Abstract
Suicide is the second leading cause of death, worldwide, for those between the ages of 24 and 44 y old. In 2013, more than 41,000 suicides occurred in the United States. These statistics underscore the need to 1) understand why people die by suicide and 2) identify risk factors that are potentially modifiable. While it has been posited that sleep disturbance may represent one such factor, systematic research in this arena did not begin until the 2000s. Since that time, sleep disturbance has been reliably identified as a risk factor for suicidal ideation, suicide attempts, and suicide. While insomnia, nightmares, and other sleep disorders have each been found to contribute to the risk for suicidal ideation and behavior, it is also possible that these factors share some common variance. One possibility is that sleep disturbance results in being awake at night, and being awake at night also confers risk. The hypothesis proffered here is that being awake when one is not biologically prepared to be so results in “hypofrontality” and diminished executive function, and that this represents a common pathway to suicidal ideation and behavior. Such a proposition is highly testable under a variety of possible protocols. The current review summarizes the extant literature on suicide rates by time-of-day, and discusses circadian, psychosocial, and neuro-cognitive explanations of risk. Such a focus promises to enhance our understanding of how sleep disturbance may confer risk, allows for the identification of future lines of research, and further justifies the need for interventions that promote good sleep continuity among at-risk individuals.
Keywords: Sleep disturbance, Suicide, Circadian patterning, Hypofrontality, Executive function
Introduction
In recent years, sleep disturbance has been identified as an indicator of risk for suicidal ideation, suicide attempts, and suicide. To date, more than 40 studies have evaluated this association. Each has varied in its design, assessment methods (i.e., of sleep and suicidal behaviors), study suicide outcome measures, samples, and the extent to which other, important suicide risk factors were covaried. These studies were summarized meta-analytically in 2012 [1]. The results from this quantitative review were that sleep disturbance (in general) is associated with increased risk for suicidal ideation, suicide attempts and death by suicide (RR = 2.79, 95% CI = 2.44–3.19) [1]. Depression was not found to moderate these associations. These findings were echoed by a more recent systematic review conducted by Bernert and colleagues (2015) [2] which included 18 well-controlled empirical investigations. Here again, insomnia, poor sleep quality, and nightmares, were significantly and independently predictive of increased risk for suicidal ideation, suicide attempts, and death by suicide.
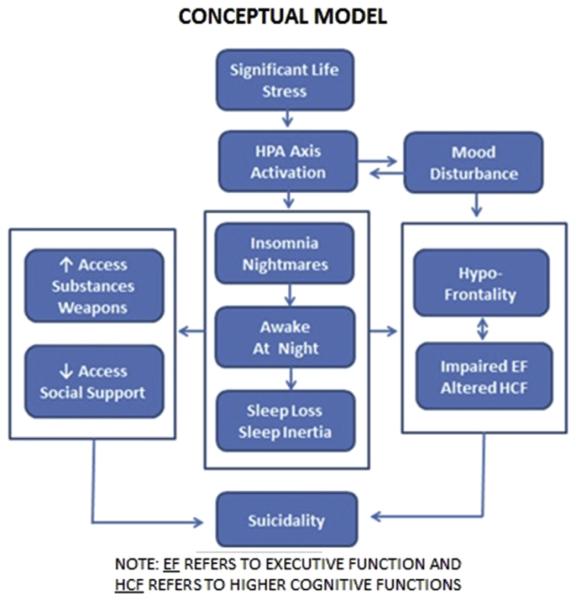
Interestingly, in at least the meta-analysis, insomnia, nightmares, and “other” sleep disorders were found to have comparable risk ratios (relative risk ratios are 2.84, 2.61, and 2.72 respectively) with respect to suicidal ideation and behavior, in general. While several studies have suggested that insomnia and nightmares confer unique risk for suicidality (ideation, attempt, and death by suicide) [3–5], it remains possible (if not likely) that the various forms of sleep disturbance also share common variance. One possibility is that sleep disturbance results in being awake at night, and it is the fact of being awake at night that confers some proportion of the risk. Given this point of view, the emergent question is, “What about being awake at night confers risk?”. One possibility is that being awake at night may be associated with increased utilization of alcohol and other substances, reduced social support, and easier access to weapons. These factors alone likely confer increased risk for suicide. Another possibility is that insomnia and/or nightmares contribute to suicidal ideation and behavior by intensifying the individual's sense of hopelessness, isolation, and distress relative to the inability to sleep. Still yet another possibility is that being awake at night (when one is not biologically predisposed to be awake) may result in a decrease in frontal lobe function (i.e., hypoactivation of the frontal lobes due to circadian effects, sleep loss/sleep deprivation, and/or sleep inertia). Hypofrontality, in turn, may result in diminished problem solving abilities and increased impulsive behavior, both of which may be expected to increase the risk for suicide. More succinctly, being awake at night, when one is not biologically prepared to be awake, likely results in “hypofrontality1” and diminished executive function. These factors are likely to interact with other sleep considerations (e.g., sleep loss) and social factors (e.g., lack of social support at night) and place the individual at greater risk for suicidal ideation and behavior, especially in those already at risk due to depression and/or adverse life circumstances. This concept is schematically represented in Fig. 1.
