This review summarize the studies deciphering the mechanisms that govern osteoblast differentiation in the context of in vitro formation of bone-like nodules, including morphologic and molecular events as well as cellular contributions to mineral nucleation, occurring during osteogenic differentiation of stem cells. Also highlighted are the limitations of current translational applications of stem cells and opportunities to use the bone-like nodule model for bone regenerative therapies.
Keywords: Bone-like nodule, Osteoblast, Differentiation, Stem cells, Extracellular matrix mineralization
Abstract
Harnessing the differentiation of stem cells into bone-forming cells represents an intriguing avenue for the creation of functional skeletal tissues. Therefore, a profound understanding of bone development and morphogenesis sheds light on the regenerative application of stem cells in orthopedics and dentistry. In this concise review, we summarize the studies deciphering the mechanisms that govern osteoblast differentiation in the context of in vitro formation of bone-like nodules, including morphologic and molecular events as well as cellular contributions to mineral nucleation, occurring during osteogenic differentiation of stem cells. This article also highlights the limitations of current translational applications of stem cells and opportunities to use the bone-like nodule model for bone regenerative therapies.
Significance
Harnessing the differentiation of stem cells into bone-forming cells represents an intriguing avenue for the creation of functional skeletal tissues. Therefore, a profound understanding of bone development and morphogenesis sheds light on the regenerative application of stem cells in orthopedics and dentistry. In this concise review, studies deciphering the mechanisms that govern osteoblast commitment and differentiation are summarized. This article highlights the limitations of current translational applications of stem cells and the opportunities to use the bone-like nodule model for bone regenerative therapies.
Introduction
Bone formation initiates from the mesenchymal condensation that prefigures each future skeletal element. These events have been described in vitro, where progenitors form three-dimensional structures usually called bone-like nodules. Bone-like nodules arise from colony-forming units-osteoprogenitor that proliferate, condense, and differentiate into osteoblasts (OBs) [1]. Once OBs are fully differentiated, they have to fulfill a function to mineralize the deposited extracellular matrix (ECM). In the stem cell and regenerative medicine field, OB differentiation has been extensively studied, with conflicting results that can be related to inadequate OB differentiation. Most differentiated cells produce only ECM and form spontaneous dystrophic minerals, as a result of precipitation of phosphates and calcium ions in culture media [2, 3].
OB commitment and ECM mineralization must be evaluated through the combination of morphologic and phenotypic criteria and suitable mineral analyses. A better understanding of the successive steps and molecular mechanisms involved in osteoblastic differentiation appears to be crucial to optimize bone regenerative therapies. This concise review focuses exclusively on bone-like nodules to highlight cellular and molecular events leading to successful OB differentiation.
Bone-Like Nodule Morphology and Ultrastructural Features
OB commitment comprises a great diversity of cellular morphologies, including fibroblastic, polygonal, cuboidal, and stellar shapes, which characterize mesenchymal stem cells (MSCs), preosteoblasts, OBs, and osteocytes, respectively [4]. The successive steps involved in the formation of bone-like nodules include ∼6-day fibroblastic cells, ∼9-day cuboidal cells, and >10-day refringent material visible to the naked eye [5]. The initial cell density of bone-like nodules varies from 5 to 35 cells per nodule. Its size and thickness increase during the course of differentiation but not indefinitely [5]. Twenty-day-old nodules comprise approximately 100 cells per nodule, with a thickness of 70–100 µm. Ultrastructural investigations of >20-day-old bone-like nodules showed woven bone architecture [1, 6].
Cells
Perpendicular sections of nodules show a continuous layer of polarized cuboidal cells connected by gap junctions at the central dome region. Their cytoplasm contains abundant rough endoplasmic reticulum, mitochondria, and well-developed Golgi. Of the total cuboidal cells, 5%–10% have a vacuole-rich cytoplasm and numerous blebs, microvilli, and other membrane protrusions at their upper edge [7]. Stellate-shaped cells and some apoptotic bodies have been observed in the inner part of the nodule. Stellate cells were completely surrounded by dense fibers and vesicles and displayed 70% reduction of cell body volume compared with cuboidal cells. Stellate cells display a reduction of organelles but prominent membrane extensions through ECM, which can form gap junctions with neighboring cells [6].
