Mesenchymal stromal cell-conditioned media (MSC-CM) inhibited proinflammatory cytokine expression, restored alternative activated microglia phenotype markers, and enhanced phagocytosis in lipopolysaccharide (LPS)-stimulated microglia. Transforming growth factor-β (TGF-β) in MSC-CM played a major role in these effects by inhibiting the nuclear factor-κB pathway and restoring the TGF-β pathway in LPS-stimulated microglia. Recombinant TGF-β also induced effects similar to those of MSC-CM in LPS-stimulated microglia.
Keywords: Mesenchymal stem cells, Microglia, TGF-β, Neurodegenerative diseases
Abstract
The regulation of microglial cell phenotype is a potential therapeutic intervention in neurodegenerative disease. Previously, we reported that transforming growth factor-β (TGF-β) levels in mesenchymal stromal cells (MSCs) could be used as potential biological markers to predict the effectiveness of autologous MSC therapy in patients with amyotrophic lateral sclerosis. However, the underlying mechanism of TGF‐β in MSCs was not fully elucidated in determining the functional properties of microglia. In this study, we aimed to clarify the role of TGF‐β that is involved in MSC effectiveness, especially focusing on microglia functional properties that play a pivotal role in neuroinflammation. We found that MSC-conditioned media (MSC-CM) inhibited proinflammatory cytokine expression, restored alternative activated microglia phenotype markers (fractalkine receptor, mannose receptor, CD200 receptor), and enhanced phagocytosis in lipopolysaccharide (LPS)-stimulated microglia. In addition, TGF-β in MSC-CM played a major role in these effects by inhibiting the nuclear factor-κB pathway and restoring the TGF-β pathway in LPS-stimulated microglia. Recombinant TGF-β also induced similar effects to MSC-CM in LPS-stimulated microglia. Therefore, we propose that MSCs can modulate the functional properties of microglia via TGF-β secretion, switching them from a classically activated phenotype to an inflammation-resolving phenotype. The latter role may be associated with the inhibition of neuroinflammatory processes in neurodegenerative disorders.
Significance
The results of this study showed that microglia functional properties may be modulated depending on the composition and quantity of mesenchymal stromal cell (MSC)-secreting factors. Transforming growth factor (TGF)-β is proposed as a modulator of microglia functional properties among MSC-secreting factors, and this study aligns with a previous clinical study by these same authors. TGF-β releasing capacity could be an important factor enhancing the therapeutic efficacy of MSCs in clinical trials.
Introduction
Microglia are the resident innate immune cells within the central nervous system (CNS) and participate in normal CNS functional plasticity by changing their morphology, surface receptor expression, and production of cytokines. Microglia also play a role in the progression and resolution of disease [1]. Microglia display various activation states induced by stimuli that arise from injured neurons and surrounding glia, which is one aspect of cell death mechanisms in neurodegenerative diseases [2, 3]. In addition, microglia play a crucial role in disease pathogenesis during neurodegenerative diseases such as Alzheimer’s disease [4], Parkinson’s disease [5], multiple sclerosis (MS) [6], and amyotrophic lateral sclerosis (ALS) [7, 8]. These diseases share a common mechanism: neuroinflammation. As with macrophages, it has been suggested that microglia can exhibit a classically activated phenotype (M1) or an alternatively activated phenotype (M2) [2, 3]. M1 exerts toxic effects by secreting proinflammatory cytokines—such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and nitric oxide (NO)—and plays a role as a major component of the neuroinflammatory response following CNS injury. In contrast to M1, M2 is involved in the maintenance of CNS homeostasis, phagocytizing apoptotic bodies, releasing neurotrophic factors, and reducing proinflammatory cytokines [9]. Markers such as fractalkine receptor (CX3CR1), mannose receptor (CD206), and CD200 receptor (CD200R) detect the M2 phenotype [10], and CX3CR1 plays a critical role in controlling microglial neurotoxicity [11]. Complementary crosstalk between constitutive CX3CL1 release on neurons and CX3CR1 expression on microglia establishes a unique communication system to regulate the activation of microglia [11]. Considering that the persistent activation of microglia (M1-like phenotype) has been demonstrated in chronic neurodegenerative diseases, properly terminating M1 microglial activation and skewing their functional phenotype toward a protective one might contribute to neuronal protection, repair, and renewal in neurodegenerative diseases [12]. Thus, the regulation of microglial cell phenotype could be a potential therapeutic intervention.
Initially, mesenchymal stem or stromal cells (MSCs) attracted public attention because of their multipotency. These cells are capable of differentiating into multiple mesenchymal lineages, including adipocytes, chondroblasts, and osteoblasts [13]. However, recent preclinical data have demonstrated that exogenously administered MSCs exert multifaceted therapeutic effects via immunomodulation and trophic factor secretion rather than via cell replacement, transdifferentiation, or fusion [14–16]. MSCs secrete various cytokines and neurotrophic factors required for tissue repair that are therapeutically useful, leading to improved neurological function, such as neurogenesis, angiogenesis, and synaptogenesis [17, 18]. On the basis of these MSC abilities, an accumulating body of clinical trials has shown that MSCs are safe and may be an alternative therapeutic strategy for treating intractable neurological diseases with unmet medical needs, including ALS, multiple sclerosis, and other degenerative diseases [19–23].
Although numerous studies have reported the positive effect of MSCs in various intractable neurological diseases, the mechanism has not been elucidated. In addition, considering that there are currently no U.S. Food and Drug Administration-approved Biologics License Applications for any MSC-based products, despite considerable interest and effort [24], it is important to clarify which factors in MSCs are involved in therapeutic efficacy. Recently, we proposed biological markers that are associated with the efficacy of autologous MSCs administered intrathecally in 37 ALS patients [25]. We found that vascular endothelial growth factor, angiogenin, and transforming growth factor-β (TGF-β) secretion were decreased in MSCs of nonresponder ALS patients. In addition, MSCs of nonresponders are less effective than are those of responders on motor function and survival time in mutant SOD1G93A transgenic mice (ALS mice) when MSCs were transplanted into the cisterna magna, suggesting that these factors could be used as potential biological markers to predict the effectiveness of autologous MSC therapy. TGF-β is a well-known pleiotropic cytokine that can transit microglia from an activated state to a resting microglial phenotype associated with tissue repair and CNS homeostasis, leading to the resolution of microglia-mediated inflammation and wound healing [9]. However, there are few reports clarifying the role of MSCs secreting TGF-β on primary cultured microglia and focusing on the microglial functional phenotype. In the present study, our results show that TGF-β in MSC-conditioned media (MSC-CM) plays a primary role in skewing M1 toward inflammation-resolving M2 cells by inhibiting the nuclear factor (NF)-κB pathway and restoring the TGF-β pathway in lipopolysaccharide (LPS)-stimulated microglia.