Fig. 1.
A conceptual model of the association of sleep disturbance and suicidality.
Below we review the evidence that suicide may exhibit a circadian pattern, the possibility that suicide may occur disproportionately in individuals who are awake at night, and the data that exist with respect to sleep deprivation and circadian effects on frontal lobe activity, executive function, and mood. As part of the review on sleep deprivation effects on mood we address the potential paradox that sleep deprivation is reliably found to exert positive effects on mood and yet may nonetheless interact with time of night to increase the risk for suicidal ideation and behavior. We conclude with a summary of the related evidence, with suggestions for future research, and raise the possibility that the perspective put forward here may be transdiagnostic with respect to psychiatric disorders.
Existing literature on nocturnal wakefulness & rates of death by suicide
To date, nine studies ([3–10]) have investigated the temporal patterning of death by suicide across the 24 h day and found that the peak frequency for suicide occurs during the day. These studies are briefly summarized below.
Vollen & Watson (1975) [6]
An archival chart study was carried out on 42 individuals that had committed suicide while being cared for at a VA hospital from 1957 to 1971. Data from the in-patient sample showed that the majority of the suicides occurred between 15:00 and 18:00h (n = 12). The probability that 75 percent of a set of suicides would occur during one 3-h period of a 16-h waking day was assessed using a critical ratio test for the difference between two proportions. The resulting critical ratio was 1.55. Thus, the data suggest that suicides may be more likely to occur between 15:00 and 18:00h.
Barraclough (1976) [7]
One hundred suicides, as defined by coroner inquests, occurring from 1966 to 1968 in West Sussex or in Borough of Portsmouth were included in the sample. The data from 52 cases were classified as occurring in one of four 6 h blocks (00:00 to 05:59h; 06:00 to 11:59h; 12:00 to 17:59h; and 18:00 to 23:59h). The incidence by time of day data were 6, 16, 15, and 15, respectively. While not specifying the manner of statistical analysis, the authors concluded that there was not sufficient evidence to suggest that suicide varies by time of day. Surprisingly, the 50% reduction in the mean incident rate from 00:00 to 06:00h was not highlighted.
Williams & Tansella (1987) [8]
Using national data on suicide collated and published by the Istituto Centrale di Statistica (ISTAT) in Rome, the temporal patterning of 25,987 suicides was evaluated for suicides occurring from 1974 to 1983. When assessed in terms of five time bands (01:00–05:59h; 06:00–10:59h; 11:00–15:59h; 16:00–20:59h; 21:00-00:59h) the peak frequencies were at 06:00h and 16:00h.
Maldonado & Kraus (1991) [9]
Time of death data (from coroner's autopsy and investigative reports) from Sacramento County California were acquired for a time period extending from 1925 to 1983. 4190 suicide deaths were identified during the study period. When assessed in terms of six time bands (3–4 h intervals) and measured as percent deviations from the mean, the overall peak frequencies were at 12:00 to 16:00h and the fewest suicide deaths were at 04:00 to 08:00h. The investigators also suggest that significant differences were observed for sex, age, and year of the study.
Gallerani et al. (1996) [10]
Data for 223 cases of suicide from 1983 to 1992 in Ferrara Italy were assessed for time of day effects. The determination of the hour of suicide was precise or “presumptive” in 115 cases. The incidents were profiled by hour and grouped into four 6-h periods (night, morning, afternoon, and evening). The data were analyzed both by means of chi-square test for goodness of-fit and by single cosinor analysis. A specific pattern, characterized by a significant peak in the late morning early afternoon hours was found for the entire sample and the sex subgroups. A graphical representation of the hourly data provided in tabular form shows a gradual rise across the morning hours (starting at 05:00 to 06:00), a peak at noon to 13:00h (although the peak is trimodal), and a steady decline from 17:00 to 18:00h. The lowest incident rates are clearly at night starting at 19:00 to 20:00h with a trough at 04:00 to 05:00h.
Altamura et al. (1999) [11]
In this study, a broad perspective was taken on the potential temporal distribution of suicide including the assessment of the relevance of month, seasons, day of the week, and time of the day. Data on suicides in Cagliari (Italy) from 1990 to 1994 were analyzed by means of spectral analysis, cosinor and multiple regression analysis. With respect to time of day, three rhythms were evident, i.e., 24 h (circadian), 8 h and 1 h, explained 63.9% of the variance in the number of suicides by time of the day. Peak numbers in number of suicides were found between 08:30h and 12:30h. Age and sex were not found to be related to the observed circadian patterning.
Preti and Miotto (2001) [12]
Incidence of suicide was assessed in a national cohort of 14,743 suicides occurring from 1994 to 1997 in Italy. Three time periods were evaluated (morning, afternoon, evening/night) both for main effects and interactions with age and sex. The investigators reported that a clear diurnal variation in the distribution of suicides over time was observed for both sexes, with a peak in the late morning (08:00–11:00h), and a subsequent decrease to a trough in the night hours. Age and sex interactions were noted.
