Abstract
Control of stereochemistry in photocycloaddition reactions remains a substantial challenge, and almost all successful catalytic examples to date have involved [2+2] photocycloadditions of enones. We report a method for the asymmetric [3+2] photocycloaddition of aryl cyclopropyl ketones that enables the enantiocontrolled construction of densely substituted cyclopentane structures that are not synthetically accessible using other catalytic methods. These results show that the dual-catalyst strategy developed in our laboratory broadens synthetic chemists’ access to classes of photochemical cycloadditions that have not previously been feasible in enantioselective form.
Graphical abstract
Stereocontrolled cycloadditions are valued in synthetic chemistry both as methods to construct the ring systems that are ubiquitous in chiral bioactive compounds and as model reactions to evaluate new concepts in enantioselective synthesis.1 Control over the absolute stereochemistry of photochemical cycloadditions, however, remains a substantial challenge without a general solution.2 A relatively small number of highly enantioselective organocatalytic3 and Lewis acid4 catalyzed photocycloadditions have been described in the past several years, but these successful methods have been focused upon [2+2] cycloadditions of enones. No strategies for photocatalytic stereocontrol have emerged that appear to be broadly applicable to the asymmetric catalysis of other classes of photocycloaddition reactions.
Our laboratory recently reported a dual catalyst system for enantioselective [2+2] photocycloaddition using a chiral Lewis acid in tandem with a transition metal photoredox catalyst.5 The success of this strategy relies upon the ability to tune the structure of the stereocontrolling chiral catalyst for optimal selectivity without adversely affecting the performance of the photocatalyst. We speculated, therefore, that this combination of catalytic strategies might successfully control the stereochemical behavior of many of the reactions now known to be amenable to photoredox catalysis.6,7,8
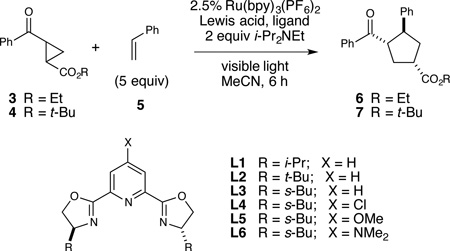
We therefore became interested in designing an asymmetric version of the photocatalytic [3+2] cycloaddition between aryl cyclopropyl ketones and alkenes that our laboratory reported several years ago (Scheme 1).9 While enantioselective cycloadditions of highly activated “donor–acceptor” cyclopropanes are known,10 no catalytic asymmetric [3+2] cycloadditions of less activated cyclopropyl ketones have yet been reported. Our photocatalytic process involves photoreduction of a Lewis acid activated aryl cyclopropyl ketone (1) to afford a ring-opened distonic radical anion that can react in an intramolecular fashion with a wide range of alkene partners. While this methodology enables the facile synthesis of structurally diverse cyclopentane-containing polycyclic compounds (e.g. 2), the reaction gives high yields only in an intramolecular context, requires stoichiometric La(OTf)3 as a Lewis acid catalyst, and employs 5 equiv of TMEDA as both a ligand for La3+ and a reductive quencher of Ru*(bpy)32+, rendering the use of a chiral diamine ligand unattractive. In this communication, we report the development of an enantioselective photocatalytic [3+2] cycloaddition that successfully address all of these challenges.
Scheme 1. Precedent and project objectives.
Table 1 outlines optimization studies for a model asymmetric [3+2] cycloaddition between phenyl ketone 3 and styrene. We initially examined conditions based upon those we reported for the racemic intramolecular reaction, employing 2.5 mol% Ru(bpy)32+ as the photocatalyst, 1 equiv La(OTf)32+ as a Lewis acid co-catalyst, and i-Pr2NEt as a reductive quencher. Consistent with our prior observations, the intermolecular reaction was sluggish, and the addition of chiral ligands strongly inhibited the reaction (entries 1 and 2). A screen of other Lewis acidic metal triflate complexes revealed that Gd(III) pybox complexes11 could provide somewhat better conversions and, gratifyingly, experimentally significant enantioselectivity, with a maximum ee of 59% using the s-Bu-substituted pybox ligand L3 (entries 3–5).