Materials and Methods
Primary Rat Microglial Culture and Identification
All procedures on animals were performed in accordance with the Hanyang University guidelines for the care and use of laboratory animals (HY-IACUC- 2014-0092A). Primary microglial cells were enriched in vitro using the shaking method described by Giulian and Baker [26]. Briefly, 2-day-old Sprague Dawley rats were sacrificed and soaked in 75% ethanol for 1 minute. Cerebral hemispheres were dissected out, following standard techniques and anatomical landmarks, and then meninges were peeled off. The hippocampus, basal ganglion, and olfactory bulb were carefully removed with microsurgical instruments under a microscope, and the remaining cortical tissue was minced with a pair of microsurgical scissors. The shredded tissue was then incubated with 1 ml trypsin and 1 ml phosphate-buffered saline (PBS) for 15 minutes in a 37°C water bath with occasional swirling. After centrifuging at 300 g for 3 minutes, the cells were plated into 75-cm2 flasks that had been coated with poly-l-lysine. Mixed glial cells were cultured in Dulbecco’s modified Eagle’s medium-high glucose (DMEM-HG) containing 10% fetal bovine serum (FBS) and 0.1% Glutamax at 37°C in 5% CO2 in air and 95% humidity. The culture medium was replaced with 15 ml fresh growth medium after 24 hours. Subsequently, one half of the volume of culture medium was replaced with an equal volume of fresh growth medium twice per week. At the end of this period, stratification had been reached, and the microglial cells in the upper layer could be harvested. On day 11, flasks were placed on a MaxQ 2000 orbital shaker (Thermo Fisher Scientific Life Sciences, Waltham, MA USA, https://www.thermofisher.com) within the incubator and shaken for 1 hour at 200 revolutions per minute (rpm). The medium, containing detached microglia, was collected and centrifuged at 190 g for 8 minutes at 23°C. Cells were resuspended with microglial complete culture medium (DMEM, 15% FBS, 0.1% Glutamax, 5 μg/ml insulin, and 1% penicillin/streptomycin) and transferred to poly-l-lysine-coated plates at a density of 2.5 × 105 cellsper milliliter. Flow cytometry analysis using fluorescein (FITC) mouse antirat CD11b antibody (#554982; BD Pharmingen, San Diego, CA, http://www.bdbiosciences.com) or FITC mouse IgG isotype (#553478, BD Pharmingen) confirmed the pure microglia isolation. In an immunofluorescence study, expression of the typical microglia marker (#ab128797, rabbit anti-CD11b, 1:200; Abcam, Cambridge, UK, http://www.abcam.com) indicated greater than 99% of the isolated cells were microglia. Glial fibrillary acidic protein (#Z0334, rabbit anti-GFAP, 1:200; Dako, Carpenteria, CA, http://www.dako.com), an astrocyte marker, stained less than 1% of the cells. Tetramethylrhodamineisothiocyanate conjugated anti-rabbit IgG (#T2769; Thermo Fisher Scientific) and Alexa-488 conjugated anti-rabbit IgG (#A11008, Invitrogen) were used as secondary antibodies, respectively.
Rat MSC Conditioned Media and Drug Treatment in Primary Cultured Microglia
Rat (Sprague Dawley) MSCs were purchased from Gibco (#S1601-100; Thermo Fisher Scientific). MSC characterization was already confirmed in the manufacture datasheet, and cells were cultured as per the manufacturer’s indication. Briefly, cells were cultured at a concentration of 4 × 103 cells per cm2 in DMEM-low glucose with Glutamax-I and supplemented with 10% MSC-qualified FBS and 5 μg/ml gentamicin (Thermo Fisher Scientific) at 37°C with 5% CO2. The medium was refreshed twice a week, and the cells were subcultured before reaching 70%–80% confluence. MSCs were used at their fourth passage. MSCs (5 × 105 ml) were plated into six-well plates (9.5 cm2). After 24 hours, the medium was replaced, and the cells were incubated for another 48 hours, at the end of which time (MSC number; 1–2 × 106 ml) the medium was collected and centrifuged at 1,500 rpm for 10 minutes at 4°C and filtered through 0.22-µm filters (Merck Millipore, Billerica, MA, http://www.emdmillipore.com) before use. The obtained medium was defined and used as MSC-conditioned medium (MSC-CM) in our study. For microglia activation, 100 ng/ml lipopolysaccharide (LPS; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) was added to the culture medium for 24, 48, or 72 hours. LPS-stimulated microglia were treated for 24, 48, or 72 hours with MSC-CM. TGF-β1 receptor (TGF-β1R) inhibitor (SB505124; Sigma-Aldrich), CX3CL1 neutralizaing antibody (ab#8021; Abcam), whose stocks were diluted in complete medium, were mixed with MSC-CM. The recombinant TGF-β1 (PHG9024; Thermo Fisher Scientific) or recombinant CX3CL1 (400-26; PeproTech, Rocky Hill, NJ, https://www.cedarlanelabs.com) were treated for 24 hours. Control samples were given equivalent volumes of complete medium. Thus, the primary cultured microglial cells were divided into the following nine groups: control (group 1, in DMEM-HG containing 10% FBS), 100 ng/ml LPS treatment for 24 hours (group 2), MSC-CM treatment in group 2 (group 3), MSC-CM treatment with 2 μM TGF-β1R inhibitor in group 2 (group 4), siMSC-CM (conditioned medium that was obtained from MSCs transfected with TGF-β1 siRNA) treatment in group 2 (group 5), MSC-CM treatment with 1 μg/ml CX3CL1 antibody in group 2 (group 6), MSC-CM treatment with 1 μg/ml CX3CL1 antibody and 2 μM TGF-β1R inhibitor in group 2 (group 7), 24 hours of 10 ng/ml recombinant TGF-β1 (rTGF-β1) treatment in group 2 (group 8), and 24 hours of 10 ng/ml recombinant CX3CL1 treatment in group 2 (group 9). To determine the concentration-dependent effect of rTGF-β1 on microglia functional phenotype, we treated 300, 700, 1,000, and 1,400 pg/ml of rTGF-β in LPS-stimulated microglia.
TGF-β1 siRNA Transfection to MSC
MSCs were stably transfected with FlexiTube small interfering RNA (siRNA) for rat TGF-β1 (NM_021578) or negative-control siRNA (#SI03650318) using HiPerFect Transfection Reagent (#301705; Qiagen, Hilden, Germany, https://www.qiagen.com). The siRNA transfection was performed in serum-free medium, following the manufacturer’s instruction. The most efficient target sequence for RNA interference was selected from among four sequences provided by Qiagen (#SI02044987, #SI02044994, #SI02045008, and #SI04717951). All siRNAs were tested for mRNA knockdowns by real-time polymerase chain reaction (PCR).
Quantitative Real-Time PCR
The microglia cells were detached to quantify gene expression after drug treatment. Total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific) and evaluated with a Nano-drop (ND-2000; Thermo Fisher Scientific). cDNA was synthesized by using an EcoDry cDNA kit (Clontech, Mountain View, CA, http://www.clontech.com). We analyzed TNF-α (PPR06411F; Qiagen), IL-1β (PPR06480B; Qiagen), IL-6 (PPR06483B; Qiagen), inducible nitric oxide (iNOS) (PPR44835A; Qiagen), CX3CR1 (PPR06709A; Qiagen), CD206 (Mannose receptor: PPR65066A; Qiagen), TGF-β1 (pPPR06430B; Qiagen), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (PPR06557B; Qiagen). cDNA was amplified using Power SYBR Green PCR Master Mix with primers in an Applied Biosystems Step One Plus system (Thermo Fisher Scientific) at 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. A melting curve was generated to examine the specificity of the amplification. The relative quantity levels were calculated with the 2−ΔΔ Ct method using GAPDH as the interval standard control. The reported results are based on three independent experiments on separate batches of cells.
Nitrate Assay
Nitrate levels were measured using a nitrate assay kit (R&D Systems, Minneapolis, MN, https://www.rndsystems.com). The nitrate level in the supernatant was measured and analyzed according to the manufacturer’s instruction.