ECM
Nodules showed fibers with the very well-defined striation signature of type-I collagen (COL-I) [6, 8]. Among randomly distributed collagen fibrils, numerous discrete structures called matrix vesicles (200–300 µm in diameter), as well as feathery and wispy needle-like crystals associated with the collagen fibrils, have been observed [6, 9]. In situ time-course studies followed by Raman microspectroscopy showed that mineralization within bone-like nodules occurred at 15 and 22 days [7]. Mineral analyses highlighted two mineral phases: (a) amorphous calcium phosphate accumulation in the intracellular and extracellular compartments and (b) carbonated hydroxyapatite crystal deposition within collagen fibrils [7, 10, 11].
Regulating Factors of Bone-Like Nodule Formation
MSCs and OBs isolated from trabecular bone are used in bone-regenerative medicine. Despite the relevance of human-derived cells for clinical applications, the formation of bone-like nodules from these cells may not occur systematically in culture, perhaps because of the large variability of patient-related factors, isolation sites, and techniques. It is generally accepted that osteogenic differentiation efficiencies of stem cells vary depending on the donor’s health status, gender, and age. Although the number and function of colony-forming units-osteoprogenitor are largely preserved with age, mitogenic responsiveness and colony formation are reduced with age [12]. In addition, MSCs obtained from female patients show lower colony-formation numbers compared with those from male patients [13]. It has been reported that estrogen can modulate osteoblastic differentiation via estrogen receptor-α and -β [14], which might contribute to the gender-dependent differentiation efficiency.
MSCs obtained from the femur had a greater potential for osteogenic differentiation than those obtained from the iliac crest. The differential daily mechanical load on the bones from which the cells are isolated was reported to contribute to the improved osteogenic differentiation of bone marrow-derived MSCs [15]. Surprisingly, studies describing bone-like nodules have used human primary fetal bone cells isolated from calvaria, femur, and iliac woven bone in spite of their different embryologic origin (Table 1). Human fetal cells showed a stronger osteogenic potential than adult cells [16, 17]. The absence of bone-like