Houwelingen & Beersma (2001) [13]
In this study the investigators focus on the occurrence of train suicides across the 24 h day and on the possibility that day length or total hours of sun light may impact suicide rates. Detailed information on the exact time of suicides was obtained in individuals from the Netherlands. Exact timing for 2830 suicides was possible given the public manner of the suicide (train suicides). Data was obtained from the Netherlands Railways archives from 1980 to 1994. Daily patterns in train suicides show systematic variations of two kinds. First, independently of time of year, suicide rates at night are about 10% of their daytime values. Second, there are two daily peaks in the patterns, which shift their timing over the year, with one peak occurring shortly after sunset, and the other one consistently occurring 9–10 h earlier. Depending on the time of year, these peaks are at 08:00–11:00h and 05:00–08:00h.
Erazo et al. (2004) [14]
The goal of this investigation was to evaluate the effects of gender on the temporal patterning of suicide by season, day of week, and time of day. Data were drawn from a German National Central Registry of all accidents on the German railway net (STABAG) between 1997 and 2002. Circadian data were presented by gender and season. As with prior studies, the incidence of suicide was lowest at night (00:01–06:00h), as compared to the remainder of the 24 h day. The circadian effects showed some degree of interaction with gender and season such that suicides in females peaked in the morning hours (6:00 to 12:00h) and this effect was particularly pronounced in the summer. The winter distribution of suicide was bimodal with peaks at 06:00–09:00h and 18:00–24:00h.
Summary of Findings and Limitations
In summary, the extant data demonstrate a variation in the rate of suicide across the 24-h day. Some studies show this pattern to be bimodal, while others show a single diurnal peak. When considered together, the modal peak rate is from 10:00 to 14:00h. All nine studies reviewed here suggest (directly or indirectly) that the rate of suicide at night is a faction of that which occurs during the day. For example, Houwelingen & Beersma (2001) [13] suggest that 10% of suicides occur at night (22:00 to 06:00h). This particular estimate is likely to be a conservative one given that train suicides necessarily vary with train schedules which are likely less frequent at night. Although seminal, and remarkably consistent in their findings, the above studies have one or more of several limitations. First, very few studies differentiate between time of death and time of fatal injury. While often difficult to discern, the two variables may have very different temporal distributions. Second, not all studies assess for sex and age differences and/or how mode of suicide may assort differently with respect to the circadian patterning of suicide. Third, the method of binning time (e.g., hour by hour vs. 2,4, or 6 h blocks) and/or analyzing effects over time (simple frequency plots, spectral analysis, cosinor and/or multiple regression analysis) may be responsible for the observed phase, amplitude, and modality differences in the data. Fourth, and perhaps most important, none of the analyses take into account the proportion of the population awake at each time interval. Since suicide represents an intentional behavior that can only be performed when awake, the population that is at risk of suicide varies by time of day. Further the probability of the incident events is not equal from 1 h to the next, thus the simple calculation of frequencies or rates per hour (or time block) has the potential to provide a skewed estimate how suicide risk varies across the circadian day and night. That is, it may be true that the absolute frequency of suicide is lower at night but the risk is nevertheless disproportionately higher at night than would be expected given the proportion of the population that is awake. In order to address this issue, proportion data by time needs to be weighted by not only the sum total of suicides but also by the percentage of people likely to be awake at each given hour.
A recent study by our group [15] evaluated the incidence of suicide by clock time while accounting for the proportion of the population that is likely awake at each given hour. Archival analyses were conducted estimating the time of fatal injury using the National Violent Death Reporting System (NVDRS) [16] and the proportion of the American population awake per hour across the 24 h day using the American Time Use Survey (ATUS) [17]. The NVDRS dataset, compiled by the Centers for Disease Control and Prevention (CDC), includes details on violent deaths from 18 participating US states and includes time of fatal injury data for a total of n = 35,332 documented suicides. The ATUS is a database maintained by the US Bureau of Labor Statistics (BLS) and is an annual survey that assesses a range of activities across the 24-h day in a representative sample of Americans. The survey is conducted by phone and requires respondents to retrospectively profile their activities hour by hour for the last 24 h. Responses are coded into standardized categories. The primary variable of interest was the report of “sleep.” The primary results from this study were that 1) 20% of suicides occurred at night and 2) when accounting for the proportion of the population that is awake at each given hour, suicide is much more likely to occur at night then during the morning, afternoon or evening hours. This finding was consistent across age groups, sex, race/ethnicity groups, and for depressed and non-depressed individuals. More specifically, the mean hourly incident rate from 06:00–23:59h was 2.2% ± 0.7%, while the mean incident rate from 24:00–05:59h was 10.3% ± 4.9%. The maximum incident rate was at 2:00–2:59h (16.3%). Hour-by-hour observed values differed from those that would be expected by chance (p < 0.0001), and when 6-h blocks were examined, the observed frequency at night was 3.6 times higher than would be expected by chance (p < 0.0001). In sum, these data suggest that being awake at night confers greater risk for suicide than being awake at other times of the day. This finding, in turn, highlights the possibility that sleep loss and/or circadian factors may moderate the observed association between nocturnal wakefulness and death by suicide [15].