Flow Cytometry Analysis
After MSC-CM treatment of LPS-stimulated microglia for 72 hours, the microglia were immune-labeled by using antibodies against the surface proteins CD86, CX3CR1, CD206, and CD200R. Indirect immunofluorescence flow cytometry was performed using the following antibodies: rabbit anti-CD86 (1:100, #ab53004, Abcam), rabbit anti-CX3CR1 antibody (1:100, ab#8021; Abcam), and rabbit anti-CD206 antibody (1:100, #ab64693; Abcam), and mouse anti-CD200R-PE (1:100, #ab34135; Abcam). The microglia cells were washed with Stain buffer (BD Pharmingen) and incubated with primary antibody for 30 minutes at RT, washed with calcium-magnesium-free [PBS(−)], resuspended, and fixed with 1% paraformaldehyde (Wako, Osaka, Japan, http://www.wako-chem.co.jp) in PBS(−). After primary staining, the microglia cells were washed with stain buffer and stained with anti-rabbit Alexa 488-conjugated secondary antibodies (#A11008, Thermo Fisher Scientific). The expression ratio of CD86, CX3CR1, CD206, and CD200R was calculated with the fluorescent intensity of each fluorochrome [27]. All data were collected on a FACS Canto II flow cytometer (BD Biosciences) and were analyzed with FACS Diva and FlowJo software (BD Biosciences).
Immunocytochemistry
iNOS, CX3CR1, and CD206 were used as microglia phenotype markers. Iba-1 was used as a microglia marker. Microglia were seeded on glass coverslips in 24-well plates and cultured with LPS for 24 hours. Cells were washed with PBS and cultured with PBS or MSC-CM for 72 hours and then fixed in 4% formaldehyde and permeabilized with 0.1% Triton X-100 for 5 minutes. Indirect immunofluorescence was performed using the following antibodies: rabbit anti-iNOS antibody (#sc-8310,1:200; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com), rabbit anti-CX3CR1 antibody (ab#8021, 1:200; Abcam), rabbit anti-CD206 antibody (#ab64693, 1:200, Abcam), and mouse anti-Iba-1 antibody (#019-19741, 1:200; Wako). Cells were incubated in primary antibodies diluted in 0.1% Triton-X 100 in PBS containing 5% normal goat serum at 4°C overnight. After rinsing three times with PBS(−) for 5 minutes, anti-rabbit Alexa 546- and anti-mouse Alexa 488-conjugated secondary antibodies (Thermo Fisher Scientific) were used for detection. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Cells without the addition of primary antibody served as negative controls. Fluorescent images were taken with a confocal microscope (TCS SP5; Leica, Wetzlar, Germany, http://www.leica-microsystems.com).
Phagocytosis Assay
For the quantification of microglial phagocytosis, primary microglia were seeded at a concentration of 3 × 105 on 35-mm glass-bottom cell culture dishes as a control. The microglia were treated with 100 ng/ml LPS for 24 hours (LPS), and the LPS group was incubated in the presence or absence of the same concentration of MSC-CM for 24, 48, and 72 hours (LPS + MSC-CM). At the end of each treatment, cells were incubated with 10 µl of red-dyed fluoresbrite microspheres red fluorescent latex beads (2 μm; Sigma-Aldrich) for 30 minutes at 37°C. Phagocytic activity was then stopped by adding 2 ml ice-cold PBS. The cells were washed twice with ice-cold PBS, fixed, and counterstained with DAPI. Cells were analyzed by confocal microscopy (TCS SP5; Leica). The number of phagocytosed beads per cell indicated phagocytic activity.
Western Blot
Cells were washed twice in cold PBS (Biochrom, Berlin, Germany, http://www.biochrom.de) and incubated for 10 minutes on ice in radioimmunoprecipitation assay buffer. Protein concentration of the cell lysates was determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, http://www.bio-rad.com). Samples containing equal amounts (50 μg) of protein were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Piscataway, NJ, http://www.gelifesciences.com). The membranes were blocked with 5% skim milk and then incubated with specific primary antibodies. We used antibodies against rabbit anti-CX3CR1(ab#8021, 1:1000; Abcam), mouse antip-IκB (SC-8404; Santa Cruz), and rabbit anti-IκB (#SC-371, 1:1000; Santa Cruz), rabbit antip-Smad2/3 (#8828, 1:1000; Cell Signaling, Danvers, MA, http://www.cellsignal.com), Smad2/3 (#8685, 1:1000; Cell Signaling), and GAPDH (SC-25778, 1:1,000; Santa Cruz). The membranes were washed with Tris-buffered saline containing 0.05% Tween-20 and then processed using a horseradish peroxidase (HRP)-conjugated secondary anti-rabbit or -mouse antibody (Amersham Pharmacia Biotech) followed by enhanced chemiluminescence (ECL) detection (Amersham Pharmacia Biotech). The Western blot results were quantified with an image analyzer (Quantity One-4,2,0; Bio-Rad) and normalized to GAPDH immunostaining, as previously described [28]. The reported results are based on three independent experiments on separate batches of cells.
Enzyme-Linked Immunosorbent Assays Analysis and Lactate Dehydrogenase Assay
For quantification of TGF-β1 and CX3CL1 levels in the supernatant of all groups and in MSC-CM, we performed enzyme-linked immunosorbent assays (ELISA) using commercially available kits on supernatants derived from each culture condition. Rat TGF-β1 ELISA (R&D Systems) and rat CX3CL1 ELISA kits (Abcam) were used according to the manufacturer’s instructions. Lactate dehydrogenase (LDH) assay (#TB163; Promega, Madison, WI, http://www.promega.com) was performed according to the manufacturer’s instructions. The reported results are based on three independent experiments on separate batches of cells.
Statistical Analysis
The data are presented as the means ± SEM. The statistical significance of the differences between the groups was assessed using Student’s t test and one-way analysis of variance using GraphPad Prism Version 5.0 software for Mac OS X (GraphPad Software, San Diego, CA, http://www.graphpad.com). Bonferroni’s post hoc analysis was performed when p values were <.05.
Results
LPS Increased Proinflammatory Cytokines in Primary Cultured Microglia
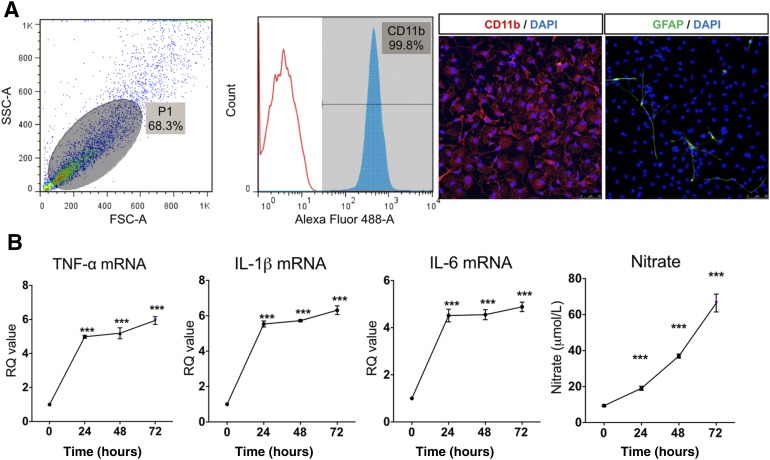
After microglia culture, we confirmed that 99% of cells were CD11b-positive by flow cytometry. By immunofluorescence, expression of the typical microglia marker (CD11b) was presented in greater than 99% of the isolated cells. GFAP, an astrocyte marker, was observed in less than 1% of the cells (Fig. 1A). To examine the effect of LPS in primary cultured microglia, we applied 100 ng of LPS for 24 hours, 48 hours, and 72 hours. LPS increased proinflammatory cytokine expression such as TNF-α, IL-1β, IL-6, and iNOS in primary cultured microglia. LPS treatment for 24 hours was sufficient to induce cytokine expression. In line with iNOS expression, nitrate accumulated gradually in culture supernatant in a time-dependent manner (Fig. 1B). Thus, we confirmed that 24 hours LPS (100 ng/ml) could induce a sufficient inflammatory reaction in microglia.
Figure 1.
