate deficits in all three core symptom domains of autism. Replicating these findings across species bolsters the face and construct validity of this approach.
Translatable Biomarkers of Autism
The lack of a biological marker for ASD has hindered pathophysiological investigation in preclinical models. Although there has been success investigating social interactions and repetitive behaviors in mice, assessing language impairment has been especially difficult (56). To address these limitations, this study employs a novel approach toward preclinical targets for autism. We have characterized two distinct measures of impaired auditory processing in autism, which likely reflect the abnormal neural dynamics of ASD more closely than behavioral criteria alone. Using analogous methods, we demonstrate that the VPA mouse model of autism recapitulates both biomarkers. These measures predicted the severity of behavioral deficits in mice. Modeling ASD endophenotypes of impaired brain functioning in mice allows direct investigation of the neural mechanisms of these phenotypes and provides rational targets for pharmacologic intervention.
Given that valproic acid is a class I histone deacytlase (HDAC) inhibitor, it is not surprising that exposure to this drug during development causes persistent alterations in gene expression profiles (57). Koloszi et al. demonstrated reduced mRNA levels of the X-linked autism risk-gene neuroligin-3 in adult mice exposed to VPA in utero (15). Our results extend previous findings to show that NLGN3 is associated with neural deficits in the VPA model. Although we did not find group differences in NLGN3 protein levels, a highly significant relationship between gamma synchronization deficits and reduced NLGN3 expression was observed. In mice, previous studies have shown that NLGN3 downregulation significantly reduced the number of inhibitory interneurons, which are critical for gamma synchrony (58). In accordance, Gogolla et al. demonstrated a loss of cortical fast-spiking interneurons in VPA exposed mice, a finding which is consistent across many rodent models of autism (20). These results indicate that the balance of excitation to inhibition is likely disrupted in the VPA model and suggest that restoring this balance may rescue phenotypic deficits.
We explored this hypothesis by assessing the effect of MPEP, a potent non-competitive mGluR5-receptor antagonist, on PPI and electrophysiological measures. The rationale for selecting MPEP was based on the fact that mGluR5 antagonists have shown promise in clinical trials of Fragile X syndrome, which shares a significant overlap with ASD (59). mGluR5 antagonists have demonstrated remarkable reversal of phenotypic deficits in preclinical models of FXS, including core ASD symptoms like social impairments (21, 60, 61). Recent work has shown that MPEP is effective in reversing phenotypic deficits related to core ASD deficits in an idiopathic mouse model of autism (62). Finally, we have recently shown that other glutamate receptor antagonists modulate gamma oscillations in mice (63). Based on this literature, we hypothesized that MPEP would be a relatively novel, promising pharmacologic compound to assess in our model. Indeed, MPEP rescued PPI deficits in the VPA model, nearly identical to the effect seen in Fmr1 knockout mice (60). It did so without affecting startle curve amplitudes suggesting it is not overly sedating. MPEP selectively reduced N1 amplitudes, indicating a reduction in cortical excitability, but did not rescue N1 latency delays. There was a trending interactive effect of MPEP and VPA exposure on gamma PLF, boosting gamma synchrony in the VPA group while reducing gamma synchrony in SAL treated mice. We hypothesize that this differential effect reflects the tightly coupled balance of excitation and inhibition in driving cortical oscillatory activity. In control mice, where this balance is optimal, we expect that reducing NMDA current with MPEP would disrupt neural synchrony. However, in VPA mice where this interplay favors excitation, we speculate that MPEP would restore appropriate balance and thus promote gamma synchrony.
In conclusion, this study demonstrates the translational potential of STG M100 auditory evoked responses and gamma-band phase-locking in parallel human and mouse studies of autism. While the present study focused on early gamma frequency responses given relevant cognitive correlates, known circuit generators, and the putative gamma abnormalities in ASD, future studies will investigate neural synchrony in lower frequency ranges and at later latencies. A limitation of this study is the different stimulus parameters between humans and mice. Human tones were chosen based on the salience of this frequency range for human language (3). Since mice cannot hear these tones well, higher frequencies at corresponding places in the mouse hearing spectrum were chosen. Future work will investigate additional stimulus parameters in both clinical and preclinical settings.