nodules has been attributed to heterogeneity in the osteogenic response of adult MSCs and the presence of a heterogeneous population with phenotypic differences in adult OBs. In view of the ethical concerns of using human fetal tissues, few studies have tried to use osteoprogenitor immortalized line cells such as 1.19 (hFOB) [18] and h-MSC-TERT [19]. Although this accomplishes the goal of creating immortal cell lines, several reports of malignant transformation using these genetically engineered MSC cell lines indicate the limitation of using these osteoprogenitor cells as in vitro models of normal stem cell differentiation.
Table 1.
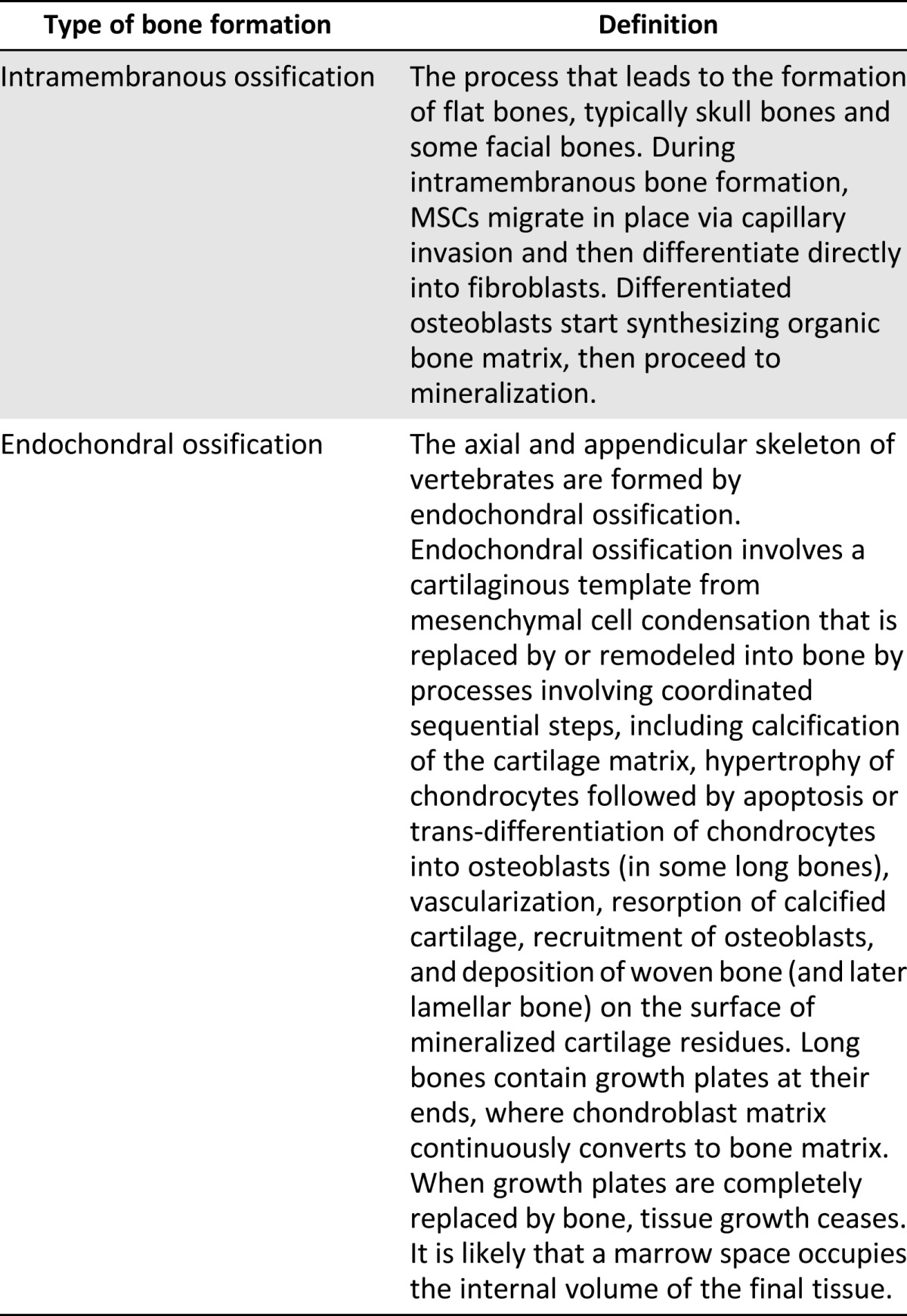
Cellular condensations and the initiation of osteogenesis: bone formation by two distinct modes of ossification, depending on origin
In addition to human cell models, cells isolated from other species have been proposed as an alternative in vitro research model. Using murine fetal progenitors, studies demonstrated that osteogenic potential could also be affected by harvesting protocols. Progenitors released from enzymatic digestion showed an enhancement of OB differentiation through the formation of bone-like nodules compared with explant outgrowth [11, 20]. These results could be explained by the heterogeneity of cell populations from which outgrowth occurs in animal as well as human bone tissue explants [12].
Bone-like nodule assays, used as quantitative function assays for the presence of bone progenitors, showed the existence of at least two populations of progenitors in terms of their responses to stimuli. One population appears to be capable of constitutively differentiating in vitro when cultured in standard osteogenic culture medium (β-glycerophosphate and ascorbic acid), whereas the other population commits into OBs only in the presence of additional inductive stimuli such as dexamethasone and bone morphogenetic protein-2 (BMP-2). Finally, in addition to biochemical factors, the formation of bone-like nodules is influenced by the culture substrate, including its topography and nature [21]. Additional information on other regulatory factors, including inflammation, angiogenesis, extracellular forces, and the application of a coculture system to elucidate the effects of environmental factors on stem cells for bone regeneration, have been intensively reviewed by others [22–24].