Lipopolysaccharide (LPS)-induced inflammatory reaction in primary cultured microglia. (A): Characteristics of primary cultured microglia by flow cytometry and immunocytochemistry. The primary cultured cells were 99.8% CD11b positive, confirming pure microglia isolation. Glial fibrillary acidic protein, an astrocyte marker, was observed in less than 1% of the cells. (B): LPS, 100 ng/ml, was applied to primary cultured microglia for 24, 48, and 72 hours. LPS increased mRNA expression of TNF-α, IL-1β, IL-6, and inducible nitric oxide at all time points. Nitrate concentration gradually increased with LPS treatment duration. The data are means ± SEM of three independent experiments. ∗∗∗, p < .001 in comparison with control and 0 hour. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; FSC-A, forward scatter; GFAP, glial fibrillary acidic protein; hr, hour; IL, interleukin; RQ, relative quantity; SSC-A, side scatter; TNF, tumor necrosis factor.
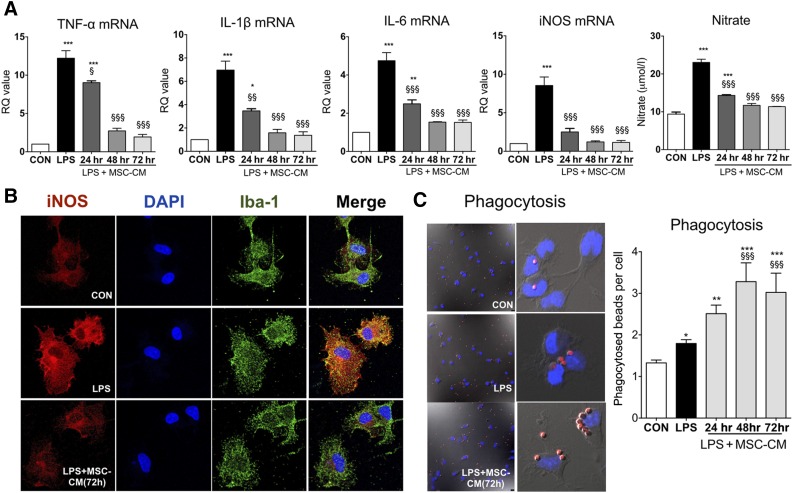
Inflammatory Reaction in LPS-Stimulated Microglia Was Suppressed by MSC-CM
To determine the role of soluble factors that are secreted by MSCs, we collected the supernatant (MSC-CM) after 48 hours of MSC culture. The cell number was 1–2 × 106 cell/ml at that time. MSC-CM was applied for 24, 48, and 72 hours to LPS-stimulated microglia, and mRNA expression was analyzed after MSC treatment. As shown in Figure 2A, LPS significantly increased TNF-α, IL-1β, IL-6, and iNOS mRNA levels, and nitrate secretion in microglia. MSC-CM completely inhibited the inflammatory reaction of LPS-stimulated microglia in a time-dependent manner. By immunofluorescence, we confirmed the effect of MSC-CM on iNOS expression in LPS-stimulated microglia (Fig. 2B). MSC-CM enhanced phagocytosis in LPS-stimulated microglia (Fig. 2C), in accordance with a previous study [29].
Figure 2.
MSC-CM suppressed the inflammatory reaction and enhanced phagocytosis in LPS-stimulated microglia. (A): To determine the effect of MSC-CM on the level of cytokines in LPS-stimulated microglia, we applied MSC-CM for 24, 48, and 72 hours to LPS-stimulated microglia. LPS increased mRNA expression of TNF-α, IL-1β, IL-6, and iNOS, and nitrate secretion in LPS-stimulated microglia, whereas MSC-CM inhibited the inflammatory reaction. (B): Immunostaining shows that MSC-CM significantly suppressed iNOS expression in LPS-stimulated microglia. The microglia and nuclei were stained with Iba-1 and DAPI, respectively. (C): LPS-stimulated microglia ingested more latex beads than did untreated controls, and the phagocytic activity of microglia was significantly enhanced when the MSC-CM was applied to LPS-stimulated microglia following LPS treatment. The data are means ± SEM of three independent experiments. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001; in comparison with control. §, p < .05; §§, p < .05; §§§, p < .001; in comparison with LPS. Abbreviations: CON, control; DAPI, 4′,6-diamidino-2-phenylindole; hr, hour; iNOS, inducible nitric oxide; IL, interleukin; LPS, lipopolysaccharide; MSC-CM, mesenchymal stromal cell-conditioned media; RQ, relative quantity; TNF, tumor necrosis factor.
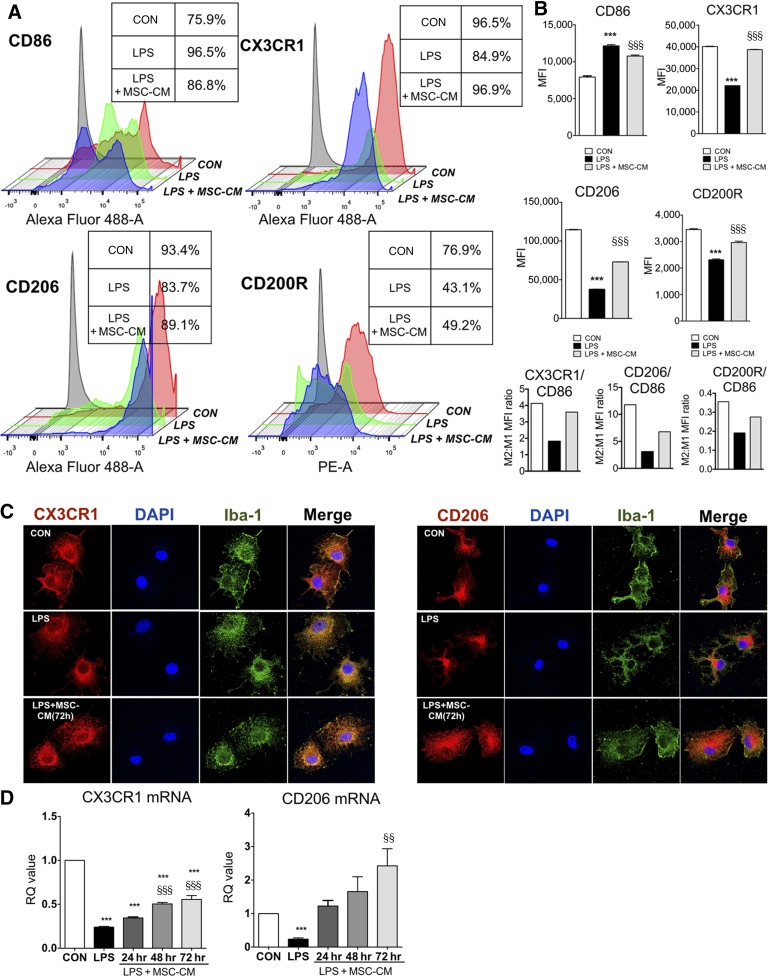
MSC-CM Rescues Both CX3CR1 and CD206 Expression, Which Were Reduced in LPS-Stimulated Microglia
Microglia phenotypes are associated with effector function, and the transition from M1 to M2 phenotype reflects the resolution of inflammation. To investigate the effect of MSC-CM on microglia phenotype, we used flow cytometry analysis, immunofluorescence, and quantitative PCR. As is shown in Figure 3, in flow cytometry analysis, we found that treatment with MSC-CM restored M2 markers such as CX3CR1, CD206, and CD200R, which were reduced by LPS while MSC-CM inhibited CD86 (M1 marker), showing that the M2:M1 mean fluorescence intensity ratio (CX3CR1, CD2006, CD200R: CD86) was restored by MSC-CM in LPS-stimulated microglia. In addition, the microglia apoptosis in all groups was not detected in LDH assay (supplemental online Fig. 1). By immunofluorescence, the reduction of CX3CR1 and CD206 was observed mainly in microglial process of LPS-stimulated microglia, which was rescued by MSC-CM. The mRNA levels of both CX3CR1 and CD206 were also rescued in an incubation time-dependent manner.