Circadian and homeostatic effects on frontal EEG activity and executive function
Sleep deprivation & circadian effects on EEG
In the present model (See Fig. 1), it is hypothesized that individuals that are awake during the typical sleep period exhibit an increased level of hypofrontality that occurs as a result of the interaction of homeostatic and circadian factors. As measured by electroencephalography (EEG), it would be expected that individuals awake during the nocturnal phase of the 24 h day (as compared to the diurnal phase of the 24 h day) would exhibit hypofrontality (more frontal cortex theta and delta activity and less beta and gamma activity). Further, such increases would be more evident in individuals with depression. To date, several areas of research exist which support the viability of this perspective. Under the conditions of experimentally induced sleep deprivation, the waking EEG of heathy non-depressed subjects during the sleep deprivation period has been reliably found to include higher waking delta, theta, and theta/alpha activity. Such activity has been found to be most pronounced in frontal areas, increased with the duration of sleep deprivation, and was associated with increasing self-reported fatigue [18,19]. More recently, it has been shown that sleep deprivation leads to a loss of functional connectivity in forebrain regions [20]. Under the conditions of a constant routine (total sleep deprivation where light, diet, body position, etc. are held constant), sleep loss increases frontal delta EEG power density [21]. This suggests that “frontal brain regions are particularly vulnerable to the effects of elevated sleep pressure (‘prefrontal tiredness’)”. When evaluated in depressed and non-depressed women, frontal delta EEG was elevated in women with major depressive disorder (MDD) across the whole of the 24 h day with a peak elevation between 04:00 and 08:00h. This suggests that women with MDD exhibit an exaggerated form of hypofrontality and that this phenomenon peaks at time that overlaps with the time frame of interest (the peak in suicide that occurs from 00:00 to 05:00) [22]. These effects maybe further exaggerated by sleep inertia thus creating increased vulnerability to hypofrontality in the period extending from when the nocturnal awakening occurs to up to 25 min following such awakenings [23]. Under the conditions of a 90 min day protocol (wake periods for 60 min and sleep periods for 30 min alternating over 1–3 d' time), beta activity (C3–C4 NREM EEG) exhibits a robust circadian rhythmicity in healthy subjects with a peak during the diurnal phase and trough during the nocturnal phase of the of the 24 h day. This patterning of activity suggests that waking frontal beta activity during the nocturnal phase may be similarly suppressed [24]. Taken together, such findings support the possibility that subjects awake at night exhibit a form of hypofrontality that may be detected with electroencephalography.
Sleep deprivation and circadian effects on executive function
In the present model, it is hypothesized that individuals that are awake during the circadian night exhibit will impaired cognitive function. These impairments likely occur as a result of the interaction of homeostatic and circadian factors. That is, as sleep debt accrues and the individual continues to be awake into the typical sleep period, cognitive performance declines [18,25,26]. A schematic representation is provided in Fig. 2.
Fig. 2. Cognitive performance/executive function across the 24 h day with and sleep loss.
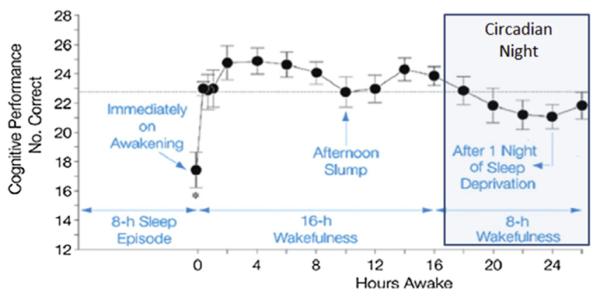
Note: Fig. 2 is adapted, with permission, from Wright et al. PMID 22529774.
While there is considerable evidence that sleep deprivation leads to slowing of response speed and increased variability in performance, particularly for simple measures related to alertness, attention and vigilance e.g., [27–30], the findings with respect to executive function are less clear [31,32]. Broadly construed, executive functioning refers to a variety of cognitive processes that are ascribed to the prefrontal cortex, including organization of information, prioritization, detection of novelty, assessment of risk, divergent thinking, problem solving and planning, and the inhibition of behavioral responding. As measured with neuropsychological tests, executive functioning is regularly assessed in terms of capacities related to response inhibition and task switching, and updating. In forced desynchrony (no sleep deprivation) and in constant routine protocols (total sleep deprivation), inhibitory control as measured with the Stroop has been found to be reliably compromised during the second half of the biological night with the worst performance at the end of the biological night [25,33,34]. Similar findings have been observed, although less reliably, for switching and updating using a variety of tasks including the Stroop task with shifting criteria [25]. Additional studies have shown that sleep deprivation decreases the individual's ability to make emotional judgments [31], changes risk preferences [34], and decreases the ability to incorporate multiple pieces of information into a single decision [34]. While these findings are not without exception [35], they nonetheless support the possibility that subjects awake at night experience diminished capacity with respect to executive function.
Sleep deprivation and circadian effects on mood
It has long been hypothesized that mood varies with time of day, alone or in interaction with amount of prior sleep. Experimental work on this subject also suggests that where the sleep period is positioned during the 24 h day and individual factors (person's “Chronotype”) are also of relevance with respect to the circadian patterning of mood. With these caveats in mind, it is generally held that the nadir for positive mood or happiness is aligned with temperature minima which generally occurs in normally entrained individuals at about 04:00h [e.g., 36,37].