From MSCs to OBs: Multistep Molecular Events
Expression of Osteoblast Markers
Signals triggering MSC commitment into progenitors are mediated by the expression and activation of cell-specific transcription factor genes, including Runt-related transcription factor-2 (Runx-2) and Osterix, which are up-regulated during the transition from progenitors to osteocytes [25]. Although the role of Runx-2 protein during OB commitment has been extensively studied, its subcellular localization has not been highlighted during the kinetic formation of bone-like nodules.
Cell proliferation is a fundamental requirement for development of tissues. At the early stage of nodule formation, up-regulation of c-myc and c-fos (known as cell cycle and cell proliferation genes as well as histone genes), confirms high cell proliferation [26]. DNA assays showed that each nodule arises from a single progenitor. Concomitant with mitosis, the gene expression of ECM including procollagen, fibronectin, BMPs (2, 4, and 6), transforming growth factor-β1, and β1-integrin and cytoskeleton proteins, are also up-regulated [27]. The expression of bone-specific ECM genes such as osteonectin (ON) and bone sialoprotein (BSPII), two COL-I binding proteins involved in ECM remodeling, also increased at the proliferative stage [28]. Immunostaining of 5-day-old bone progenitors showed an intense ON signal at the cytoplasm and a fair BSP signal at the Golgi region [29].
The multilayered cells downregulate cell cycle genes and translocate cyclin E to the nucleus and cyclin B to the cytoplasm [26, 30]. These nonproliferative cells start to up-regulate the genes of ECM-related noncollagenous proteins (such as osteopontin [OPN] and BSPII), remodeling matrix-related protein (such as matrix metalloproteinases [MMPs] and their tissue inhibitors [TIMPs]), genes of the specific enzymes (such as alkaline phosphatase [ALP] and nucleoside diphosphate [NDP] kinase), and genes of the skeletal regulatory molecules (such as parathyroid hormone related-protein and its receptor [PTHrP/PTH1R], fibroblast growth factor-receptor-1 [FGF-R1], and platelet-derived growth factor-receptor-α [PDGFRα]). The temporal expression of these genes as well as their abundance remain controversial, however. Some studies reported, first, an overexpression of both of ALP and OPN [26, 31], whereas others showed a significant up-regulation of FGF-R1, PTH1R, and PDGF-Rα before that of ALP [32, 33]. The function of OPN allows the control of interaction between cells and ECM, particularly in light of its RGD sequence (Arg-Gly-Asp) mediating cell attachment. The expression of ALP and NDP during ECM remodeling suggests that their corresponding proteins are involved in preparation of the ECM for mineralization [34, 35]. General structural and biological properties of noncollagenous proteins secreted by mature OBs have been reviewed by Clarke [36]. Eleven-day-old nodules showed a colocalization of labeled PTH1R and ALP on the cell surface of cuboidal cells. Some fibroblastic internodular cells fairly express a membrane-localized ALP but are negative for PTH1R.
Cell multilayering and ECM accretion involve changes in cell adhesion and may require reorganization of the ECM. Although the secretion of inactive and active gelatinases (MMP-2- and -9) as well as collagenase (MMP-13) has been described in bone-like nodules, no studies have documented the timing of MMP-2, -9, and -13 mRNA expression, especially during the proliferative stage. In addition to the transmembrane gelatinase (MMP-14), weak expression of TIMP-1 and TIMP-2 was also detected in ALP-positive cells [31].
The late stage of nodule formation corresponds to matrix mineralization, which is actively regulated by protein and physicochemical mechanisms. Concomitant with the accumulation of minerals, the expression of several bone-related genes (such as OPN and ON) peaks at maximal levels at the onset of mineralization (16 to 20 days). OPN has several putative calcium binding sites that are known to be important for ECM mineralization [36]. In contrast to the biphasic expression pattern for OPN, osteocalcin (OCN) occurs only at the OB stage, subsequent to the initiation of ALP activity and mineral deposition [31]. OCN is therefore commonly considered a hallmark of mature OBs. The exact role of OCN in bone is not yet completely understood but prominently involves the regulation of bone mineralization and turnover [36]. In addition to MMP-2 and -9, the highest amount of MMP-14 and increased expression of two bone proteoglycans, biglycan and decorin, were found during mineralization [31]. Although OCN decreases in 35-day-old nodules, osteocyte markers including sclerostin and dentine matrix protein 1 were up-regulated in the heavily mineralized nodules and are involved in the inhibition of OB differentiation (sclerostin) and phosphate homeostasis and ECM mineralization (dentine matrix protein 1).