Figure 3.
MSC-CM restored CX3CR1, CD206, and CD200R expression in LPS-stimulated microglia. To investigate the effect of MSC-CM on the expression of CD86, CX3CR1, CD206, and CD200R in LPS-stimulated microglia, we performed flow cytometry. (A): Microglia were treated with LPS for 24 hours and MSC-CM was incubated for 72 hours. The isotype control was indicated as gray color. Control group (red), LPS group (green), and LPS + MSC-CM (blue) were indicated, respectively. The table box beside the diagram indicates the percentage of positive cells in each group. (B): Rather than divide this microglia population further by imposing strict boundaries on a continuous expression pattern, we then determined MFI of the entire remaining population for each marker, and we calculated the M2:M1 MFI ratio (CX3CR1, CD206, or CD200R/CD86). MSC-CM restored CX3CR1, CD206, and CD200R MFI, whereas it inhibited the CD86 in LPS-stimulated microglia, showing the normalization of M2:M1 MFI ratio. (C): Immunofluorescence results showed a similar pattern with flow cytometry analysis. Microglia was stained with Iba-1 and DAPI, respectively. (D): MSC-CM restored mRNA expression of CX3CR1 and CD206 in LPS-stimulated microglia following to incubation time. The data are means ± SEM of three independent experiments. §, p < .001 in comparison with control (CON); §§, p < .01; §§§, p < .001 in comparison with LPS. Abbreviations: CON, control; DAPI, 4′,6-diamidino-2-phenylindole; hr, hour; LPS, lipopolysaccharide; MFI, mean fluorescence intensity; MSC-CM, mesenchymal stromal cell-conditioned media; RQ, relative quantity.
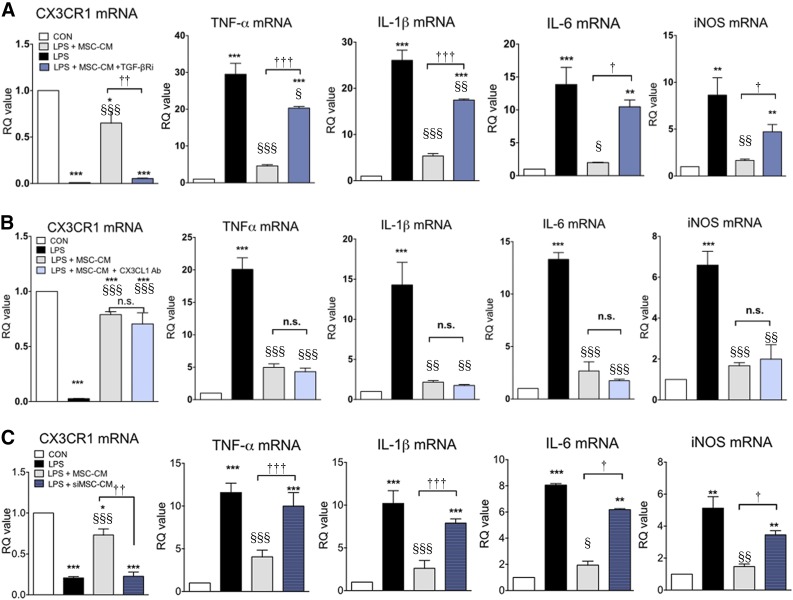
TGF-β in MSC-CM Plays a Main Role in Inhibition of Proinflammatory Cytokine Expression and Restoration of CX3CR1 Expression in LPS-Stimulated Microglia
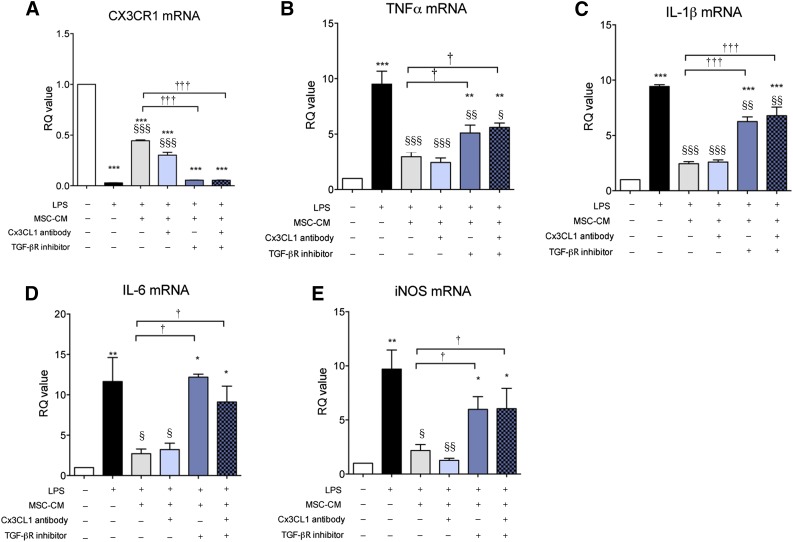
Previously, CX3CL1 was suggested to be a direct modulatory factor that can transition microglia to the M2 phenotype [29]. TGF-β is an anti-inflammatory cytokine that can resolve inflammatory reactions in microglia. We reported that TGF-β might be involved in MSC efficacy when MSCs were administered intrathecally in ALS patients [25]. Thus, we investigated the role of TGF-β and CX3CL1 in LPS-stimulated microglia, focusing on CX3CR1 and proinflammatory cytokine expression. As is shown in Figure 4, LPS increased proinflammatory cytokine expression, and MSC-CM inhibited these inflammatory reactions with restoration of CX3CR1 expression in LPS-stimulated microglia. When TGF-βR inhibitor and CX3CL1 antibody were added to MSC-CM to investigate the role of soluble TGF-β and CX3CL1on MSC-CM effect, CX3CL1 antibody treatment did not abolish the effect of MSC-CM, whereas TGF-βR inhibitor did. In addition, CX3CL1 antibody neutralized CX3CL1 completely in MSC-CM (supplemental online Figs. 2, 3). TGF-βR inhibitor alone did not affect CX3CR1, TNF-α, IL-1β, and IL-6 mRNA levels (data not shown). Furthermore, we transfected TGF-β siRNA into MSCs (siMSC) and obtained the conditioned medium from siMSC (siMSC-CM). More than 50% of TGF-β expression and secretion was reduced in siMSC (supplemental online Fig. 4) and siMCS-CM exhibited a blunted inhibitory effect on inflammatory response in LPS-stimulated microglia. These results suggest that the TGF-β signaling pathway may be involved in the effect of MSC-CM on LPS-stimulated microglia. To investigate whether double blocking of both TGF-β and CX3CL1 completely abolished the effect of MSC-CM on LPS-stimulated microglia, we added a combination treatment of both TGF-βR inhibitor and CX3CL1 antibody in MSC-CM. As is shown in Figure 5, the double blocking did not show any difference from the results of TGF-βR inhibitor alone, suggesting that TGF-β alone in MSC-CM inhibits proinflammatory cytokine expression and rescues CX3CR1 expression, similar to the results shown in Figure 4.
Figure 4.