Sleep deprivation effects on depression – a potential paradox
It is well established that acute sleep deprivation can have significant antidepressant effects [38–40]. This effect has been shown for a variety of deprivation manipulations including total sleep deprivation (TSD), partial sleep deprivation (PSD), and selective REM sleep deprivation. TSD has been the most exhaustively studied with more than 60 studies being conducted prior 1990. In a systematic review of the early literature Wu and Bunney [41] indicate that 59% of patients exhibit treatment responses to TSD. Further, they show that the effect is linear with time and that mood linearly improves across bi-hourly measures for the sleep deprivation period and the following day. As with all forms of sleep deprivation, the mood improvements are usually lost following recovery sleep (50–80% of subjects relapse following the next sleep bout). There is, however, some evidence that clinical gains can be maintained with concomitant use of antidepressant medications [40]. PSD has been shown to have similar antidepressant effects, particularly when conducted in the second half of the night (PSD-L). Generally, PSD is of approximately 4 h in duration. Whether precisely timed shorter duration forms of PSD have antidepressant effects is unknown, however 2-h forced awakenings in the middle of the night, have not been shown to produce appreciable clinical effects [40]. Finally, selective REM sleep deprivation also appears to have antidepressant effects but requires weeks, not hours, to produce changes in mood [38].
In the present conceptualization, the biological effects of sleep deprivation are hypothesized, in combination with time of day, to create a window of vulnerability with respect to suicidal ideation and behavior. At first glance, this perspective does not seem to be consistent with the well documented antidepressant effects of sleep deprivation. This potential paradox, however, can be reconciled in several ways. First, it is possible that those at marked risk for suicidal ideation and behavior may be among the individuals who are non-responders to sleep deprivation. Wu and Bunney's data are consistent with this point of view as they show worsening mood in treatment non-responders at 01:00h, 03:00h, and 05:00h. Second, even in treatment responders, the beneficial effects of sleep deprivation on mood sometimes are not noted until the early morning. Thus, mood may initially worsen across the period of sustained wakefulness before the emergence of a rebound “euphoria” following the biological night and/or with exposure to sunlight (i.e., a “it is darkest before the dawn” effect). Third, it is possible that assessing depression as a global construct has not allowed for a differentiation between mood/energy and cognitive effects. That is, it is possible that (even in treatment responders) mood and energy may improve while the individual's capacity to make reasoned judgments is impaired. This could result in a scenario where the individual is sufficiently activated as to be able to make a suicide attempt [42].
Summation
The well-replicated finding that sleep disturbance is a significant risk factor for suicidal ideation and behavior and the more recent finding that individuals are at increased risk for death by suicide at night, may provide a window on to how people decide to end their own lives. While several pathways have been suggested regarding how sleep disturbance may confer risk [43], the present review focuses on circadian fluctuations in frontal lobe/executive function. Specifically, we are proposing that the 20% of suicides that occur at night [15] occur in large measure because of a neurobiological/neuropsychological vulnerability that occurs in all humans. That is, when one is awake when not biologically prepared to be awake (i.e., not sleep sated and/or in a circadian phase that is associated with alertness and higher cognitive functioning), the consequence is a kind of brain activity that is associated with poor executive function. This places the individual in a context where they lack the resources to make reasoned choices, let alone life-and-death judgments, judgments that “in the clear light of day” might be very different. This perspective is supported by several lines of evidence including: 1) there is an relative increase in the number of suicides that are committed at night; 2) EEG “hypofrontality” occurs in association with sleep deprivation and as a function of circadian effects; 3) executive function is compromised by sleep deprivation and time of day effects; and 4) positive mood appears to be at its lowest during the circadian night and this phenomenon may or may not be exaggerated by sleep deprivation. In contrast to these supporting lines of evidence, the therapeutic outcomes of sleep deprivation on mood appear to suggest the opposite; that extended nocturnal wakefulness is protective with respect to suicide suicidal ideation and behavior. Both phenomena may be true. One potential resolution may be that the vulnerability conferred by nocturnal wakefulness occurs in the 20–40% of subjects that do not respond to the antidepressant effects of partial or total sleep deprivation.
Given that “being awake at night confers increased risk for suicide ideation and behavior for at least a significant subset of patients with depression, it follows that 1) targeted treatment for insomnia and nightmares and 2) the increased allocation/utilization of psycho-social resources at night (e.g., increased availability of peer and professional support) should have substantial value as preventive strategies for suicide. The former, appears to particularly viable given the results from a recent study by Manber and colleagues (2015) [44] which provides the first evidence that cognitive behavioral therapy for insomnia (CBT-I) can significantly reduce suicidal ideation.
Concluding remarks
It should be noted that the proposed model is focused on the incidence of suicidal ideation and behavior that occur at night. This narrow focus does not preclude the possibility that sleep loss also exerts effects on executive function during the day [45,46] and thus this also may explain why insomnia is associated with suicide via this particular pathway.
