Abstract
Background
Coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass (CPB) triggers an inflammatory reaction, leading to the development of myocardial damage and dysfunction. It is suggested that selenium (Se), an essential trace element, has a protective role against oxidative stress. Decreased intraoperative Se levels might be an independent predictive factor for postoperative multiorgan failure. In spite of its proposed advantages, however, the optimal timing and dosage are not well known.
Objectives
To determine whether 600 µg of intravenous Se administration before induction of anesthesia for CABG surgery could attenuate inflammatory reactions in an Iranian population.
Methods
This randomized triple-blind clinical trial took place in the department of cardiac surgery of an academic hospital affiliated with Guilan University of Medical Sciences (GUMS) from May 2015 to September 2015. Eighty-eight eligible patients scheduled for elective on-pump CABG surgery were divided into two groups using randomized fixed quadripartite blocks. They received either an intravenous bolus of 600 µg Se before induction of anesthesia, or normal saline as a placebo. We had four measurement time-points: just before induction of anesthesia (T0), immediately after the end of CPB (T1), 24 hours after surgery (T2), and 48 hours after surgery (T3). Interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) serum levels were measured using the enzyme-linked immunosorbent assay (ELISA).
Results
Data from a total of 81 patients were analyzed: group S (n = 41) and group C (n = 40). There was no significant difference between the two groups with regard to baseline characteristics. In both groups, CPB caused markedly increased IL-6, TNF-α, and CRP plasma concentrations compared with baseline (P = 0.0001). However, the pattern of changes was not significantly different between group S (P = 0.068) and group C (P = 0.26). The IL-6 and TNF-α change trends were significant in each group (P=0.0001). However, comparing the two groups showed no significant difference. With regard to IL-6, there was no significant difference between the two groups at the time-points of T1 (P = 0.34), T2 (P = 0.17), and T3 (P = 0.056), and the same was found for TNF-α at T1 (P = 0.34), T2 (P = 0.17), and T3 (P = 0.056). With regard to CRP, the trend of the changes was significant in each group (P = 0.0001). However, comparing two groups showed a borderline significant difference between them at T1 (P = 0.039), but not at T2 (P = 0.075) or T3 (P = 0.11).
Conclusions
This study revealed that the administration of 600 μg of intravenous Se immediately before induction of anesthesia was safe, but when compared to a placebo, no predominant clinical effects or modifications in the systemic inflammatory response induced by on-pump CABG were observed.
Keywords: Coronary Artery Bypass Graft, Selenium, Inflammatory Response, CRP, IL-6, TNF-α
1. Background
In coronary artery bypass graft (CABG) surgery, reperfusion of the ischemic heart causes myocardial injury, which is exacerbated by cardioplegic arrest. During reperfusion injury, neutrophils are activated and an inflammatory response is triggered, leading to cytokine release and reactive oxygen species (ROS) generation (1-4). The imbalance between the ROS products and the ability of antioxidants to neutralize them results in oxidative stress. Increased oxidants, as a prognostic indicator, leads to a poor outcome and postoperative organ dysfunction (5-7).
In addition to reperfusion injury, cardiopulmonary bypass also provokes oxidative stress (8-12). The other responsible factors are endotoxemia caused by splanchnic hypoperfusion injury and gut translocation of endotoxins, contact between blood cells and the foreign surfaces of the CPB circuit, temperature fluctuations, blood-product transfusions, and surgical trauma (13-17). In addition, CABG surgery is increasingly being performed on an elderly population with a high incidence of co-morbidities, such as diabetes, heart failure, previous myocardial infarction, and renal impairment. These conditions are also associated with an enhanced degree of oxidative stress (18, 19). Despite considerable advances in surgical techniques and myocardial preservation strategies, undesirable inflammatory reactions still remain a problem, resulting in major complications (8, 18).
Antioxidant (AOX) enzymes, such as catalase superoxide dismutase and glutathione peroxidase, which are involved in the body’s defense against oxidative stress, require trace elements as essential cofactors. Therefore, reduced concentrations of these elements in the circulation and tissues might be correlated with a systemic inflammatory response and perioperative mortality (20). Selenium (Se), as a cost-effective and simple-to-use metabolic agent, is an essential trace element with antioxidant and anti-inflammatory effects. This micronutrient plays an important role in human health outcomes, and suboptimal levels might contribute to damage to key proteins (19, 21-25).
Se influences the inflammatory signaling pathways that modulate ROS and inhibits the nuclear factor-kappa B (NF-κB) cascade, leading to suppressed production of TNF-α and interleukins (18). It also increases enzymatic activity and reduces lipid peroxidation (26). Previous studies have demonstrated that Se levels are depleted in CABG under CPB, resulting in an insufficient AOX capacity, which is correlated with the development of multiorgan failure (8). The rationale behind the present study was provided by several reports indicating the anti-inflammatory and antioxidative potential of Se, which has been considered an attractive complementary option for supporting patients after surgery (8, 15, 18, 23). However, due to the limited data in this area, answering questions about the optimization of Se dosages for promoting health benefits, the timing of doses, the selection of subjects, and the presumed adverse effects remains an elusive goal. Hence, its preemptive administration prior to surgery must be done cautiously.
It is notable that most related studies have investigated the effects of Se under conditions other than CABG surgery. Despite the importance of this issue, almost no similar studies are found in our country. Regarding the differences of serum Se levels and the consequential required dosage among different areas, it was worthwhile to carry out this study in an Iranian population.
2. Objectives
The aim of the present study was to determine whether 600 µg of intravenous Se administration immediately before induction of anesthesia could modulate oxidative stress due to CABG surgery in an Iranian population.
3. Methods
3.1. Setting
This randomized triple-blind clinical trial took place at Dr. Heshmat hospital from May 2015 to September 2015. This governmental and academic center is affiliated with Guilan University of Medical Sciences (GUMS) in Rasht, Iran. It is a specialized referral hospital for different types of cardiac surgeries with 180 beds, featuring different departments, such as angiography, angioplasty, echocardiography, electrophysiology, and surgical wards for men, women, and pediatric patients. The trial protocol was approved by the university’s research ethics committee and was registered in the Iranian Registry of Clinical Trials (IRCT) with number 2015040813456N3.
3.2. Study Participants
Before enrollment in the study, informed consent was obtained from all patients.
3.3. Inclusion Criteria
The inclusion criteria were ASA class I and II, candidacy for elective CABG as an isolated procedure using CPB, age of 30 - 65 years, 3-vessel disease, and an ejection fraction of > 40% - 45%.
3.4. Exclusion Criteria
The exclusion criteria were emergent or urgent surgery, myocardial infarction in the previous six months, antioxidant supplementation during the previous month, concomitant malignant disease, uncontrolled diabetes, thyroid disease, liver or renal dysfunction, pregnancy, major trauma or major surgery in the previous three months, and inability to give informed consent.