TGF-β mediates the effect of MSC-CM on LPS-stimulated microglia. To determine which soluble factor was involved in the effect of MSC-CM on LPS-stimulated microglia, we treated LPS-stimulated microglia with MSC-CM + a TGF-βR inhibitor (A), a CX3CL1 antibody (B), or siMSC-CM (siMSC-CM indicates conditioned medium that was obtained from TGF-β siRNA-transfected MSCs) (C). LPS increased TNF-α, IL-1β, IL-6, and iNOS mRNA levels, and MSC-CM inhibited the increased cytokines and led to CX3CR1 restoration. TGF-β inhibition abolished the effect of MSC-CM on LPS-stimulated microglia, whereas CX3CL1 antibody did not. siMSC-CM did not affect LPS-stimulated microglia. The data are means ± SEM of three independent experiments. ∗, p < .01; ∗∗, p < .001; in comparison with control (CON). §, p < .05; §§, p < .01; §§§, p < .001; in comparison with LPS. †, p < .05; ††, p < .01; †††, p < .001; in comparison with two groups (LPS + MSC-CM vs. LPS + MSC-CM + antibody/inhibitor or siMSC-CM). Abbreviations: CON, control; iNOS, inducible nitric oxide; IL, interleukin; LPS, lipopolysaccharide; MSC-CM, mesenchymal stromal cell-conditioned media; n.s., not significant; RQ, relative quantity; TGF, transforming growth factor; TNF, tumor necrosis factor.
Figure 5.
TGF-βR inhibition alone can abolish the effect of MSC-CM in LPS-stimulated microglia. To investigate whether CX3CL1 plays a role in the anti-inflammatory effect of TGF-β in MSC-CM, we added a CX3CL1 antibody, a TGF-βR inhibitor, and both together to MSC-CM. LPS reduced CX3CR1 (A) and increased TNF-α mRNA expression (B), IL-1β mRNA expression (C), IL-6 mRNA expression (D), and iNOS mRNA expression (E), and. MSC-CM rescued these changes. The TGF-βR inhibitor alone showed the same effect on LPS-stimulated microglia, regardless of the presence of the CX3CL1 antibody. When the TGF-βR inhibitor was added to the MSC-CM, the effect of the MSC-CM on TNF-α, IL-1β, IL-6, iNOS, and CX3CR1 was abolished. The data are means ± SEM of three independent experiments. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001; in comparison with control. §, p < .05; §§, p < .01; §§§, p < .001; in comparison with LPS. †, p < .05; †††, p < .001; compared between two groups. Abbreviations: iNOS, inducible nitric oxide; IL, interleukin; LPS, lipopolysaccharide; MSC-CM, mesenchymal stromal cell-conditioned media; RQ, relative quantity; TGF, transforming growth factor; TNF, tumor necrosis factor.
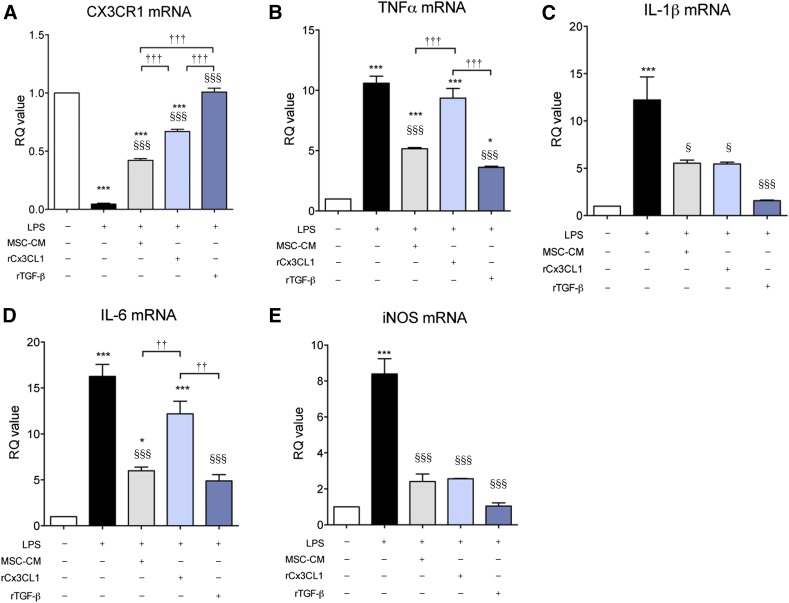
Recombinant TGF-β Mimics MSC-CM on LPS-Stimulated Microglia
Confirming that TGF-β plays a major role in the effects of MSC-CM on LPS-stimulated microglia, we investigated whether recombinant TGF-β (rTGF-β) had similar effects as MSC-CM. To maximize the effect of rTGF-β and to match the concentration of rCX3CL1 (10 ng/ml) that was used in a previous study [29], a high concentration (10 ng/ml) of rTGF-β was used. As is shown in Figure 6, rTGF-β suppressed the expression of all proinflammatory cytokines in LPS-stimulated microglia, similar to the effect of MSC-CM. Interestingly, rCX3CL1 (10 ng/ml) inhibited IL-1β and iNOS expression and restored CX3CR1 expression but did not affect TNF-α and IL-6 expression [29]. Furthermore, we confirmed that rTGF-β mimics MSC-CM effect in a concentration-dependent manner (supplemental online Fig. 5). These results and those of a previous study [29] suggest that CX3CL1 and TGF-β both regulate microglia effector function in LPS-stimulated microglia, depending on the composition and concentration of factors that MSCs secrete.
Figure 6.
Recombinant TGF-β showed an effect similar to that of MSC-CM on LPS-stimulated microglia. Recombinant TGF-β (rTGF-β, 10 ng/ml) induced similar effects as did MSC-CM. On the basis of a previous study, recombinant CX3CL1 (rCX3CL1, 10 ng/ml) was also tested. The rTGF-β and rCX3CL1 were applied for 24 hours. rTGF-β rescued the reduced CX3CR1 expression (A) and inhibited the increased gene expression of TNF-α (B), IL-1β (C), IL-6 (D), and iNOS (E), similar to MSC-CM in LPS-stimulated microglia. rCX3CL1 (10 ng/ml) also inhibited IL-1β and iNOS expression and restored CX3CR1 expression but did not affect TNF-α and IL-6 expression. The data are means ± SEM of three independent experiments. ∗, p < .05; ∗∗∗, p < .001; in comparison with control. §, p < .05; §§§, p < .001; in comparison with LPS. ††, p < .05; †††, p < .001; compared between two groups. Abbreviations: iNOS, inducible nitric oxide; IL, interleukin; LPS, lipopolysaccharide; MSC-CM, mesenchymal stromal cell-conditioned media; RQ, relative quantity; TGF, transforming growth factor; TNF, tumor necrosis factor.
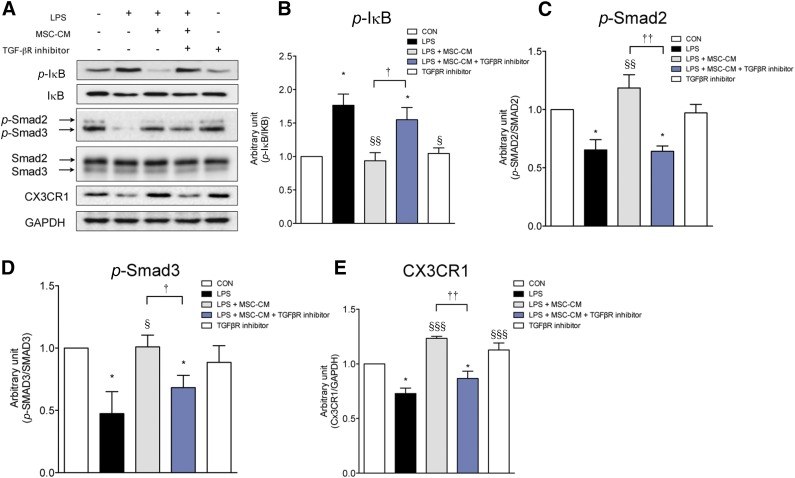
MSC-CM Inhibits the NF-κB Pathway and Rescues CX3CR1 Expression via the TGF-β Signaling Pathway in LPS-Stimulated Microglia
As is shown in Figure 7, LPS increased p-IκB and reduced p-smad2/3 and CX3CR1 expression. MSC-CM normalized these alterations in LPS-stimulated microglia. A TGF-βR inhibitor—which alone did not affect CX3CR1, p-IκB, and p-smad2/3 expression levels—abolished the effects of MSC-CM, suggesting that MSC-CM inhibited the NF-κB pathway and rescued CX3CR1 expression via the TGF-β pathway in LPS-stimulated microglia. In addition, these results suggest that TGF-β in MSC-CM plays a central role in the inhibition of proinflammatory cytokine expression and the rescue of CX3CR1 expression in LPS-stimulated microglia.