Table 1.
Optimization studies.
 | |||
|---|---|---|---|
| Entry | Conditionsa | Yieldb | dr; % ee |
| 1 | 100 % La(OTf)3 | 25 % | 14:1; --- |
| 2 | 100 % La(OTf)3,100% L2 | 5 % | --- ; --- |
| 3 | 100 % Gd(OTf)3, 100 % L1 | 15 % | 3:1; 38 % |
| 4 | 100 % Gd(OTf)3, 100 % L2 | 96 % | 3:1; 45 % |
| 5 | 100 % Gd(OTf)3, 100 % L3 | 25 % | 2:1; 59 % |
| 6 | 100 % Gd(OTf)3, 200 % L3 | 36 % | 3:1; 63 % |
| 7 | 100 % Gd(OTf)3, 200 % L4 | 0 % | ---;--- |
| 8 | 100 % Gd(OTf)3, 200 % L5 | 90 % | 2:1; 64 % |
| 9 | 100 % Gd(OTf)3, 200 % L6 | 89 % | 2:1; 85 % |
| 10 | 10 % Gd(OTf)3, 20% L6 | 96 % | 2:1; 79 % |
| 11 | 10 % Gd(OTf)3, 20% L6, 0 °C | 80% | 3:1; 85 % |
| 12 | 10 % Gd(OTf)3, 20% L6, −20 °C | 41 % | 3:1; 91 % |
| 13c | 10 % Gd(OTf)3, 20 % L6, 0 °C | 86 % | 3:1; 90 % |
| 14c,d | 10 % Gd(OTf)3, 20 % L6, 0 °C | 95 % | 3:1; 93 % |
Reactions carried out on 0.045 mmol scale, irradiating with a 23 W CFL for 6 h.
Yields determined by 1H-NMR using phenanthrene as an internal standard.
Using 4 instead of 3.
Using 1 equiv of i-Pr2NEt.
Analysis of reaction progress revealed that Gd(OTf)3 and i-Pr2NEt slowly formed an inactive Lewis acid-base complex over several hours, which coincided with a concomitant decrease in the rate of product formation. We increased the ligand-to-metal ratio in an attempt to slow formation of a deactivated complex, albeit with little beneficial effect (entry 6). As an alternative strategy, we wondered if we might stabilize the active Gd-pybox complex by increasing the coordinating ability of the chiral ligand.12 Indeed, while chloride-substituted ligand L4 resulted in no product formation, electron-rich methoxy-substituted ligand L5 provided 6 in excellent yield (entry 8). Dimethylamino-substituted ligand L6 provided optimal rate and stereoselectivity (entry 9), and with this ligand, the Lewis acid loading could be decreased to 10 mol% with little effect on ee (entry 10). Lowering the temperature to 0 °C resulted in an increase in the enantioselectivity to 85% ee (entry 11). The ee was further improved at −20 °C, but we observed an increased proportion of an undesired reductive ring-opening product (entry 12). Increasing the bulk of the ester substituent provided somewhat higher ee at 0 °C (entry 13), and the occurrence of the reductive ring-opening side-product could be minimized by lowering the concentration of i-Pr2NEt (entry 14). Under these optimized conditions, cycloadduct 7 was obtained in 95% yield, 93% ee, and 3:1 d.r..13
We next conducted an exploration of the scope of the enantioselective cycloaddition under these conditions. Table 2 outlines the effect of varying the structure of the alkene reaction partner.14 We have proposed a stepwise cycloaddition initiated by radical addition of a ring-opened distonic radical anion to an alkene. Consistent with this proposal, simple aliphatic alkenes are not reactive. However, a variety of electronically modified styrenes react smoothly and with good ee (8–10). Potentially reactive aryl halides are well tolerated (11), providing a handle for derivatization of the enantioenriched cycloadducts. The enantioselectivity is relatively insensitive to the position of substituents on the aryl ring (12). While heterocycles containing Lewis basic heteroatoms resulted in a loss in stereoselectivity, alkenes bearing less basic heterocycles such as carbazoles react smoothly with good ee (13). Internal olefins, unfortunately, were unreactive under these reaction conditions; however, 1,1-disubstituted styrenes react smoothly and provide excellent ee (14–16). Finally, dienes are also competent reaction partners, affording vinyl cyclopentane products in good ee (17, 18).