3.5. Sample Size
With a margin of error of α = 0.05 and β = 10%, an expected power of 90%, and a Z value of 1.28, it was calculated that a sample of at least 40 patients in each group was required. We decided to include 44 patients in each group.
3.6. Randomization and Blinding
Eighty-eight eligible patients were randomly allocated to either the Se group (S) or the control group (C) using randomized fixed quadripartite blocks. Our subjects had an equal probability of being assigned to each of the two groups. Se and normal saline were injected intravenously as a single bolus dose by a responsible anesthesiologist who monitored the patients and was aware of the type of injection. However, the patient and the investigator who recorded the data were blinded, as was the lab technician who recorded the results. Therefore, this study was a triple-blind clinical trial.
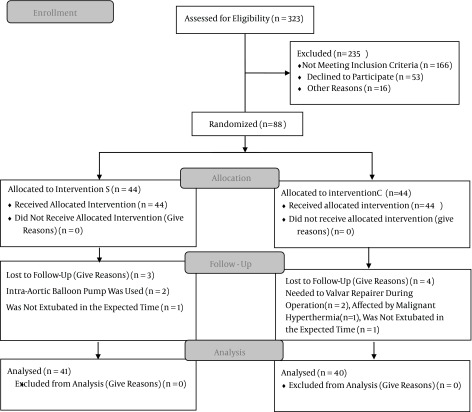
Figure 1. CONSORT 2010 Flow Diagram.
3.7. Intervention
Immediately before the induction of anesthesia, the patients in group S received an intravenous bolus of 600 μg of Se (vial 50 mcg/mL, Fellbach, Germany) diluted with 20 cc of sodium chloride 0.9%, while group C received the same amount of normal saline for the placebo. The patients were recruited one day before the surgery; to ensure physical and mental health, a medical history and physical exam were conducted. Venous blood was obtained for baseline biochemical serum measurements, as well as for CRP, IL-6, and TNF-α. All samples were taken in the morning between 7:30 a.m. and 8:30 a.m.
3.8. Anesthesia and Surgical Methods
Anesthesia was performed in accordance with a standardized protocol for CABG surgery in both groups. Surgery always started in the morning between 8:00 a.m. and 9: 00 a.m. to avoid bias caused by the circadian rhythm’s effect on circulating stress hormones. As premedication, the subjects in both groups received oral lorazepam 1 mg the night before surgery and one hour before transferring to the operating room, and intramuscular morphine 0.1 mg/kg 30 minutes before transferring to the operating room. On arrival in the operating room, an intravenous 18-gauge catheter was inserted into the forearm vein. Standard monitoring, including electrocardiography with both leads II and V5 with automated ST-segment analysis, pulse oximetry, invasive arterial blood pressure, central venous pressure, nasopharyngeal thermometer, bispectral index (BIS), end-tidal CO2 (EtCO2), urine output (UOP), and non-invasive blood pressure (NIBP) was performed at intervals of 3 minutes. Anesthesia was induced with 0.05 mg/kg of midazolam and 2 µg/kg of sufentanil. After neuromuscular blockade was achieved with 0.2 mg/kg of cisatracurium, the trachea was intubated. Anesthesia was maintained with continuous infusion of propofol 50 - 150 mg/kg/minutes, sufentanil 0.1 - 0.3 µg/kg/hour, and cisatracurium 0.6 mg/kg/hour. An initial dose of 300 u/kg of heparin was administered to achieve an activated coagulation time of > 480 seconds, then CPB was performed. In order to induce cardiac arrest, a cold cardioplegic solution was injected into the coronary arteries during pump-time. Throughout the CPB period, a mean blood pressure of 50 - 70 mmHg was maintained by administration of vasodilators or vasopressors, and hematocrit was kept between 21% and 27%. During the surgery, a body temperature of 32 - 34°C and a BIS value of 40 - 60 were maintained. The patients were ventilated with 100% oxygen to an EtCO2 concentration of 35 - 40 mmHg. The patients underwent median sternotomy, and a standard technique was used to establish the heart-lung pump (standard membrane oxygenator, Medtronic). At the end of the procedure, protamine was used in a ratio of 1: 1 to fully reverse the heparin. After the vascular graft was complete and the patients had stable vital signs, they were disconnected from the heart-lung pump and transferred to the coronary care unit (CCU). Weaning off of ventilator support and tracheal extubation was performed within 6 - 8 hours, after the standard criteria were fulfilled, including stable vital signs, being awake and cooperative, normal sinus rate, normal electrolytes (Na/K/Mg), normal arterial blood gas (ABG), and a body temperature of 36 - 37°C. For patients who complained of pain, morphine 0.1 mg/kg was administered. Any complication was recorded for 48 hours of follow-up.
3.9. Inflammatory Marker Measurements
An experienced and trained lab technician who was employed for this purpose closely monitored lab tests, performing and recording the results. All lab tests were conducted uniquely in our hospital laboratory.
The samples targeting IL-6, TNFα, and CRP were collected four times: before the induction of anesthesia (T0), just after the end of CPB (T1), 24 hours (T2) after surgery, and 48 hours (T3) after surgery. The first sample was obtained from a venous puncture and the others were collected through arterial catheters. All blood samples were 6 mL, and K2 EDTA was used as the anticoagulant. The samples were immediately placed in ice and within a few minutes, the plasma was separated by centrifuging at 1200 g for 5 minutes. Plasma samples were stored below -20°C until analysis. Serum concentrations of IL-6 (pg/mL), TNFα (pg/mL), and CRP (mg/L) were measured by using the sandwich enzyme-linked immunosorbent assay (ELISA) (BioTek-ELX800). IL-6 and TNF-α human kits (Bender medSystems eBioscience, Germany) and CRP kits (Audit Diagnostic, Ireland) were used. The data were collected and the results were compared between the two groups.
3.10. Statistical Analysis
All of the statistical analyses were performed using SPSS statistical software, version 16 (SPSS Inc., Chicago, IL, USA). The chi-square test was used to compare the categorical variables between the two groups. The K-S test was applied to determine the normality of the variables, followed by parametric tests. The independent t test was performed to compare and assess the parametric data between the two groups, and the repeated-measures test was used to compare the parametric data at four measurement time-points. The data were expressed as mean ± standard deviation. P values of < 0.05 were considered statistically significant.
4. Results
In the present study, 88 eligible subjects were divided into selenium (S) and control (C) groups. In group S, intra-aortic balloon pumps were used for two patients and one could not be extubated within the expected time. In group C, one patient experienced malignant hyperthermia, two patients required valve repair during surgery, and one could not be extubated within the expected time. After excluding these cases from the survey, data from 81 patients were finally analyzed.