Supplementary Material
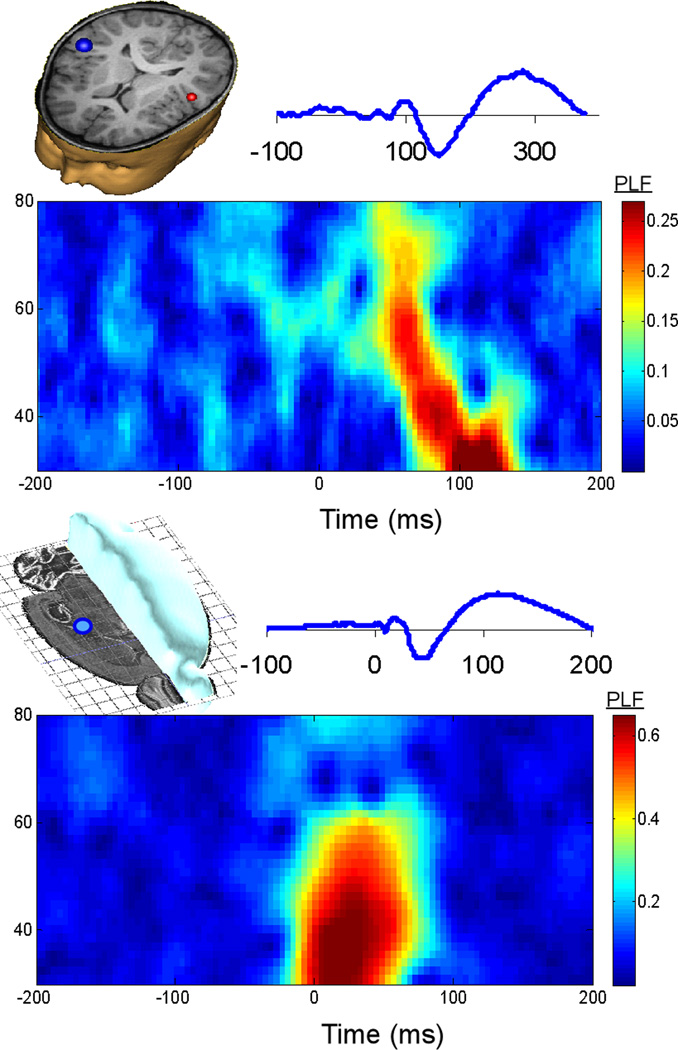
Fig 1.
Representative control subjects from clinical (top) and preclinical (bottom) studies. An auditory evoked response is shown above a plot of gamma phase-locking (PLF).
Fig 4.
Biomarkers are related to the expression of the autism risk gene neuroligin 3 and may be useful therapeutic targets. A) A western blot of neuroligin 3 and β-actin indicates no group differences between SAL and VPA treated adult mice (p=0.1). B) However, NLGN3 protein expression (optical density) significantly predicts gamma phase-locking (R2=0.46, p=0.035, corrected). Note, the VPA-group outlier fits the linear correlation, as it also does in the regression of gamma PLF and PPI (Fig 3B). C) The effect of the mGluR5 antagonist MPEP was assessed on gamma PLF. The plot demonstrates the change in phase-locking (%) after drug administration. A trending interactive effect across SAL and VPA groups suggests that MPEP reduced PLF in the SAL group but boosted gamma synchrony in VPA exposed mice. D) Across both groups, MPEP reduced cortical excitability as measured by N1 amplitude. E) MPEP increased PPI across VPA and SAL groups. Dashed lines indicate that MPEP normalizes PPI deficits in VPA exposed mice. All figures indicate mean ± s.e.m.
Acknowledgments
This study was funded by National Institutes of Health grants R01-DA023210 (SJS), T32-MH017168 (MJG), and R01-DC008871 (TPR) as well as from the Nancy Lurie Marks Family Foundation (TPR) and Autism Speaks (TPR).
Dr. Siegel reports having received grant support from Eli Lilly, AstraZeneca, NuPathe and Pfizer that is unrelated to the content of this paper and consulting payments from NuPathe Inc., Merck, Sanofi and Wyeth that are unrelated to the content of this work.