Finally, the proposed model has the potential to be transdiagnostic. That is, being awake at night (with or without corresponding sleep loss) may confer increased risk for any behavior that requires the individual to be motivated not to engage in and/or that requires exceptional levels of impulse control.2 Accordingly, being awake at night may also have implications for other psychiatric/ behavioral disorders including eating, addictive, paraphilic, and obsessive compulsive disorders. The present perspective suggests that the incidence of such abnormal behaviors should be highest during the circadian night and/or symptoms worst during the day given sleep disturbance. In all cases, the implication being that improved sleep will improve clinical outcomes within these domains.
Practice points.
1) Identification of sleep disturbance may serve as means to identify risk for suicidality.
2) Identification of wakefulness during the traditional sleep period may also serve as means to identify risk for suicidality.
3) Treatment for sleep disturbance may reduce the risk for suicidal ideation and behavior.
4) Allocating increased mental health resources during the circadian night may serve to reduce the incidence of suicide attempts and/or death by suicide.
Research agenda.
“Is it a bad thing to be awake when reason sleeps?”
The exploration of the proposed model could proceed along any of several directions. First, sleep deprivation studies along with repeated measure assessments could be used to assess the combined effects of sleep loss and time of day on forebrain activity and executive function. Second, it may be equally informative to assess hypofrontality and executive function using any one of several chronobiologic paradigms including a constant routine protocol (CR [sleep deprivation]), alone or in parallel with 90 min day protocol (NMD [minimal sleep deprivation after 24 h]). This would allow a precise delineation of how ideation, hypofrontality, and executive function (in depressed subjects and nondepressed subjects) vary hour-by-hour across the 24 h day, and most importantly, hour-by-hour across the biological night. Third, evaluating inpatients who have been hospitalized for suicide attempts (using any of the protocols deemed feasible along with measures of forebrain activity and executive function) would allow for a strong test of the hypothesis given its assessment in the most relevant population. This would be especially illustrative if conducted in a manner similar to Keilp et al. [47], who evaluated executive function in depressed inpatients with no, low, and high lethality suicide attempts [48]. Fourth, the use of positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) imaging may more successfully resolve “hypofrontality” as it occurs with either sleep deprivation or chronobiologic protocols. Finally, the larger issue of circadian variation in suicidal ideation may be productively assessed epidemiologically using an ecological momentary assessment approach like that reported by Ben-Zeev and colleagues [49,50]. Regardless of which next step is taken, all such efforts will serve to elucidate how suicide ideation and risk varies across the 24 h day and why simply being awake at night may represent a risk factor (and likely a modifiable one) for suicidal ideation and behavior.
Acknowledgments
The empirical work regarding the circadian patterning of suicide has been ongoing since 2013 and includes the efforts and talents of a larger group of investigators than those that contributed to the present theoretical review including: David Dinges, Mathias Basner, Ruben Gur, David Roalf, Knashawn Morales, Phil Gehrman, and Ninad Chaudhary. The following are in receipt of grants as follows: Michael Perlis (R01AG041783, R01AT003332, R01MH077900); Michael Grandner (R21ES022931, K23HL110216); Gregory Brown (R01MH086572); Mathias Basner (National Space Biomedical Research Institute through NASA NCC-958); Subhajit Chakravorty (IK2CX000855); Michael Thase (R01MH082794); and David Dinges (R01NR004281).
Abbreviations
- ATUS
American time use survey
- BLS
bureau of labor statistics
- C3
central EEG site (left), over the sensory-motor area
- C4
central EEG site (right), over the sensory-motor area
- CBT-I
cognitive behavioral therapy for insomnia
- CDC
centers for disease control and prevention
- CR
constant routine
- EEG
electroencephalography
- fMRI
functional magnetic resonance imaging
- MDD
major depressive disorder (depression)
- NES
night eating syndrome
- NMD
ninety minute day
- NREM
non-rapid eye movement sleep (Stages 1, 2, 3, 4)
- NVDRS
national violent death reporting system
- PET
positron emission tomography
- PSD
partial sleep deprivation
- REM
rapid eye movement
- SRED
sleep related eating disorder
- TSD
total sleep deprivation
Footnotes
Hypofrontality most often refers to low levels of cerebral blood flow within the frontal cortex (i.e., hypo-perfusion). In the present proposal the term refers to, more generally, diminished activation within this region.
The broader conceptualization was formulated in discussion with Jennifer Lundgren (PhD FAED, Associate Professor and Chair, Department of Psychology, University of MissourieKansas City) regarding how such concepts may be applied to nocturnal eating syndrome (NES) and sleep-related eating disorder (SRED).
Conflict of interest
The authors do not have any conflicts of interest to disclose.
References
* The most important references are denoted by an asterisk.