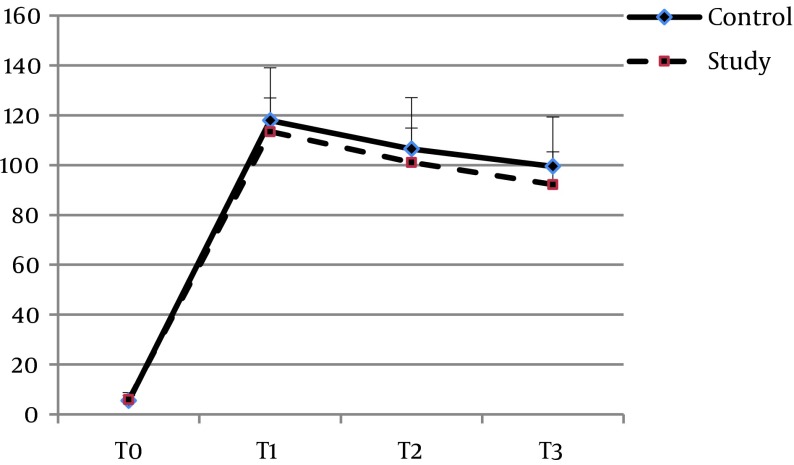
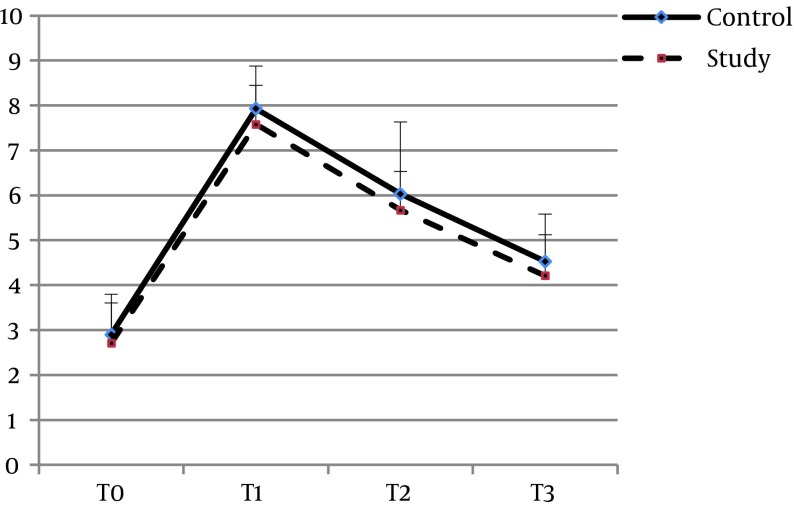
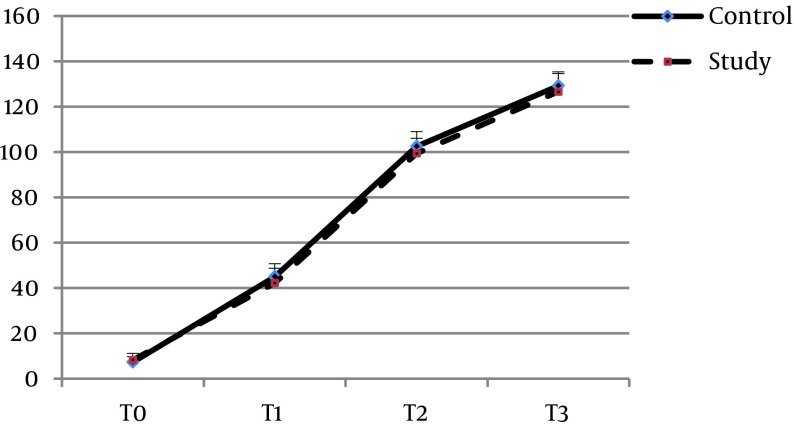
The mean age of the patients in groups S and C were 54.65 ± 8.46 and 58.2 ± 8.29 years, respectively (P = 0.061). Twenty-one (51.2%) of the patients in group S and 19 (47.5%) in group C were women (P = 0.738). There was no significant difference between the two groups with regard to the other baseline characteristics, including BMI (P = 0.8), ejection fraction (P = 0.67), surgery duration (P = 0.15), pump time (P = 0.45), clamp time (P = 0.46), creatinine clearance (P = 0.258), and serum creatinine (P = 0.37) (Table 1). Baseline plasma concentrations of IL-6 (P = 0.507), TNF-α (P = 0.352), and CRP (0.095) also showed no significant differences. The pattern of changes in serum levels of IL-6 and TNF-α over time was not significantly different between the two groups (P = 0.068 and P = 0.26, respectively) (Figures 2 and 3). With regard to IL-6, there was no significant difference between the two groups at the time-points of T1 (P = 0.34), T2 (P = 0.17), or T3 (P = 0.056), and the same was found for TNF-α at T1 (P = 0.34), T2 (P = 0.17), and T3 (P = 0.056). The trend of changes in CRP was significant in each group (P = 0.0001). However, comparing the two groups, there was a borderline significant difference between them at T1 (P = 0.039), but not at T2 (P = 0.075) or T3 (P = 0.11) (Table 2, Figure 4). None of the patients in the Se group experienced adverse effects due to the intervention.
Table 1. Patients’ Preoperative Characteristics.
| Variable | Control Group (n = 40) | Selenium Group (n = 41) | P Value |
|---|---|---|---|
| Age, y | 58.2 ± 8.29 | 54.65 ± 8.46 | T = 1.9, P = 0.061 |
| Gender, No. (%) | P = 0.738 | ||
| Male | 21 (52.5) | 20 (48.8) | |
| Female | 19 (47.5) | 21 (51.2) | |
| BMI, kg/m 2 | 27.32 ± 2.37 | 27.16 ± 3.03 | T = 0.25, P = 0.8 |
| Ejection fraction, % | 47.87 ± 3.9 | 47.31 ± 7.42 | t = 0.42, P = 0.67 |
| Surgery duration, hour | 2.78 ± 0.28 | 2.88 ± 0.36 | t = 1.43, P = 0.15 |
| Pump time, minute | 60.45 ± 18.08 | 57.87 ± 12.41 | t = 0.74, P = 0.45 |
| Clamp time, minute | 36.72 ± 10.16 | 35.17 ± 8.59 | t = 0.743, P = 0.46 |
| Creatinine clearance | 60.5 ± 3.57 | 61.41 ± 3.65 | t = 1.13, P = 0.258 |
| Serum creatinine | 110.27 ± 3.49 | 100.95 ± 3.26 | t = 0.9, P = 0.37 |
Figure 2. Changes in IL-6 Concentrations (pg/mL) at Four Points.
T0, before induction of anesthesia; T1, at the end of CPB; T2, 24 hours after surgery; T3, 48 hours after surgery.
Figure 3. Changes in TNF-α Concentrations (pg/mL) at Four Points.
T0, before induction of anesthesia; T1, at the end of CPB; T2, 24 hours after surgery; T3, 48 hours after surgery.