Table 2.
Alkene Substrate Scopea
Reactions irradiated with a 23 W CFL for 6 h. Yields reported are the combined isolated yields of all diastereomers. Major diastereomer shown.
The scope of this reaction with respect to the aryl ketone component is summarized in Table 3. The aryl moiety tolerates significant electronic perturbation: both electron-rich and electron-deficient substituents provided the corresponding cyclopentanes in good yield and excellent ee (19–22). Heteroaryl cyclopropyl ketones are also tolerated (23), although the ee suffers if the heterocycle is positioned to provide an alternate site for Lewis acid chelation. Arene substituents at the 3-position have minimal impact on the selectivity of the reaction (24). However, 2-substituents have a large deleterious effect, which would be expected if the ketone were coordinated to the chiral Lewis acid in the enantioselectivity-determining step. The ester moiety can be replaced by a ketone with minimal impact on the stereoselectivity (25), but a methyl-substituted cyclopropane provides poor ee (26). On the other hand, cyclopropyl ketones bearing geminal β-dialkyl substituents afford excellent ee, although higher Lewis acid concentrations were required for optimal rate (27, 28).
Table 3.
Cyclopropane Substrate Scopea
Yields reported are the combined isolated yields of all diastereomers. Major diastereomer shown.
Reaction conducted using 20 mol% Gd(OTf)3 and 30 mol% L6 at −20 °C for 48 h.
Scheme 2 depicts our working model for the mechanism of this reaction. Photoexcitation of Ru(bpy)32+ and reductive quenching by i-Pr2NEt affords Ru(bpy)3+. Subsequent electron transfer to phenyl ketone 4 occurs only upon activation with the chiral Gd(III) Lewis acid; the resulting ketyl radical ([Gd]-4•−) undergoes reversible ring-opening followed by slow stepwise cycloaddition with styrene to afford product ketyl radical [Gd]-7•−. Formation of the neutral product 7 could occur either by chain-propagating electron transfer to another equivalent of substrate or by chain-terminating reduction of the photogenerated amine radical cation.
 |
(1) |
 |
(2) |
 |
(3) |
Scheme 2. Proposed mechanism for enantioselective [3+2] cycloaddition.
The mechanism proposed in Scheme 2 is supported by several lines of evidence. First, a reaction with deuteriumlabeled styrene d2-5 gives an inverse secondary kinetic isotope effect (kH/kD = 0.78) consistent with a rate-limiting intermolecular C–C bond-forming step (eq 1).15 Second, Tanko has reported that the ring-opening of similar cyclopropyl ketyl radicals is reversible and endergonic.16 To validate this expectation, we monitored a reaction starting with the cis isomer of 4 and found that the cyclopropane was completely isomerized to the trans isomer within 1 h, well before the reaction was complete. This reversible cleavage is consistent with the observation that racemic β,β’-disubstituted cyclopropane 29 undergoes stereoconvergent cycloaddition to cyclopentyl ketone 30 in good diastereoselectivity and excellent ee (eq 2).17 The cycloaddition of unsymmetrically substituted cyclopropyl ketone 31 also provides excellent stereoselectivity as well as exclusive chemoselectivity for the formation of enantioenriched cyclopentane 32 and not its constitutional isomer (eq 3).
While the scope of this new asymmetric [3+2] cycloaddition is complementary to the established enantioselective reactions of donor-acceptor cyclopropanes, the aryl ketone moiety required for the initial one-electron reduction process imposes an undesirable limitation on scope. Thus we wondered if the aryl ketone could be removed with retention of stereochemistry through a Baeyer–Villiger cleavage. Indeed, the p-methoxyphenyl ketone cycloadduct 19 undergoes completely regioselective oxidation to afford 33 in good yield, the activated ester of which is poised for further manipulation into diverse carboxylic acid derivatives. Under identical conditions, p-trifluoromethylphenyl ketone 34 undergoes regiocomplementary oxidation to afford benzoate ester 35. Thus, the applicability of this [3+2] photocycloaddition method to reactions of electronically varied aryl ketones provides a flexible strategy for the conversion of the enantioenriched products to a diverse array of five-membered carbocyclic derivatives.