- [*1].Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012 Sep;73(9):e1160–1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- [*2].Bernert RA, Kim JS, Iwata NG, Perlis ML. Sleep disturbances as an evidence-based suicide risk factor. Curr Psychiatry Rep. 2015 Mar;17(3):554. doi: 10.1007/s11920-015-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cukrowicz KC, Ainhoa O, Pinto Jennifer V, Bernert Rebecca A, Krakow Barry, Joiner Thomas E., Jr The impact of insomnia and sleep disturbances on depression and suicidality. Dreaming. 2006 Mar;16(1):1–10. [Google Scholar]
- [4].Sjostrom N, Hetta J, Waern M. Persistent nightmares are associated with repeat suicide attempt: a prospective study. Psychiatry Res. 2009 Dec 30;170(2–3):208–11. doi: 10.1016/j.psychres.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [5].Nadorff MR, Nazem S, Fiske A. Insomnia symptoms, nightmares, and suicidal ideation in a college student sample. Sleep. 2011 Jan;34(1):93–8. doi: 10.1093/sleep/34.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vollen KH, Watson CG. Suicide in relation to time of day and day of week. Am J Nurs. 1975 Feb;75(2):263. doi: 10.1097/00000446-197502000-00018. [DOI] [PubMed] [Google Scholar]
- [7].Barraclough BM. Time of day chosen for suicide. Psychol Med. 1976 May;6(2):303–5. doi: 10.1017/s0033291700013866. [DOI] [PubMed] [Google Scholar]
- [8].Williams P, Tansella M. The time for suicide. Acta Psychiatr Scand. 1987 May;75(5):532–5. doi: 10.1111/j.1600-0447.1987.tb02829.x. [DOI] [PubMed] [Google Scholar]
- [9].Maldonado G, Kraus JF. Variation in suicide occurrence by time of day, day of the week, month, and lunar phase. Suicide Life Threat Behav. 1991;21(2):174–87. Summer. [PubMed] [Google Scholar]
- [10].Gallerani M, Avato FM, Dal Monte D, Caracciolo S, Fersini C, Manfredini R. The time for suicide. Psychol Med. 1996 Jul;26(4):867–70. doi: 10.1017/s0033291700037909. [DOI] [PubMed] [Google Scholar]
- [11].Altamura C, VanGastel A, Pioli R, Mannu P, Maes M. Seasonal and circadian rhythms in suicide in Cagliari, Italy. J Affect Disord. 1999 Apr;53(1):77–85. doi: 10.1016/s0165-0327(98)00099-8. [DOI] [PubMed] [Google Scholar]
- [12].Preti A, Miotto P. Diurnal variations in suicide by age and gender in Italy. J Affect Disord. 2001 Aug;65(3):253–61. doi: 10.1016/s0165-0327(00)00232-9. [DOI] [PubMed] [Google Scholar]
- [13].van Houwelingen CA, Beersma DG. Seasonal changes in 24-h patterns of suicide rates: a study on train suicides in The Netherlands. J Affect Disord. 2001 Oct;66(2–3):215–23. doi: 10.1016/s0165-0327(00)00308-6. [DOI] [PubMed] [Google Scholar]
- [14].Erazo N, Baumert J, Ladwig KH. Sex-specific time patterns of suicidal acts on the German railway system. An analysis of 4003 cases. J Affect Disord. 2004 Nov 15;83(1):1–9. doi: 10.1016/j.jad.2004.04.012. [DOI] [PubMed] [Google Scholar]
- [15].Perlis ML, Grandner MA, Basner M, Chakravorty S, Brown GK, Morales KW, et al. When accounting for wakefulness, completed suicides exhibit an increased likelihood during circadian night. Sleep. 2014;37:A268–9. Abstract Supplement. [Google Scholar]
- [16].National Center for Injury Prevention and Control . National violent death reporting system (NVDRS) Centers for Disease Control and Prevention; Atlanta, GA: 2013. [Google Scholar]
- [17].Bureau of Labor Statistics . American time use survey fact sheet. Bureau of Labor Statistics; Washington, DC: 2013. [Google Scholar]
- [18].Bonnet M. Acute sleep deprivation. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 5 Saunders; Philadelphia: 2010. pp. 54–66. [Google Scholar]
- [19].Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995 Dec;18(10):890–4. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- [20].Verweij IM, Romeijn N, Smit DJ, Piantoni G. Van Someren EJ, van der Werf YD, editors. Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci. 2014;15:88. doi: 10.1186/1471-2202-15-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Munch M, Knoblauch V, Blatter K, Wirz-Justice A, Cajochen C. Is homeostatic sleep regulation under low sleep pressure modified by age? Sleep. 2007 Jun 1;30(6):781–92. doi: 10.1093/sleep/30.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Frey S, Birchler-Pedross A, Hofstetter M, Brunner P, Götz T, Münch M, et al. Young women with major depression live on higher homeostatic sleep pressure than healthy controls. Chronobiol Int. 2012 Apr;29(3):278–94. doi: 10.3109/07420528.2012.656163. [DOI] [PubMed] [Google Scholar]
- [23].Groeger JA, Lo JC, Burns CG, Dijk DJ. Effects of sleep inertia after daytime naps vary with executive load and time of day. Behav Neurosci. 2011 Apr;125(2):252–60. doi: 10.1037/a0022692. [DOI] [PubMed] [Google Scholar]
- [24].Niggemyer KA, Begley A, Monk T, Buysse DJ. Circadian and homeostatic modulation of sleep in older adults during a 90-minute day study. Sleep. 2004 Dec 15;27(8):1535–41. doi: 10.1093/sleep/27.8.1535. [DOI] [PubMed] [Google Scholar]