Table 2. IL-6, TNF-α, and CRP Concentrations Measured at Four Pointsa.
| Variable Group | T0 | T1 | T2 | T3 | P Value | P Value |
|---|---|---|---|---|---|---|
| IL-6, pg/mL | ||||||
| Control | 5.51 ± 3.29 | 117.2 ± 21.1 | 106.5 ± 20.65 | 99.5 ± 19.8 | F = 1217.7, P = 0.0001 | F = 3.19, P = 0.068 |
| Study | 5.89 ± 1.59 | 113.4 ± 13.6 | 101.1 ± 13.8 | 92.2 ± 13.2 | F = 2081.9, P = 0.0001 | |
| P Value | P = 0.507 | P = 0.34 | P = 0.17 | P = 0.056 | ||
| TNF-α, pg/mL | ||||||
| Control | 2.9 ± 0.9 | 7.93 ± 0.95 | 6.03 ± 1.06 | 4.53 ± 1.05 | F = 1452.3, P = 0.0001 | F = 1.32, P = 0.26 |
| Study | 2.71 ± 0.89 | 7.58 ± 0.87 | 5.67 ± 0.86 | 4.21 ± 0.91 | F = 2706.2, P = 0.0001 | |
| P Value | P = 0.352 | P = 0.089 | P = 0.1 | P = 0.146 | ||
| CRP, mg/L | ||||||
| Control | 7.27 ± 2.36 | 45.02 ± 5.64 | 102.05 ± 6.44 | 129.22 ± 6.12 | F = 9643.7, P = 0.0001 | F = 4.09, P = 0.017 |
| Study | 8.26 ± 2.90 | 42.19 ± 6.45 | 99.41 ± 6.68 | 126.65 ± 8.01 | F = 5691.9; P = 0.0001 | |
| P Value | P = 0.095 | P = 0.039 | P = 0.075 | P = 0.11 |
aT0, before induction of anesthesia; T1, at the end of CPB; T2, 24 hours after surgery; T3, 48 hours after surgery.
Figure 4. Changes in CRP Concentrations (mg/L) at Four Points.
T0, before induction of anesthesia; T1, at the end of CPB; T2, 24 hours after surgery; T3, 48 hours after surgery.
5. Discussion
Patients undergoing on-pump CABG surgery are affected by various ischemic stimuli: induction of cardioplegic arrest, microembolic events, reperfusion of the myocardium by surgical revascularization, and termination of cardioplegic arrest (18). There has been increasing evidence that the application of CPB using a heart-lung machine for open heart surgery may be the cause of some of this morbidity and is associated with pre- and postoperative stress responses, which triggers postoperative organ dysfunction (4, 27). It is proposed that pretreatment with antioxidants such as Se could attenuate this inflammation. Se is involved in the anti-inflammatory process and increases antioxidant capacities via at least 25 selenoproteins, among which selenoprotein P, the glutathione peroxidases, thioredoxin reductases, and iodothyronine deiodinases are the best known. The redox-regulating properties of Se-dependent glutathione peroxidase play a pivotal role in its benefits. Se has multiple AOX defense lines and is involved in apoptosis, cell cycling, and microvascular tone. Moreover, Se inhibits lipopolysaccharide-induced pro-inflammatory gene expression by modulating NF-κB (17, 28-33).
However, despite promising results in several studies indicating the relationship between preoperative Se administration and cardiovascular outcomes, other clinical trials show conflicting results (21, 34, 35). Indeed, recent clinical studies and case reports have shown that Se supplementation in patients who are not carefully selected may be harmful, and several adverse health effects of this metabolic agent have been reported in humans, including carcinogenesis, cytotoxicity, and genotoxicity. We can also point to its toxic effects on endocrine function, including synthesis of thyroid hormones, growth hormone, and insulin-like growth factor-1. Hepatotoxicity, gastrointestinal disturbances, natural-killer-cell dysfunction, brittle nails, hair loss, a garlic smell to the breath, vomiting, dizziness, and pulmonary edema are also reported. Laboratory investigations indicate that Se has a bivalent effect on the risk of cancer, with the risk either increased or decreased (18, 21, 22, 35-39). Most of the mentioned adverse effects are due to chronic exposure; however, due to the old age and co-morbidities in patients undergoing CABG surgery, it is wise to investigate the lowest dose possible that has beneficial effects. Hence, the lack of a complete understanding of the pharmacodynamics, safety, and presumed toxicity of Se limits its routine application in humans. Clinical studies have used a wide range of Se doses and have demonstrated that ≤ 500 μg failed to improve clinical outcomes in various conditions (22, 23, 40, 41).
In this context, the authors tested the hypothesis that an intravenous bolus of 600 μg of Se, which is only slightly over its lower limit of efficacy, could blunt cytokine-release following CABG surgery. Cardiac surgery is an ideal model for studying the efficacy of preemptive Se because the onset of oxidative injury is predictable and we can reproducibly prompt ischemia-reperfusion injury, as the onset and duration of ischemia are under the control of the surgeon. Therefore, on-pump CABG with cardioplegic arrest and elective global ischemia represents an ideal situation for evaluating the potential injury induced by oxidative stress (8, 10). Accumulating evidence suggests that Se concentrations decrease following surgery and remain low for several days to weeks. This reduction is associated with the elevation of ROS and the severity of illness (6, 19, 20, 42-44). On the other hand, post-injury normalization of Se status is unable to reduce oxidative stress (20). Therefore, it is postulated that a preemptive approach is preferred. It has been reported that the duration of surgery influences the release of neuroendocrine hormones, and decreased intraoperative Se levels are correlated with CPB duration (20, 45). Although the surgeries were performed by different surgeons, the mean duration of surgery did not differ statistically significantly between the two groups.
Considering the following actions, certain inflammatory markers could be our therapeutic targets. TNF-α, as a pro-inflammatory mediator, rises rapidly after major surgery, facilitating the activation of other cytokines and the initiation of the inflammatory cascade (1). In on-pump CABG, IL-6 serum concentration is a significant predictor of worsened outcomes and is correlated with the degree of myocardial damage and left ventricular dysfunction (2, 13, 15). CRP is a marker of the acute-phase response in patients undergoing CABG surgery, and its serum levels are correlated with early postoperative complications, such as acute ischemic events and stenosis of the graft. Recent evidence suggests that CRP is a powerful predictor of heart failure after myocardial infarction via activating the complement and coagulation cascades (46). In agreement with previous studies, our findings also suggested that IL-6, TNFα, and CRP were involved in ischemic reperfusion injury, as they were markedly increased after CPB began. There was a trend of Se being higher in parts of our evaluations, but it did not reach statistical significance except at one time-point (T1), according to CRP. We could not find a meaningful difference between the study and control groups. It seems that the authors’ hypothesis has not been proved. Hence, larger doses or repeated and more prolonged treatment durations before surgery may be necessary in order to achieve the optimal condition.