These studies have several important implications. From a practical perspective, this method provides an asymmetric catalytic method to assemble structurally complex five-membered carbocycles, which are a class of compounds that remain challenging to prepare in enantioenriched form. More broadly, these results demonstrate that the combination of chiral Lewis acid and photoredox catalysis offers a robust and potentially general approach to photochemical stereocontrol that is broadly applicable to the increasing number of powerful transformations achievable using photoredox catalysis.
Supplementary Material
Scheme 3. Cleavage of the aryl ketone moiety.
Acknowledgments
We thank Ilia Guzei for determination of the absolute configuration of 11 by X-ray crystallographic analysis. The authors are grateful to the NIH (GM098886) for financial support and to Sigma–Aldrich for a generous gift of RuCl3. The NMR facilities at UW–Madison are supported by a generous gift from the Paul J. Bender fund.
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting Information is available free of charge via the Internet at http://pubs.acs.org”: Detailed experimental procedures and compound characterization data (PDF); X-ray crystallographic data for 11.
REFERENCES
- 1.(a) Walsh PJ, Kozlowski MC. Fundamentals of Asymmetric Catalysis. Sausalito: University Science Books; 2009. [Google Scholar]; (b) Ojima I. Catalytic Asymmetric Synthesis. Hoboken: Wiley; 2010. [Google Scholar]; (c) Gawley RE, Aubé J. Principles of Asymmetric Synthesis. 2nd. Chapter 6. Oxford: Elsevier; 2012. [Google Scholar]
- 2.For reviews on asymmetric photochemical synthesis, see: Rau H. Chem. Rev. 1983;83:535–547. Inoue Y. Chem. Rev. 1992;92:741–770. Brimioulle R, Lenhart D, Maturi MM, Bach T. Angew. Chem. Int. Ed. 2015;54:3872–3890. doi: 10.1002/anie.201411409.
- 3.(a) Müller C, Bauer A, Bach T. Angew. Chem. Int. Ed. 2009;48:6640–6642. doi: 10.1002/anie.200901603. [DOI] [PubMed] [Google Scholar]; (b) Müller C, Bauer A, Maturi MM, Cuquerella MC, Miranda MA, Bach T. J. Am. Chem. Soc. 2011;133:16689–16697. doi: 10.1021/ja207480q. [DOI] [PubMed] [Google Scholar]; (c) Maturi MM, Wenninger M, Alonso R, Bauer A, Pöthig A, Riedle E, Bach T. Chem. Eur. J. 2013;19:7461–7472. doi: 10.1002/chem.201300203. [DOI] [PubMed] [Google Scholar]; (d) Alonso R, Bach T. Angew. Chem. Int. Ed. 2014;53:4368–4371. doi: 10.1002/anie.201310997. [DOI] [PubMed] [Google Scholar]; (e) Vallavoju N, Selvakumar S, Jockusch S, Sibi MP, Sivaguru J. Angew. Chem. Int. Ed. 2014;53:5604–5608. doi: 10.1002/anie.201310940. [DOI] [PubMed] [Google Scholar]; (f) Maturi MM, Bach T. Angew. Chem. Int. Ed. 2014;53:7661–7664. doi: 10.1002/anie.201403885. [DOI] [PubMed] [Google Scholar]
- 4.(a) Guo H, Herdtweck E, Bach T. Angew. Chem. Int. Ed. 2010;49:7782–7785. doi: 10.1002/anie.201003619. [DOI] [PubMed] [Google Scholar]; (b) Brimioulle R, Guo H, Bach T. Chem. Eur. J. 2012;18:7552–7560. doi: 10.1002/chem.201104032. [DOI] [PubMed] [Google Scholar]; (c) Brimioulle R, Bach T. Angew. Chem. Int. Ed. 2014;53:12921–12924. doi: 10.1002/anie.201407832. [DOI] [PubMed] [Google Scholar]; (d) Brimioulle R, Bach T. Science. 2013;342:840–843. doi: 10.1126/science.1244809. [DOI] [PubMed] [Google Scholar]
- 5.Du J, Skubi KL, Schultz DM, Yoon TP. Science. 2014;344:392–396. doi: 10.1126/science.1251511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For an application of tandem Lewis acid/photoredox catalysis to an asymmetric conjugate addition reaction, see: Espelt LR, McPherson IS, Wiensch EM, Yoon TP. J. Am. Chem. Soc. 2015;137:2452–2455. doi: 10.1021/ja512746q.