Footnotes
Financial Disclosures
Drs. Edgar and Roberts report no biomedical financial interests or potential conflicts of interest. Mr. Gandal and Mr. Ehrlichman report no biomedical financial interests or potential conflicts of interest. Ms Mehta reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Geschwind DH. Advances in autism. Annual review of medicine. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgar JC, Keller J, Heller W, Miller GA. Psychophysiology in research on psychopathology. In: Tassinary LG, Cacioppo JT, Bemtson GG, editors. Handbook of Psychophysiology. 3. New York: Cambridge University Press; 2007. [Google Scholar]
- 3.Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, et al. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 2010;3:8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uhlhaas PJ, Singer W. What do disturbances in neural synchrony tell us about autism? Biological psychiatry. 2007;62:190–191. doi: 10.1016/j.biopsych.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends in cognitive sciences. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 7.Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of a utism. Development and psychopathology. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- 8.Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. Journal of medical genetics. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Developmental medicine and child neurology. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- 10.Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71:1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- 11.Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: additional evidence of an association. Developmental medicine and child neurology. 2001;43:202–206. [PubMed] [Google Scholar]
- 12.Markram H, Rinaldi T, Markram K. The intense world syndrome - an alternative hypothesis for autism. Frontiers in neuroscience. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33:901–912. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- 14.Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- 15.Kolozsi E, Mackenzie RN, Roullet FI, deCatanzaro D, Foster JA. Prenatal exposure to valproic acid leads to reduced expression of synaptic adhesion molecule neuroligin 3 in mice. Neuroscience. 2009;163:1201–1210. doi: 10.1016/j.neuroscience.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi T, Kulangara K, Antoniello K, Markram H. Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13501–13506. doi: 10.1073/pnas.0704391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. The Journal of comparative neurology. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, brain, and behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicology and teratology. 2000;22:319–324. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 20.Gogolla N, LeBlanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. Journal of Neurodevelopmental Disorders. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiology of disease. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods. 2004;134:921. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandal MJ, Ehrlichman RS, Rudnick ND, Siegel SJ. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience. 2008;157:95–104. doi: 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, et al. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochemical research. 2004;29:1179–1188. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- 26.Connolly PM, Maxwell CR, Kanes SJ, Abel T, Liang Y, Tokarczyk J, et al. Inhibition of auditory evoked potentials and prepulse inhibition of startle in DBA/2J and DBA/2Hsd inbred mouse substrains. Brain research. 2003;992:85–95. doi: 10.1016/j.brainres.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 27.Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, et al. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- 28.Bodarky CL, Halene TB, Ehrlichman RS, Banerjee A, Ray R, Hahn CG, et al. Novel environment and GABA agonists alter event-related potentials in N-methyl-D-aspartate NR1 hypomorphic and wild-type mice. The Journal of pharmacology and experimental therapeutics. 2009;331:308–318. doi: 10.1124/jpet.109.150938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeste SS, Nelson CA., 3rd Event related potentials in the understanding of autism spectrum disorders: an analytical review. Journal of autism and developmental disorders. 2009;39:495–510. doi: 10.1007/s10803-008-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruneau N, Roux S, Adrien JL, Barthelemy C. Auditory associative cortex dysfunction in children with autism: evidence from late auditory evoked potentials (N1 wave-T complex) Clin Neurophysiol. 1999;110:1927–1934. doi: 10.1016/s1388-2457(99)00149-2. [DOI] [PubMed] [Google Scholar]
- 31.Gage NM, Siegel B, Callen M, Roberts TP. Cortical sound processing in children with autism disorder: an MEG investigation. Neuroreport. 2003;14:2047–2051. doi: 10.1097/00001756-200311140-00008. [DOI] [PubMed] [Google Scholar]
- 32.Kasai K, Hashimoto O, Kawakubo Y, Yumoto M, Kamio S, Itoh K, et al. Delayed automatic detection of change in speech sounds in adults with autism: a magnetoencephalographic study. Clin Neurophysiol. 2005;116:1655–1664. doi: 10.1016/j.clinph.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Maziade M, Merette C, Cayer M, Roy MA, Szatmari P, Cote R, et al. Prolongation of brainstem auditory-evoked responses in autistic probands and their unaffected relatives. Archives of general psychiatry. 2000;57:1077–1083. doi: 10.1001/archpsyc.57.11.1077. [DOI] [PubMed] [Google Scholar]