Given the importance of this issue, several studies have been performed to investigate the efficacy of factors that contribute to reducing the inflammatory response due to CABG surgery. In 2013, Stoppe et al. (8) performed a study in which patients undergoing elective cardiac surgery received an intravenous bolus of 2000 µg of Se after induction of anesthesia. They claimed that this preemptive dose of Se was associated with a decrease in SOFA scores; however, it failed to improve the postoperative outcomes for the patients. This study differed from ours because we enrolled patients undergoing CABG under CPB, while their participants all underwent elective cardiac surgeries, which of course induced different levels of stress response. The Se dosage was also different. In contrast to our findings, in a clinical trial conducted by Altaei et al. in 2012 (1), it was reported that Se treatment (140 mg × 3 capsules per day) three days before CABG significantly reduced the serum levels of IL-6 and TNF-α. Comparing the two studies, it is notable that in addition to their differences with regard to Se dosage and the route of administration, both on-pump and off-pump CABG patients were enrolled the study and the evaluations were only pre- and postoperative.
Leong et al. (19) believe that when antioxidants function as a network, they act more effectively than as single agents. They reported that preoperative metabolic therapy (coenzyme Q10, lipid acid, Se, orthotic acid, and omega 3) reduced oxidative stress and the incidence of myocardial damage in elective CABG surgery. However, the efficacy of each component was not clear. Their results were not in line with the present study, which might be explained by the differences in methods. Their subjects were selected from among patients who were candidates for elective CABG and/or valve surgery, and the Se was orally administered at a lower dosage but for at least two weeks before surgery. Duncan et al. showed that a systemic inflammatory response was associated with a 50% reduction in serum Se concentrations when CRP concentrations were > 80 mg/dL (18). Stoppe et al. (20) showed that post-operative Zn and Se concentrations had predictable accuracy for the later development of multiorgan failure. Poulsen et al. (15) investigated the anti-inflammatory effect of EPO in CPB, but their results rejected this hypothesis. Their evaluation was based on TNFα, IL-6, and IL-10. Krivoy et al. (46) conducted a study to determine whether administration of high doses of atorvastatin before CABG could decrease the inflammatory response as reflected by CRP, and their findings supported this idea. Jouybar et al. (47) investigated the effects of ascorbic acid on decreasing the inflammatory cytokines IL-6 and IL-8, but their results revealed no positive effect of this antioxidant agent compared to a placebo. Angstwurm et al. (48) reported in 2008 that adjuvant treatment with high-dose Se diminished the mortality rate in patients with severe sepsis or septic shock. Valenta et al. reported that although high-dose Se in patients with sepsis reduced inflammatory markers, the mortality rate was not affected (49). Heyland et al. conducted a multicenter study on critically ill patients who received 500 µg Se in their artificial nutrition. Their results were disappointing and reported no beneficial effects from this intervention on the 28-day mortality rate (50). Andrews et al. (41) performed a clinical trial searching for the effects of Se on 505 adults in intensive care units, who received Se (500 µg/day) for seven days. They found a reduction rate in new infections but no effect on the mortality rate. Landucci et al. conducted a systemic review and meta-analysis of nine randomized trials and concluded that high-dose Se might be have an effect on 28-day mortality in critically ill patients. They also found that Se supplementation at doses of < 500 µg/day had no significant beneficial effects (51). Brodin et al. investigated the pharmacokinetics and toxicity of Se in cancer patients by administering it at different doses for 2 - 4 weeks. The reported adverse effects were fatigue, nausea, and cramps in the fingers and legs; the incidence rate of chemotherapy-induced toxicity was higher in cases treated with < 3 mg/m2 of Se (52).
Several factors might be responsible for these disparate results. The biology of Se is complex, cell-tissue dependent, and is often affected by other metabolic pathways and by the disease process itself (21). We do not know the exact predominant factors (such as the cardiopulmonary bypass or the surgical trauma) in the initiation of the systemic inflammatory response in cardiac surgeries. In fact, no single pharmacological or technical intervention is adequate to entirely suppress the associated adverse clinical outcomes and inflammatory response due to surgery (3). The exact biomarkers reflecting the severity of the stress response have not yet been identified. Moreover, in cardiac surgery, the inflammatory reaction varies considerably among patients, and the kinetics of exogenous administration of Se and its pathophysiological role in multiorgan injury are poorly understood. The genetic background of individuals also contributes to the stress response. It remains speculative whether the present genotype and phenotype of patients might affect Se distributions. Furthermore, it is largely unknown whether the underlying genotype might influence the physiologic response to Se complements (3, 53, 54).
Varying doses, preparations, routes of administration, surgeon experience, anesthetic agents, and methods (21, 53) are also partly responsible for the disparate findings (2, 28, 29, 55, 56). Several Se studies have focused on cancer, the endocrine system, cerebrovascular disease, immune function, the respiratory system, pregnancy, the gastrointestinal system, and other conditions, but not on-pump CABG surgery. Moreover, a number of previous studies evaluated the combination of Se with other minerals or vitamins. This indicates the strength and novelty of the present study; however, comparing our findings with those of similar studies and discussing the conflicting results was limited (37, 52, 57-76).
5.1. Strengths
To the authors’ knowledge, despite the importance of this issue, no similar studies have been performed purely to investigate the efficacy of low-dose Se on the stress response in CABG surgery. This could be considered a strength of the present study.
5.2. Weaknesses
Considering the fact that the human pharmacodynamic conditions are unknown, earlier Se treatment before surgery or repeated doses may be necessary to achieve an anti-inflammatory response. The mentioned time-points might not have been optimal to recognize the peak values; for instance, between T1 (just after the end of CPB) and T2 (24 hours after surgery), there may have been a valuable time-point. In addition, a short follow-up duration of only up to 48 hours after surgery could be considered a weakness of this study.
5.3. Limitations
First, this was a single-center study involving a limited number of surgeries, up to four per day. Second, the sample size was small. Third, the evaluated inflammatory markers were restricted, and it cannot be ruled out that the results might be different if other cytokines were evaluated. Therefore, these results might not be generalizable.
5.4. Suggestions
We hope that the present findings underlie new studies in the future. In order to report reliable and generalizable results about the anti-inflammatory properties of Se, further large, well-planned, multicenter trials with longer follow-up durations in different populations and with other types of surgeries are still needed. More complete data on the safety, optimal dosage, and timing are required before making recommendations for pre-, peri- or postoperative Se administration.
5.5. Conclusions
The results of the present study showed that 600 µg of intravenous Se was safe with no side effects, but did not support the superiority of this treatment as an anti-inflammatory agent in on-pump CABG surgery.
Acknowledgments
We thank the research and technology vice-chancellorship of Guilan University of Medical Sciences (GUMS).