Figure 7.
MSC-CM inhibits the NF-κB pathway and rescues CX3CR1 expression via the TGF-β signaling pathway in LPS-stimulated microglia. (A): LPS increased p-IκB expression and reduced CX3CR1 expression and Smad2/3 phosphorylation by Western blot. MSC-CM restored these changes in LPS-stimulated microglia. However, the effect of MSC-CM was abolished when TGF-βR inhibitor was applied with MSC-CM. TGF-βR inhibitor alone did not affect CX3CR1 expression, IκB, or Smad2/3 phosphorylation in primary cultured microglia. The p-IκB (B), p-smad2 (C), p-smad3 (D), and CX3CR1 (E) expression levels were measured using densitometry. The data are means ± SEM of three independent experiments. ∗, p < .05, compared with control. §, p < .05; §§, p < .01; §§§, p < .001; in comparison with LPS. †, p < .05; ††, p < .001; compared between two groups. Abbreviations: CON, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LPS, lipopolysaccharide; MSC-CM, mesenchymal stromal cell-conditioned media; TGF, transforming growth factor.
Discussion
Considering that neuroinflammation is one of the most striking hallmarks of neurodegenerative disease and that microglia play pivotal roles in neuroinflammation, it is important to investigate whether MSCs can modulate microglial functional phenotypes and which factors produced by MSCs are responsible. We reported that soluble factors such as prostaglandin E2, indoleamine-2,3 dioxygenase, and TGF-β secreted by MSCs have immunomodulatory functions, which may resolve inflammatory reactions and lead to the prevention of neuronal damage [30]. In the present study, our results suggest that TGF-β secreted by MSCs plays a central role on skewing the classically activated M1 phenotype toward a protective M2 phenotype by inhibiting the NF-κB pathway and leading to a reduction of proinflammatory cytokine expression in LPS-stimulated microglia. Previously, we proposed that TGF-β might be a biological marker associated with the efficacy of MSCs administered intrathecally to ALS patients [25]. Interestingly, the TGF-β concentration in microglia culture medium was similar to the cerebrospinal fluid (CSF) of ALS patients (TGF-β: 600–800 pg/ml) before intrathecal MSCs injection (supplemental online Fig. 2). In addition, the TGF-β concentration (1744.9 ± 95.8 pg/ml) in the LPS + MSC-CM group was a little higher than was the CSF of ALS patients at 1 month after intrathecal 1 × 106 MSC injection (TGF-β: 1200–1300 pg/ml) (supplemental online Fig. 3). Thus, our in vitro result seems to adequately reflect our phase I clinical trial results that intrathecal MSC injection increased TGF-β concentration in CSF [23]. With all results taken together, we speculate that TGF-β may be one of the main factors regulating microglial function and determining the efficacy of MSCs in ALS and other neurodegenerative diseases involving microglia.
On the basis of a previous study showing that CX3CL1 could shape microglia effector functions in the murine microglia cell line N9 [29], we investigated the role of CX3CL1 and TGF-β in primary cultured microglia following LPS treatment. In contrast to the result of a previous study that used mouse MSCs and N9 cell line, our results showed that a CX3CL1 neutralizing antibody did not block the phenotype transition effect of MSC-CM in LPS-stimulated microglia. In addition, regardless of the presence of CX3CL1 in MSCs, TGF-β alone sufficiently modulated microglia effector function. On the other hand, recombinant CX3CL1 (10 ng/ml) could shape microglia effector function, in agreement with the previous study [29]. MSCs can secret different composition and capacity of soluble factors, depending on microenvironment [14, 28, 29, 31], and N9 cell line is different from primary microglia culture on microglia signature expression [32]. Thus, we concluded that this discrepancy might be related to potential microglia character, MSC culture condition, and microenvironment. Our MSC-CM contained sufficient TGF-β (1747 ± 29.3 pg/ml) but had less CX3CL1 (15.9 ± 0.1 pg/ml) than that (15 ng/ml) of the previous study [29]. When the MSC number reached 1.3 × 107 (7 days after 2 × 105 MSC seeding), the maximum cell number in a 75T flask, the CX3CL1 concentration was 477.75 pg/ml, which was still less than 10 ng/ml (data not shown). Only 1–2 × 106 MSCs have been administered in most clinical and animal studies, because it is rare to isolate more than 1–2 × 106 MSC/kg weight from adults, and most studies have shown maximum efficacy and negligible mortality at the dose of 1 × 106 MSCs [20, 23, 25, 33]. Therefore, it seems unlikely that the CX3CL1 concentration in the CSF would reach 10 ng/ml when 1–2 × 106 MSCs are administered intrathecally, although we cannot exclude the possibility that the primed MSCs in an inflammatory microenvironment could acquire an immunomodulatory phenotype, enhancing the release of CX3CL1 and TGF-β, as in previous studies [25, 30]. Collectively, these results suggest that CX3CL1 in our MSC-CM is not the main contributor to the M2 transition but is a candidate factor that can also reduce proinflammatory cytokine expression and rescue the M2 phenotype. In addition, our results indicate that TGF-β alone in MSC-CM is sufficient to suppress the inflammatory reaction and restore a M2 microglia phenotype. Thus, we propose that the amount and quantity of soluble factors such as TGF-β and CX3CL1 may contribute to MSC efficacy in neurodegenerative diseases involving microglia. However, further study will be required to clarify which soluble factor secreted by human MSC contributes to the functional properties of human microglia.
The NF-κB pathway plays a pivotal role in inflammatory reactions, and a recent study has demonstrated that microglia induce motor neuron death via the classical NF-κB pathway [7]. Classical NF-kB consists of the p65/p50 heterodimer and is a pivotal regulator in inflammatory reactions, driving proinflammatory cytokine gene expression in microglia [34]. TGF-β signaling, a potent deactivator of microglia, is thought to act as an anti-inflammatory cytokine [9] and down-regulates cytokine production in LPS-stimulated microglia via Smad phosphorylation [35]. The relationship between CX3CL1 (fractalkine) and its receptor (CX3CR1) is tightly regulated in the brain. CX3CL1 is known to modulate a complex network of paracrine and autocrine interactions between neurons and microglia, controlling microglia neurotoxicity [11]. A recent study showed that activation of the NF-κB pathway inhibited CX3CR1 expression in microglia via yy1 and led to attenuated functional responses of microglia to CX3CL1 [36]. Our results also showed that MSC-CM inhibited the NF-κB pathway, restored Smad2/3 phosphorylation, and normalized CX3CR1 expression in LPS-stimulated microglia. A TGF-βR inhibitor abolished the MSC-CM effects, and siMSC-CM did not affect the microglia functional phenotype. We conclude from these results that MSC-secreted TGF-β restores the reduced CX3CR1 expression via inhibition of the NF-κB pathway by TGF-β1R-related Smad2/3 phosphorylation in LPS-induced microglia. However, further study will be required to confirm whether TGF-β2 or 3R-related signaling pathways are also involved in microglia functional phenotype.