Cell-ECM and Cell-Cell Interactions
Proliferating progenitors do not form a contact-inhibited monolayer but have the ability to form multilayered 3D structures in which each cell interacts specifically with both neighboring cells and its own ECM. The ECM plays a key regulatory role in the cessation of proliferation and initiation of signaling cascades through cell-ECM and cell-cell interactions to activate the expression of OB-related genes and mediate the changes in cell shapes. The changes in cell morphology during OB commitment are associated with ECM-integrin-cytoskeleton interactions. The unique combinations of integrin subunits determine the type of ECM molecules recognized by cells, the first step of ultimate cell fate determination, and therefore play an important role in matrix mineralization [37, 38]. Proteins containing RGD sequences, such as COL-I and OPN, are up-regulated earlier than other ECM proteins (such as OCN), confirming their role in mediating cell attachment via integrins. Similarity in the distribution pattern of integrins between calvaria tissue and bone-like nodules has been established [39]. The temporal distribution pattern of α5/β1 showed few differences among proliferation, remodeling, and mineralization stages. But interfering α5/β1-ECM interactions suppress the osteogenic potential of progenitors [39]. The expression of α3/β1 and α8/β1 was maintained at a lower level after day 10 of culture, suggesting a possible downregulation of these receptors over time, and their disturbance blocked the up-regulation of ALP and OCN. However, cells retain the capacity to continue the osteogenic potential once the perturbation is suppressed [39]. α2/β1-collagen binding requires the maintenance of COL-I fibrillar conformation and the transcription of OCN, suggesting its involvement at the onset of mineralization [40]. The formation of focal adhesion complex, subsequent to integrin/ECM binding, ensures polymerization of actin. The functional relationship between the progression of cell morphology during OB differentiation and dynamic reorganization of the cytoskeletal network has been demonstrated [38]. Fibroblastic cells display thin actin fibers, running in parallel to the longitudinal cell axis; in contrast, cuboidal cells exhibit thick, nonaligned actin fibers at the outermost periphery. Despite these morphologic evolutions, the microtubule-associated network remains unchanged in the cytoplasm. Additionally, changes in cytoskeleton tension have a direct effect on cell morphology and an indirect effect on mechanotransduction pathways [41]. The modification of material surface properties via changing of microtexture and nano-roughness induces the rearrangement of vinculin around the cytoplasm periphery and consequently enhances cell adherence. Changes in cell spreading and cytoskeletal tension will alter nucleus shape, chromosomal arrangement, and gene transcription. Hence, changes in cell mechanics directly influence cell gene expression profiles, leading to bone-like nodule formation [41, 42].
Cell-cell interactions and functional gap junctions were highlighted through the identification of connexin-43 and -45 [43]. Cell morphologic changes proceed with an increase in the formation of gap junctions through microtubule actions, followed by the rapid assembly of preexisting gap junction protein on the membrane into an organized structure [44]. Disturbance of gap junctions prevents calcium transport, OB maturation, and matrix mineralization. These cells failed to express ALP, BSP, and OCN, indicating the important role of gap junctions in osteogenic function [45].
Minerals During the Course of Bone-Like Nodule Formation
Stevens and colleagues highlighted step-by-step processes during bone-like nodule formation that involve both intra- and extracellular compartments in mineralization [10]. Deciphering the roles of these compartments in the mineralization of ECM could provide important benchmarks for the stages of OB differentiation.