- 8.For a recent review of dual catalysis approaches in photoredox catalysis, see: Hopkinson MN, Sahoo B, Li J-L, Glorius F. Chem. Eur. J. 2014;20:3874–3886. doi: 10.1002/chem.201304823.
- 9.Lu Z, Shen M, Yoon TP. J. Am. Chem. Soc. 2011;133:1162–1164. doi: 10.1021/ja107849y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For examples, see: Parsons AT, Johnson JS. J. Am. Chem. Soc. 2009;131:3122–3123. doi: 10.1021/ja809873u. Parsons AT, Smith AG, Neel AJ, Johnson JS. J. Am. Chem. Soc. 2010;132:9688–9692. doi: 10.1021/ja1032277. Trost BM, Morris PJ. Angew. Chem. Int. Ed. 2011;50:6167–6170. doi: 10.1002/anie.201101684. Xiong H, Xu H, Liao S, Xie Z, Tang Y. J. Am. Chem. Soc. 2013;135:7851–7853. doi: 10.1021/ja4042127. Hashimoto T, Kawamata Y, Maruoka K. Nat. Chem. 2014;6:702–705. doi: 10.1038/nchem.1998.
- 11.For examples of the use of (pybox)Gd(III) complexes in asymmetric catalysis, see: Evans DA, Wu J. J. Am. Chem. Soc. 2003;125:10162–10163. doi: 10.1021/ja0367602. Evans DA, Song H-J, Fandrick KR. Org. Lett. 2006;8:3351–3354. doi: 10.1021/ol061223i. Suga H, Ishimoto D, Higuchi S, Ohtsuka M, Arikawa T, Tsuchida T, Kakehi A, Baba T. Org. Lett. 2007;9:4359–4362. doi: 10.1021/ol701936b. Suga H, Higuchi S, Ohtsuka M, Ishimoto D, Arikawa T, Hashimoto Y, Misawa S, Tsuchida T, Kakehi A, Baba T. Tetrahedron. 2010;66:3070–3089.
- 12.(a) Nishiyama H, Yamaguchi S, Kondo M, Itoh K. J. Org. Chem. 1992;57:4306–4309. [Google Scholar]; (b) Park S-B, Murata K, Matsumoto H, Nishiyama H. Tetrahedron: Asymm. 1995;6:2487–2494. [Google Scholar]; (c) Wang H, Wang H, Liu P, Yang H, Xiao J, Li C. J. Mol. Catal. A: Chem. 2008;285:128–131. [Google Scholar]; (d) Bettencourt-Dias Ad, Barber PS, Viswanathan S, Lill DTd, Rollet A, Long G, Altun S. Inorg. Chem. 2010;49:8848–8861. doi: 10.1021/ic101034y. [DOI] [PubMed] [Google Scholar]
- 13.The d.r. reflects the ratio of the major diastereomer shown and an epimer at the ester-bearing carbon. Only a trace of a presumed third diastereomer was observable by 1H NMR analysis of the unpurified reaction mixture and could not be isolated for identification.
- 14 The absolute configuration of 11 was determined by X-ray crystallographic analysis; the configuration of other cycloadducts were assigned by analogy.
- 15.Feld M, Stefani AP, Szwarc M. J. Am. Chem. Soc. 1962;84:4451–4453. [Google Scholar]
- 16.Tanko JM, Drumright RE. J. Am. Chem. Soc. 1992;114:1844–1854. [Google Scholar]
- 17.The minor diastereomer of 31 can be isolated and resubjected to the reaction conditions with no loss of stereochemical integrity. Thus the diastereoselectivity does not reflect an equilibrium ratio.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