Footnotes
Authors’ Contribution:Abbas Sedighinejad and Vali Imantalab helped to design and conduct the study, collect data, and prepare the manuscript; Ali Mirmansouri and Ali Mohammadzadeh Jouryabi helped to conduct the study, collect data, and prepare the manuscript; Gholamreza Kanani and Nassir Nassiri Sheikhani helped to conduct the study; Zahra Atrkarroushan performed statistical analyses; Gelareh Biazar, Mohammad Haghighi, and Gelareh Biazar MD helped to collect and analyze the data.
Funding/Support:This study was financially supported by the vice-chancellorship of research and technology at Guilan University of Medical Sciences.
References
- 1.Altaei T. Protective effect of silymarin during coronary artery bypass grafting surgery. Exp Clin Cardiol. 2012;17(1):34–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Suleiman MS, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br J Pharmacol. 2008;153(1):21–33. doi: 10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohrn L, Pinto A, Steinbrenner H, Bilgic E, Boeken U, Lichtenberg A, et al. Tissue specific inflammatory response caused by cardiopulmonary bypass-acute and preventive treatment with selenium compounds as an approach to mitigate i/r injury. J Thorac Cardiovasc Surg. 2015;63(S 01):ePP21. [Google Scholar]
- 4.Fudulu D, Angelini GD. Oxidative stress after surgery on the immature heart. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/1971452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiko S. Controlling Oxidative Stress as a Potential Tool for Perioperative Management to Reduce Morbidity After Surgical Trauma. Diet Nut Crit Care. 2015:521–32. [Google Scholar]
- 6.Manzanares W, Biestro A, Galusso F, Torre MH, Manay N, Pittini G, et al. Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill? Intensive Care Med. 2009;35(5):882–9. doi: 10.1007/s00134-008-1356-5. [DOI] [PubMed] [Google Scholar]
- 7.Scholl R, Bekker A, Babu R. Neuroendocrine and immune responses to surgery. Internet J Anesthesiol. 2012;30(3) [Google Scholar]
- 8.Stoppe C, Spillner J, Rossaint R, Coburn M, Schalte G, Wildenhues A, et al. Selenium blood concentrations in patients undergoing elective cardiac surgery and receiving perioperative sodium selenite. Nutrition. 2013;29(1):158–65. doi: 10.1016/j.nut.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Milei J, Forcada P, Fraga CG, Grana DR, Iannelli G, Chiariello M, et al. Relationship between oxidative stress, lipid peroxidation, and ultrastructural damage in patients with coronary artery disease undergoing cardioplegic arrest/reperfusion. Cardiovasc Res. 2007;73(4):710–9. doi: 10.1016/j.cardiores.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Chambers DJ. Oxidative stress injury during cardiac surgery: how important is it? Cardiovasc Res. 2007;73(4):626–8. doi: 10.1016/j.cardiores.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-de-la-Asuncion J, Pastor E, Perez-Griera J, Belda FJ, Moreno T, Garcia-del-Olmo E, et al. Oxidative stress injury after on-pump cardiac surgery: effects of aortic cross clamp time and type of surgery. Redox Rep. 2013;18(5):193–9. doi: 10.1179/1351000213Y.0000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raja SG, Dreyfus GD. Modulation of systemic inflammatory response after cardiac surgery. Asian Cardiovasc Thorac Ann. 2005;13(4):382–95. doi: 10.1177/021849230501300422. [DOI] [PubMed] [Google Scholar]
- 13.Franke A, Lante W, Fackeldey V, Becker HP, Kurig E, Zoller LG, et al. Pro-inflammatory cytokines after different kinds of cardio-thoracic surgical procedures: is what we see what we know? Eur J Cardiothorac Surg. 2005;28(4):569–75. doi: 10.1016/j.ejcts.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Nussmeier N, Sarwar M. In: Anesthesia for cardiac surgical procedures. Ronald D, editor. Vol. 5. Philadelphia: Churchill Livingstone; 2015. pp. 2047–9. [Google Scholar]
- 15.Poulsen TD, Andersen LW, Steinbruchel D, Gotze JP, Jorgensen OS, Olsen NV. Two large preoperative doses of erythropoietin do not reduce the systemic inflammatory response to cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23(3):316–23. doi: 10.1053/j.jvca.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Elahi MM, Yii M, Matata BM. Significance of oxidants and inflammatory mediators in blood of patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(3):455–67. doi: 10.1053/j.jvca.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Tyszka-Czochara M, Pasko P, Zagrodzki P, Gajdzik E, Wietecha-Posluszny R, Gorinstein S. Selenium Supplementation of Amaranth Sprouts Influences Betacyanin Content and Improves Anti-Inflammatory Properties via NFkappaB in Murine RAW 264.7 Macrophages. Biol Trace Elem Res. 2016;169(2):320–30. doi: 10.1007/s12011-015-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benstoem C, Goetzenich A, Kraemer S, Borosch S, Manzanares W, Hardy G, et al. Selenium and its supplementation in cardiovascular disease--what do we know? Nutrients. 2015;7(5):3094–118. doi: 10.3390/nu7053094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong JY, van der Merwe J, Pepe S, Bailey M, Perkins A, Lymbury R, et al. Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: a randomised trial. Heart Lung Circ. 2010;19(10):584–91. doi: 10.1016/j.hlc.2010.06.659. [DOI] [PubMed] [Google Scholar]
- 20.Stoppe C, Schalte G, Rossaint R, Coburn M, Graf B, Spillner J, et al. The intraoperative decrease of selenium is associated with the postoperative development of multiorgan dysfunction in cardiac surgical patients. Crit Care Med. 2011;39(8):1879–85. doi: 10.1097/CCM.0b013e3182190d48. [DOI] [PubMed] [Google Scholar]