- [25].Valdez P, Ramirez C, Garcia A, Talamantes J, Cortez J. Circadian and homeostatic variation in sustained attention. Chronobiol Int. 2010 Jan;27(2):393–416. doi: 10.3109/07420521003765861. [DOI] [PubMed] [Google Scholar]
- [26].Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McCauley P, Kalachev LV, Mollicone DJ, Banks S, Dinges DF, Van Dongen HP. Dynamic circadian modulation in a biomathematical model for the effects of sleep and sleep loss on waking neurobehavioral performance. Sleep. 2013 Dec;36(12):1987–97. doi: 10.5665/sleep.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–90. doi: 10.1016/B978-0-12-396971-2.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mollicone DJ, Van Dongen HP, Rogers NL, Banks S, Dinges DF. Time of day effects on neurobehavioral performance during chronic sleep restriction. Aviat Space Environ Med. 2010 Aug;81(8):735–44. doi: 10.3357/asem.2756.2010. [DOI] [PubMed] [Google Scholar]
- [30].Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009 Sep;29(4):320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Killgore WDS. Effects of sleep deprivation on cognition. In: Kerkhof GA, Van Dongen HPA, editors. Human sleep and cognition, part I: basic research. Elsevier; Amsterdam: 2010. pp. 105–29. [Google Scholar]
- [32].Jackson ML, Gunzelmann G, Whitney P, Hinson JM, Belenky G, Rabat A, et al. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev. 2013 Jun;17(3):215–25. doi: 10.1016/j.smrv.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006 Sep;15(3):261–5. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- [34].McKenna BS, Dicjinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007 Sep;16(3):245–52. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- [35].Bratzke D, Steinborn MB, Rolke B, Ulrich R. Effects of sleep loss and circadian rhythm on executive inhibitory control in the Stroop and Simon tasks. Chronobiology Int. 2012 Feb;29(1):55–61. doi: 10.3109/07420528.2011.635235. [DOI] [PubMed] [Google Scholar]
- [36].Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch General Psychiatry. 1997 Feb;54(2):145–52. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- [37].Monk TH, Buysse DJ, Reynolds CF, 3rd, Jarrett DB, Kupfer DJ. Rhythmic vs homeostatic influences on mood, activation, and performance in young and old men. J Gerontol. 1992 Jul;47(4):P221–7. doi: 10.1093/geronj/47.4.p221. [DOI] [PubMed] [Google Scholar]
- [38].Benca R. Psychiatric disorders. In: Kryger M, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5 Saunders; Philadelphia: 2010. [Google Scholar]
- [39].Giedke H. The usefulness of therapeutic sleep deprivation in depression. J Affect Disord. 2004 Jan;78(1):85–6. doi: 10.1016/s0165-0327(02)00216-1. author reply 87. [DOI] [PubMed] [Google Scholar]
- [40].Benedetti F, Colombo C. Sleep deprivation in mood disorders. Neuropsychobiology. 2011;64(3):141–51. doi: 10.1159/000328947. [DOI] [PubMed] [Google Scholar]
- [41].Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990 Jan;147(1):14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- [42].Perlis RH, Beasley CM, Jr, Wines JD, Jr, Tamura RN, Cusin C, Shear D, et al. Treatment-associated suicidal ideation and adverse effects in an open, multicenter trial of fluoxetine for major depressive episodes. Psychother Psychosom. 2007;76(1):40–6. doi: 10.1159/000096363. [DOI] [PubMed] [Google Scholar]
- [*43].McCall WV, Black CG. The link between suicide and insomnia: theoretical mechanisms. Curr Psychiatry Rep. 2013 Sep;15(9):389. doi: 10.1007/s11920-013-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Trockel M, Karlin BE, Taylor CB, Brown GK, Manber R. Effects of cognitive behavioral therapy for insomnia on suicidal ideation in veterans. Sleep. 2015;38(2):259–65. doi: 10.5665/sleep.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012 Feb;16(1):83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- [46].Pollock LR, Williams JM. Problem-solving in suicide attempters. Psychol Med. 2004 Jan;34(1):163–7. doi: 10.1017/s0033291703008092. [DOI] [PubMed] [Google Scholar]
- [47].Keilp JG, Gorlyn M, Russell M, Oquendo MA, Burke AK, Harkavy-Friedman J, et al. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 2013 Mar;43(3):539–51. doi: 10.1017/S0033291712001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Keilp JG, Wyatt G, Gorlyn M, Oquendo MA, Burke AK, John Mann J. Intact alternation performance in high lethality suicide attempters. Psychiatry Res. 2014 Sep 30;219(1):129–36. doi: 10.1016/j.psychres.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ben-Zeev D, Young MA. Accuracy of hospitalized depressed patients' and healthy controls' retrospective symptom reports: an experience sampling study. J Nerv Ment Dis. 2010 Apr;198(4):280–5. doi: 10.1097/NMD.0b013e3181d6141f. [DOI] [PubMed] [Google Scholar]
- [50].Ben-Zeev D, Young MA, Depp CA. Real-time predictors of suicidal ideation: mobile assessment of hospitalized depressed patients. Psychiatry Res. 2012 May 15;197(1–2):55–9. doi: 10.1016/j.psychres.2011.11.025. [DOI] [PubMed] [Google Scholar]