In the current study, rTGF-β mimics the effect of MSC-CM on LPS-stimulated microglia. However, MSCs exert a broad spectrum of immunoregulatory effects on cells of both the innate and adaptive immune systems via soluble mediators and direct cell contact [37, 38]. We reported that when MSCs interact with human peripheral blood mononuclear cell (PBMCs), MSCs were potentiated, and more TGF-β was secreted from both MSCs and PBMCs, leading to synergic TGF-β elevation in comparison with MSCs alone [30]. However, rTGF-β in vivo has a short half-life in its soluble phase, suggesting difficulty in clinical applications [39]. Furthermore, we cannot ignore the role of various neurotropic factors that MSCs release. Taken together, we suggest that MSCs would be superior to rTGF-β in neurodegenerative diseases, although rTGF-β shapes microglial functional effects in LPS-stimulated microglia.
Although the adult resident microglia phenotype is strictly controlled in spite of LPS/interferon-γ stimulation in healthy humans [40], a comparison study between blood-derived macrophages and human adult microglia demonstrated that adult microglia up-regulate inflammatory markers such as macrophages under long-term M1 polarizing conditions [3]. In addition, most studies demonstrated that M2 phenotype markers, in contrast to M1 phenotype markers, could be modulated in adult microglia, depending on the microenvironment [3, 40]. In summary, we speculate that MSC-CM could break a chronic inflammatory cycle by skewing microglia with disease-specific characteristics toward an M2 phenotype in various neurodegenerative diseases [3, 40, 41]. Whether MSC-CM can switch functional phenotypes in adult microglia from patients should be clarified.
Conclusion
To the best of our knowledge, few studies have investigated the underlying mechanism of MSC-CM in primary cultured microglia by focusing on the functional phenotypes, except for a report demonstrating the role of CX3CL1. In this study, we found that TGF-β alone sufficiently restored M2 phenotype markers and suppressed proinflammatory cytokine expression in LPS-stimulated microglia. These results are in line with our clinical data [25], showing that TGF-β is a biomarker determining the efficacy of MSCs administered intrathecally in ALS. Therefore, we suggest that TGF-β as well as CX3CL1 released by MSCs modulate the inflammatory cycle in microglia and enhance the therapeutic efficacy of MSCs in clinical trials.
Supplementary Material
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communication Technology, and Future Planning (NRF-2014R1A1A3052516) and by the Korea Healthcare Technology Research and Development Project of the Ministry for Health and Welfare Affairs of the Republic of Korea (A120182).
Author Contributions
M.Y.N.: conception and design, manuscript writing, financial support; S.M.L.: data analysis and interpretation, manuscript writing; K.-W.O.: collection and/or assembly of data, data analysis and interpretation; K.-A.C., J.P., K.-S.K., and S.-J.L.: data analysis and interpretation, administrative support; M.-S.K.: conception and design, manuscript writing, data analysis and interpretation; S.H.K.: conception and design, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 2.Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson’s disease and amyotrophic lateral sclerosis: Are we listening? Trends Immunol. 2010;31:7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durafourt BA, Moore CS, Zammit DA, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 4.Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm (Vienna) 2010;117:949–960. doi: 10.1007/s00702-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 6.Priller J, Flügel A, Wehner T, et al. Targeting gene-modified hematopoietic cells to the central nervous system: Use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 7.Frakes AE, Ferraiuolo L, Haidet-Phillips AM, et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81:1009–1023. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: Role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- 9.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 10.Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134:1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 12.Liao B, Zhao W, Beers DR, et al. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol. 2012;237:147–152. doi: 10.1016/j.expneurol.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reyes M, Dudek A, Jahagirdar B, et al. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 16.Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 18.Koh SH, Noh MY, Cho GW, et al. Erythropoietin increases the motility of human bone marrow-multipotent stromal cells (hBM-MSCs) and enhances the production of neurotrophic factors from hBM-MSCs. Stem Cells Dev. 2009;18:411–422. doi: 10.1089/scd.2008.0040. [DOI] [PubMed] [Google Scholar]
- 19.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HY, Paik JY, Park HK, et al. Efficacy and safety of autologous bone marrow-derived mesenchymal stem cell treatment in patients with amyotrophic lateral sclerosis. J Korean Neurol Assoc. 2009;27:163–169. [Google Scholar]
- 21.Lee PH, Lee JE, Kim H-S, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- 22.Mazzini L, Ferrero I, Luparello V, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A phase I clinical trial. Exp Neurol. 2010;223:229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Oh KW, Moon C, Kim HY, et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Translational Medicine. 2015;4:590–597. doi: 10.5966/sctm.2014-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendicino M, Bailey AM, Wonnacott K, et al. MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell. 2014;14:141–145. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Kim HY, Kim H, Oh KW, et al. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: An investigator-initiated trial and in vivo study. Stem Cells. 2014;32:2724–2731. doi: 10.1002/stem.1770. [DOI] [PubMed] [Google Scholar]
- 26.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker PA, Bedi SS, Shah SK, et al. Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: Modulation of the resident microglia population. J Neuroinflammation. 2012;9:228. doi: 10.1186/1742-2094-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YS, Noh MY, Cho KA, et al. Hypoxia/reoxygenation-preconditioned human bone marrow-derived mesenchymal stromal cells rescue ischemic rat cortical neurons by enhancing trophic factor release. Mol Neurobiol. 2014;52:792–803. doi: 10.1007/s12035-014-8912-5. [DOI] [PubMed] [Google Scholar]
- 29.Giunti D, Parodi B, Usai C, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells. 2012;30:2044–2053. doi: 10.1002/stem.1174. [DOI] [PubMed] [Google Scholar]
- 30.Kwon MS, Noh MY, Oh KW, et al. The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients. J Neurochem. 2014;131:206–218. doi: 10.1111/jnc.12814. [DOI] [PubMed] [Google Scholar]
- 31.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butovsky O, Jedrychowski MP, Moore CS, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci. 2013;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morando S, Vigo T, Esposito M, et al. The therapeutic effect of mesenchymal stem cell transplantation in experimental autoimmune encephalomyelitis is mediated by peripheral and central mechanisms. Stem Cell Res Ther. 2012;3:3. doi: 10.1186/scrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell K, Shah JP, Tsytsikova LV, et al. LPS antagonism of TGF-β signaling results in prolonged survival and activation of rat primary microglia. J Neurochem. 2013;129:155–168. doi: 10.1111/jnc.12612. [DOI] [PubMed] [Google Scholar]
- 36.Duan M, Yao H, Cai Y, et al. HIV-1 Tat disrupts CX3CL1-CX3CR1 axis in microglia via the NF-κBYY1 pathway. Curr HIV Res. 2014;12:189–200. doi: 10.2174/1570162x12666140526123119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Griffin MD, Ryan AE, Alagesan S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol Cell Biol. 2012;91:40–51. doi: 10.1038/icb.2012.67. [DOI] [PubMed] [Google Scholar]
- 39.Coffey RJ, Jr, Kost LJ, Lyons RM, et al. Hepatic processing of transforming growth factor beta in the rat. Uptake, metabolism, and biliary excretion. J Clin Invest. 1987;80:750–757. doi: 10.1172/JCI113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melief J, Koning N, Schuurman KG, et al. Phenotyping primary human microglia: Tight regulation of LPS responsiveness. Glia. 2012;60:1506–1517. doi: 10.1002/glia.22370. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz M, Butovsky O, Brück W, et al. Microglial phenotype: Is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.