- 21.Speckmann B, Grune T. Epigenetic effects of selenium and their implications for health. Epigenetics. 2015;10(3):179–90. doi: 10.1080/15592294.2015.1013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, et al. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91(4):923–31. doi: 10.3945/ajcn.2009.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venardos KM, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Current med chem. 2007;14(14):1539–49. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 24.Alehagen U, Aaseth J. Selenium and coenzyme Q10 interrelationship in cardiovascular diseases--A clinician's point of view. J Trace Elem Med Biol. 2015;31:157–62. doi: 10.1016/j.jtemb.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Fink K, Moebes M, Vetter C, Bourgeois N, Schmid B, Bode C, et al. Selenium prevents microparticle-induced endothelial inflammation in patients after cardiopulmonary resuscitation. Crit Care. 2015;19:58. doi: 10.1186/s13054-015-0774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84(4):762–73. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoda MR, El-Achkar H, Schmitz E, Scheffold T, Vetter HO, De Simone R. Systemic stress hormone response in patients undergoing open heart surgery with or without cardiopulmonary bypass. Ann Thorac Surg. 2006;82(6):2179–86. doi: 10.1016/j.athoracsur.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 28.Duntas LH. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res. 2009;41(6):443–7. doi: 10.1055/s-0029-1220724. [DOI] [PubMed] [Google Scholar]
- 29.Wei Z, Yao M, Li Y, Yang Z, Feng X. Inhibition of Lipopolysaccharide (LPS)-induced inflammatory responses by selenium in bovine mammary epithelial cells in primary culture. Inflammation. 2015;38(1):152–8. doi: 10.1007/s10753-014-0017-9. [DOI] [PubMed] [Google Scholar]
- 30.Valenta J, Brodska H, Drabek T, Hendl J, Kazda A. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med. 2011;37(5):808–15. doi: 10.1007/s00134-011-2153-0. [DOI] [PubMed] [Google Scholar]
- 31.Brodska H, Valenta J, Malickova K, Kohout P, Kazda A, Drabek T. Biomarkers in critically ill patients with systemic inflammatory response syndrome or sepsis supplemented with high-dose selenium. J Trace Elem Med Biol. 2015;31:25–32. doi: 10.1016/j.jtemb.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Iglesias SB, Leite HP, Paes AT, Oliveira SV, Sarni RO. Low plasma selenium concentrations in critically ill children: the interaction effect between inflammation and selenium deficiency. Crit Care. 2014;18(3):R101. doi: 10.1186/cc13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattmiller SA, Carlson BA, Sordillo LM. Regulation of inflammation by selenium and selenoproteins: impact on eicosanoid biosynthesis. J Nutr Sci. 2013;2:e37918. doi: 10.1017/jns.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevanovic A, Coburn M, Menon A, Rossaint R, Heyland D, Schalte G, et al. The importance of intraoperative selenium blood levels on organ dysfunction in patients undergoing off-pump cardiac surgery: a randomised controlled trial. PLoS One. 2014;9(8):e37918. doi: 10.1371/journal.pone.0104222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moosmann B, Behl C. Selenoprotein synthesis and side-effects of statins. The Lancet. 2004;363(9412):892–4. doi: 10.1016/S0140-6736(04)15739-5. [DOI] [PubMed] [Google Scholar]
- 36.Edwin R, Babu RH, Subrahmanyam G, Ramalingam K, Shaik MV. A comparative study of trace element levels in coronary artery tissue of Coronary heart disease patients with serum levels in healthy individuals. 2015 [Google Scholar]
- 37.Sinha I, Karagoz K, Fogle RL, Hollenbeak CS, Zea AH, Arga KY, et al. "Omics" of Selenium Biology: A Prospective Study of Plasma Proteome Network Before and After Selenized-Yeast Supplementation in Healthy Men. OMICS. 2016;20(4):202–13. doi: 10.1089/omi.2015.0187. [DOI] [PubMed] [Google Scholar]
- 38.Sun HJ, Rathinasabapathi B, Wu B, Luo J, Pu LP, Ma LQ. Arsenic and selenium toxicity and their interactive effects in humans. Environ Int. 2014;69:148–58. doi: 10.1016/j.envint.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Zwolak I, Zaporowska H. Selenium interactions and toxicity: a review. Selenium interactions and toxicity. Cell Biol Toxicol. 2012;28(1):31–46. doi: 10.1007/s10565-011-9203-9. [DOI] [PubMed] [Google Scholar]
- 40.Berger MM, Soguel L, Shenkin A, Revelly JP, Pinget C, Baines M, et al. Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care. 2008;12(4):R101. doi: 10.1186/cc6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ. 2011;342:d1542. doi: 10.1136/bmj.d1542. [DOI] [PubMed] [Google Scholar]
- 42.Koszta G, Kacska Z, Szatmari K, Szerafin T, Fulesdi B. Lower whole blood selenium level is associated with higher operative risk and mortality following cardiac surgery. J Anesth. 2012;26(6):812–21. doi: 10.1007/s00540-012-1454-y. [DOI] [PubMed] [Google Scholar]
- 43.Ghashut RA, McMillan DC, Kinsella J, Vasilaki AT, Talwar D, Duncan A. The effect of the systemic inflammatory response on plasma zinc and selenium adjusted for albumin. Clin Nutr. 2016;35(2):381–7. doi: 10.1016/j.clnu.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Mertens K, Lowes DA, Webster NR, Talib J, Hall L, Davies MJ, et al. Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. Br J Anaesth. 2015;114(6):990–9. doi: 10.1093/bja/aev073. [DOI] [PubMed] [Google Scholar]
- 45.Stoppe C, McDonald B, Rex S, Manzanares W, Whitlock R, Fremes S, et al. SodiUm SeleniTe Adminstration IN Cardiac Surgery (SUSTAIN CSX-trial): study design of an international multicenter randomized double-blinded controlled trial of high dose sodium-selenite administration in high-risk cardiac surgical patients. Trials. 2014;15:339. doi: 10.1186/1745-6215-15-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krivoy N, Adler Z, Saloma R, Hawadie A, Azzam ZS. Targeting C-reactive protein levels using high-dose atorvastatin before coronary artery bypass graft surgery. Exp Clin Cardiol. 2008;13(4):171–4. [PMC free article] [PubMed] [Google Scholar]
- 47.Jouybar R, Kabgani H, Kamalipour H, Shahbazi S, Allahyary E, Rasouli M, et al. The perioperative effect of ascorbic acid on inflammatory response in coronary artery bypass graft surgery; a randomized controlled trial coronary artery bypass graft surgery. Int Cardivas Res J. 2012;6(1):13–7. [Google Scholar]
- 48.Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35(1):118–26. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]
- 49.Valenta J, Brodska H, Drabek T, Stach Z, Zima T, Kazda A. Selenium: an important trace element and therapeutic adjunct in critical care. Trace Elements Electrolytes. 2012;29(4) [Google Scholar]
- 50.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368(16):1489–97. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 51.Landucci F, Mancinelli P, De Gaudio AR, Virgili G. Selenium supplementation in critically ill patients: a systematic review and meta-analysis. J Crit Care. 2014;29(1):150–6. doi: 10.1016/j.jcrc.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Brodin O, Eksborg S, Wallenberg M, Asker-Hagelberg C, Larsen EH, Mohlkert D, et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients. 2015;7(6):4978–94. doi: 10.3390/nu7064978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo F, Monsefi N, Moritz A, Beiras-Fernandez A. Selenium and cardiovascular surgery: an overview. Curr Drug Saf. 2012;7(4):321–7. doi: 10.2174/1574886311207040321. [DOI] [PubMed] [Google Scholar]