Intracellular Mineral Accumulation
Intracellular mineral analysis indicates an accumulation of nano-“calcified” granules into mitochondria and cytoplasm [10]. It is well documented that calcium phosphate could accumulate in mitochondria under the control of adenosine triphosphate (ATP). Proliferating progenitors predominantly use glucose through aerobic glycolysis, whereas the metabolic pathway shifts from glycolysis to oxidative phosphorylation upon matrix maturation and mineralization. ATP generated from oxidative phosphorylation thus promotes the accumulation of minerals at the onset of matrix maturation [46]. In other hand, it has been reported that active OBs sequester calcium and phosphate ions in their cytoplasm before mineralization to facilitate the nucleation process [47]. Unlike the cytoplasm of nodules in chickens containing free crystals [48], the mammalian model showed mineral-containing vesicles. Recently, Boonrungsiman et al. showed that mineral-containing mitochondria enter in a membrane vesicle fusion process by which their mineral content is discharged [10]. Although the origin of these vesicles is controversial, some convincing reports demonstrated that they process through the Golgi and into ECM via exocytosis [48].
Extracellular Mineral Accumulation
Matrix vesicles, described above, have been identified within bone-like nodules before mineralization and could participate in the initiation of ECM mineralization [10]. Indeed, they harbor the necessary machinery in the form of enzymes, annexin-calcium binding proteins, and phospholipids to initiate mineral nucleation. Matrix vesicle composition and biochemistry are outside of the scope of this review; further information can be found in a recent report [49]. Four hypotheses regarding the biogenesis and release of matrix vesicles have been proposed: (a) they are formed and budded through a series of cellular processes, (b) they are generated from existing structures such as microvilli, (c) they result from cellular degeneration and apoptosis, or (d) they are secreted as subunits and assembled outside of cells. Data from the literature thus far collectively support the first hypothesis, which suggests that polarized OBs secrete matrix vesicles into ECM [10].
Critical Comments and Perspectives
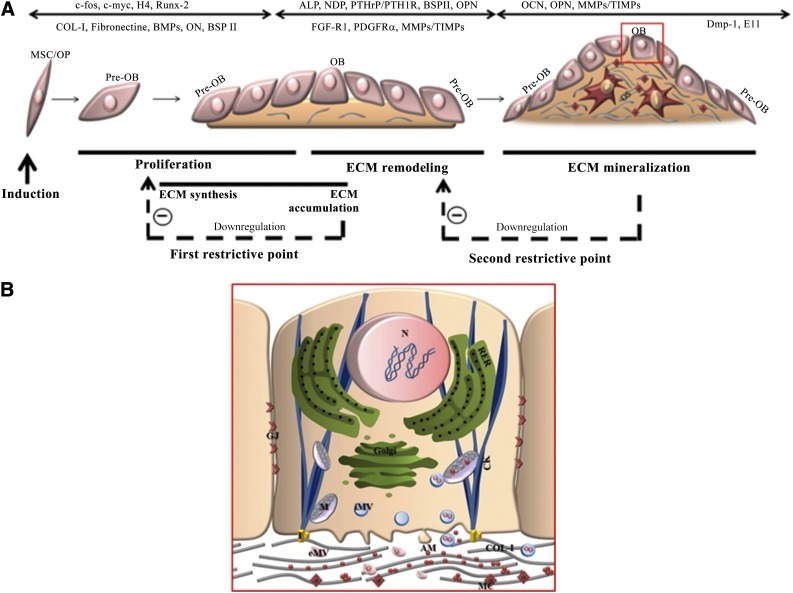
Bone regeneration represents a well-orchestrated series of biological events of stem cell recruitment, proliferation, and differentiation that can be recapitulated in vitro through bone-like nodule formation [1]. Studies of bone-like nodules have provided a detailed understanding of the differentiation-maturation events that follow a temporal sequence of cell proliferation, osteogenic differentiation, and ECM mineralization (Fig. 1). Two restriction points from which cells can progress but cannot pass without further instructive signals have been identified (Fig. 1A). Transition from cell proliferation to differentiation is mediated by crosstalk between regulatory mechanisms controlling the cell cycle and phenotypic commitment. The accumulation of COL-I promotes exit from the cell cycle and induces OB commitment, whereas ascorbic acid deprivation, while avoiding extracellular collagen accumulation, promotes cellular growth. The accumulation of COL-I also promotes the expression of genes that render the matrix competent for mineralization. Absence of extracellular COL-I prevents the increase of ALP and further ECM mineralization [50], whereas a COL-I substrate culture model showed an acceleration of OB differentiation [51]. Of note, however, the early expression of ALP by bone progenitors impairs bone-like nodule formation [52]. The sequential expression of genes encoding the OB phenotype in culture follows the temporal-spatial gene expression pattern observed in neonatal bones as determined by in situ hybridization [53] and during fetal calvaria development in vivo [54], supporting the biological relevance of using the bone-like nodule as a model to mimic intramembranous ossification. This hypothesis has been argued by recent studies from Gentleman et al. [55] that demonstrated a transitory expression of cartilage markers, which disappeared at the onset of mineralization and indicated mimicking of endochondral ossification (Table 1). Additionally, the report by Morriss-Kay and Wilkie is mostly consistent with the findings from in vivo studies describing cellular and molecular events in developing membranous and endochondral bone formation in mammalian animals [56].