- 34.Roberts TP, Khan SY, Blaskey L, Dell J, Levy SE, Zarnow DM, et al. Developmental correlation of diffusion anisotropy with auditory-evoked response. Neuroreport. 2009;20:1586–1591. doi: 10.1097/WNR.0b013e3283306854. [DOI] [PubMed] [Google Scholar]
- 35.Stufflebeam SM, Witzel T, Mikulski S, Hamalainen MS, Temereanca S, Barton JJ, et al. A non-invasive method to relate the timing of neural activity to white matter microstructural integrity. NeuroImage. 2008;42:710–716. doi: 10.1016/j.neuroimage.2008.04.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ke X, Tang T, Hong S, Hang Y, Zou B, Li H, et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain research. 2009;1265:171–177. doi: 10.1016/j.brainres.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Choudhury N, Benasich AA. Maturation of auditory evoked potentials from 6 to 48 months: Prediction to 3 and 4 year language and cognitive abilities. Clin Neurophysiol. doi: 10.1016/j.clinph.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature reviews. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 39.Brown C, Gruber T, Boucher J, Rippon G, Brock J. Gamma abnormalities during perception of illusory figures in autism. Cortex; a journal devoted to the study of the nervous system and behavior. 2005;41:364–376. doi: 10.1016/s0010-9452(08)70273-9. [DOI] [PubMed] [Google Scholar]
- 40.Milne E, Scope A, Pascalis O, Buckley D, Makeig S. Independent component analysis reveals atypical electroencephalographic activity during visual perception in individuals with autism. Biological psychiatry. 2009;65:22–30. doi: 10.1016/j.biopsych.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, et al. Excess of high frequency electroencephalogram oscillations in boys with autism. Biological psychiatry. 2007;62:1022–1029. doi: 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 42.Rojas DC, Maharajh K, Teale P, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC psychiatry. 2008;8:66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biological psychiatry. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braeutigam S, Swithenby SJ, Bailey AJ. Contextual integration the unusual way: a magnetoencephalographic study of responses to semantic violation in individuals with autism spectrum disorders. The European journal of neuroscience. 2008;27:1026–1036. doi: 10.1111/j.1460-9568.2008.06064.x. [DOI] [PubMed] [Google Scholar]
- 45.Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, et al. The Early Auditory Gamma-Band Response Is Heritable and a Putative Endophenotype of Schizophrenia. Schizophrenia bulletin. 2009 doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biological psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 47.Alonso-Bua B, Diaz F, Ferraces MJ. The contribution of AERPs (MMN and LDN) to studying temporal vs. linguistic processing deficits in children with reading difficulties. Int J Psychophysiol. 2006;59:159–167. doi: 10.1016/j.ijpsycho.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Michalewski HJ, Starr A, Zeng FG, Dimitrijevic A. N100 cortical potentials accompanying disrupted auditory nerve activity in auditory neuropathy (AN): effects of signal intensity and continuous noise. Clin Neurophysiol. 2009;120:1352–1363. doi: 10.1016/j.clinph.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leicht G, Kirsch V, Giegling I, Karch S, Hantschk I, Moller HJ, et al. Reduced early auditory evoked gamma-band response in patients with schizophrenia. Biological psychiatry. 67:224–231. doi: 10.1016/j.biopsych.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 50.Bromley RL, Baker GA, Meador KJ. Cognitive abilities and behaviour of children exposed to antiepileptic drugs in utero. Current opinion in neurology. 2009;22:162–166. doi: 10.1097/WCO.0b013e3283292401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71:1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- 52.Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. The New England journal of medicine. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markram H, Rinaldi T, Markram K. The intense world syndrome--an alternative hypothesis for autism. Frontiers in neuroscience. 2007;1:77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stromland K, Nordin V, Miller M, Akerstrom B, Gillberg C. Autism in thalidomide embryopathy: a population study. Developmental medicine and child neurology. 1994;36:351–356. doi: 10.1111/j.1469-8749.1994.tb11856.x. [DOI] [PubMed] [Google Scholar]
- 55.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain pathology (Zurich, Switzerland) 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eyal S, Yagen B, Sobol E, Altschuler Y, Shmuel M, Bialer M. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia. 2004;45:737–744. doi: 10.1111/j.0013-9580.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- 58.Gutierrez RC, Hung J, Zhang Y, Kertesz AC, Espina FJ, Colicos MA. Altered synchrony and connectivity in neuronal networks expressing an autism-related mutation of neuroligin 3. Neuroscience. 2009;162:208–221. doi: 10.1016/j.neuroscience.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 59.Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. Journal of medical genetics. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 61.McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 62.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism is Blocked by the mGluR5 Antagonist MPEP. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine Modulates Theta and Gamma Oscillations. Journal of cognitive neuroscience. 2009 doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 64.Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biological psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.