- 54.Joseph J, Loscalzo J. Selenistasis: epistatic effects of selenium on cardiovascular phenotype. Nutrients. 2013;5(2):340–58. doi: 10.3390/nu5020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun LH, Zhang NY, Zhu MK, Zhao L, Zhou JC, Qi DS. Prevention of Aflatoxin B1 Hepatoxicity by Dietary Selenium Is Associated with Inhibition of Cytochrome P450 Isozymes and Up-Regulation of 6 Selenoprotein Genes in Chick Liver. J Nutr. 2016 doi: 10.3945/jn.115.224626. [DOI] [PubMed] [Google Scholar]
- 56.Lee TW, Kowalski S, Falk K, Maguire D, Freed DH, HayGlass KT. High Spinal Anesthesia Enhances Anti-Inflammatory Responses in Patients Undergoing Coronary Artery Bypass Graft Surgery and Aortic Valve Replacement: Randomized Pilot Study. PLoS One. 2016;11(3):e0149942. doi: 10.1371/journal.pone.0149942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, et al. Low Population Selenium Status Is Associated With Increased Prevalence of Thyroid Disease. J Clin Endocrinol Metab. 2015;100(11):4037–47. doi: 10.1210/jc.2015-2222. [DOI] [PubMed] [Google Scholar]
- 58.Maasland DH, Schouten LJ, Kremer B, van den Brandt PA. Toenail selenium status and risk of subtypes of head-neck cancer: The Netherlands Cohort Study. Eur J Cancer. 2016;60:83–92. doi: 10.1016/j.ejca.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Nakken HL, Lephart ED, Hopkins TJ, Shaw B, Urie PM, Christensen MJ. Prenatal exposure to soy and selenium reduces prostate cancer risk factors in TRAMP mice more than exposure beginning at six weeks. Prostate. 2016;76(6):588–96. doi: 10.1002/pros.23150. [DOI] [PubMed] [Google Scholar]
- 60.Stolzoff M, Webster TJ. Reducing bone cancer cell functions using selenium nanocomposites. J Biomed Mater Res A. 2016;104(2):476–82. doi: 10.1002/jbm.a.35583. [DOI] [PubMed] [Google Scholar]
- 61.Cai X, Wang C, Yu W, Fan W, Wang S, Shen N, et al. Selenium Exposure and Cancer Risk: an Updated Meta-analysis and Meta-regression. Sci Rep. 2016;6:19213. doi: 10.1038/srep19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porto B, Moreira L, Haddad-Ribeiro F, Belo L, Neves MJ. Inorganic selenium acts as a sensitizer to ionizing radiation in vivo Saccharomyces cerevisiae cells. J Radioanal Nucl Chem. 2016;307(1):419–26. [Google Scholar]
- 63.Wu L, Zhang H, Xu C, Xia C. Critical Thresholds of Antioxidant and Immune Function Parameters for Se deficiency Prediction in Dairy Cows. Biol Trace Element Res. 2016:1–6. doi: 10.1007/s12011-015-0606-y. [DOI] [PubMed] [Google Scholar]
- 64.Mao S, Zhang A, Huang S. Erratum to: Selenium supplementation and the risk of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Endocrine. 2016;52(2):401. doi: 10.1007/s12020-015-0794-4. [DOI] [PubMed] [Google Scholar]
- 65.Gashu D, Stoecker BJ, Adish A, Haki GD, Bougma K, Aboud FE, et al. Association of serum selenium with thyroxin in severely iodine-deficient young children from the Amhara region of Ethiopia. Eur J Clin Nutr. 2016 doi: 10.1038/ejcn.2016.27. [DOI] [PubMed] [Google Scholar]
- 66.Mao J, Bath SC, Vanderlelie JJ, Perkins AV, Redman CW, Rayman MP. No effect of modest selenium supplementation on insulin resistance in UK pregnant women, as assessed by plasma adiponectin concentration. Br J Nutr. 2016;115(1):32–8. doi: 10.1017/S0007114515004067. [DOI] [PubMed] [Google Scholar]
- 67.Akintunde JK, Bolarin OE, Akintunde DG. Garlic capsule and selenium-vitamins ACE combination therapy modulate key antioxidant proteins and cellular adenosine triphosphate in lisinopril-induced lung damage in rats. Drug Metab Pers Ther. 2016;31(1):47–54. doi: 10.1515/dmpt-2015-0035. [DOI] [PubMed] [Google Scholar]
- 68.Chauhan SS, Ponnampalam EN, Celi P, Hopkins DL, Leury BJ, Dunshea FR. High dietary vitamin E and selenium improves feed intake and weight gain of finisher lambs and maintains redox homeostasis under hot conditions. Small Ruminant Res. 2016;137:17–23. [Google Scholar]
- 69.Donma MM, Donma O. Promising link between selenium and peroxisome proliferator activated receptor gamma in the treatment protocols of obesity as well as depression. Med Hypotheses. 2016;89:79–83. doi: 10.1016/j.mehy.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Ogawa-Wong AN, Berry MJ, Seale LA. Selenium and Metabolic Disorders: An Emphasis on Type 2 Diabetes Risk. Nutrients. 2016;8(2):80. doi: 10.3390/nu8020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakalli Cetin E, Naziroglu M, Cig B, Ovey IS, Aslan Kosar P. Selenium potentiates the anticancer effect of cisplatin against oxidative stress and calcium ion signaling-induced intracellular toxicity in MCF-7 breast cancer cells: involvement of the TRPV1 channel. J Recept Signal Transduct Res. 2016:1–10. doi: 10.3109/10799893.2016.1160931. [DOI] [PubMed] [Google Scholar]
- 72.Lavu RV, Van De Wiele T, Pratti VL, Tack F, Du Laing G. Selenium bioaccessibility in stomach, small intestine and colon: Comparison between pure Se compounds, Se-enriched food crops and food supplements. Food Chem. 2016;197(Pt A):382–7. doi: 10.1016/j.foodchem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Gao X, Zhang Z, Xing H, Yu J, Zhang N, Xu S. Selenium deficiency-induced inflammation and increased expression of regulating inflammatory cytokines in the chicken gastrointestinal tract. Biol Trace Element Res. 2016:1–9. doi: 10.1007/s12011-016-0651-1. [DOI] [PubMed] [Google Scholar]
- 74.Haque MM, Moghal MM, Sarwar MS, Anonna SN, Akter M, Karmakar P, et al. Low serum selenium concentration is associated with preeclampsia in pregnant women from Bangladesh. J Trace Elem Med Biol. 2016;33:21–5. doi: 10.1016/j.jtemb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Liu F, Cottrell JJ, Furness JB, Rivera LR, Kelly FW, Wijesiriwardana U, et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp Physiol. 2016;101(7):801–10. doi: 10.1113/EP085746. [DOI] [PubMed] [Google Scholar]
- 76.Ebner N, Foldes G, Schomburg L, Renko K, Springer J, Jankowska EA. Importance of selenium status in patients with chronic heart failure. Perspect Sci. 2015;3(1):34–5. [Google Scholar]