Figure 1.
Bone-like nodule formation. (A): Schematic overview of developmental sequences involving morphologic progressions and selective expression of genes that regulate cell proliferation and promote osteoprogenitor commitment and bone-like nodule formation. These processes were schematically illustrated by three major stages divided by two restrictive points. Downregulation of proliferation/ECM accumulation and ECM remodeling/mineral deposition corresponds, respectively, to the first and second restrictive points. Details about OB-mediated ECM mineralization (red square) are provided in (B). (B): The proposed OB-dependent mechanism of calcium phosphate nucleation includes both extracellular and intracellular matrix vesicles. Abbreviations: AM, amorphous mineral; CK, cytoskeleton; COL-I, type-1 collagen; ECM, extracellular matrix; eMV, extracellular matrix vesicle; GJ, gap junction; I, integrin; iMV, intracellular matrix vesicle; M, mitochondria; MC, mineral crystal; MSC, mesenchymal stem cell; N, nucleus; OB, osteoblast; OP, osteoprogenitor cell; RER, rough endoplasmic reticulum.
Most in vitro studies highlight the presence of mineral crystals in the ECM and demonstrate that matrix mineralization proceeds in a manner similar to what has been described in woven bone in vivo [8, 9]. However, the involvement of OBs in producing minerals has been debated for a long time and still remains unclear. Although some basic concepts of biomineralization are well established, several conflicting opinions persist in the literature involving a cell-independent [57] or cell-dependent [58] process. Stem cell and bone-like nodule models provide new insights into the contribution of cells to the initiation and progression of the nucleation event. A step-by-step process involves the accumulation of amorphous calcium phosphate in the mitochondria and intra- and the extracellular vesicles, and carbonated hydroxyapatite crystals localize to collagen fibrils (Fig. 1B) [10]. The amorphous phase is more abundant in the newly formed nodules and evolves toward a more crystalline phase, with the crystalline/amorphous ratio doubling within approximately 4 days after the initiation of mineralization. Analysis of the spatial distribution of the amorphous and crystalline phases of hydroxyapatite showed that the central parts of the nodules have a higher ratio of crystalline hydroxyapatite, whereas the amorphous phase is dominant at the edges of bone-like nodules [7]. These results are in agreement with the cellular characterization of bone-like nodules, which suggests that matrix mineralization starts in the central zone of the nodule and extends to the periphery [5].
An appropriate in vitro experimental design, including the selection of a suitable cell model, is of the utmost importance to better understand the physiologic, pathologic, and regenerative processes involved in bone formation. Twenty-five years ago, the identification of bone-like nodules provided knowledge about the sequential events leading to osteoblast differentiation. More recently, the in vitro bone-like nodule model has delivered new insights into the contributions of osteoblasts in the initiation and progression of mineralization. Growth factors, the extracellular matrix/scaffold, fluid flow, and vascularization provide the foundation for successful bone regeneration. Several studies have explored the use of MSCs encapsulated in osteoinductive scaffolds or morphogenic biomaterials to enhance the natural healing process of bone and cartilage in vivo [59]. The overall results suggest that these multipotent cells seem to be able to either differentiate within the scaffolds or secrete factors attracting neighboring autologous progenitors. Moreover, studies of the interaction between MSCs and immune cells indicate the implications of the immune system in fracture repair and the use of MSCs in enhancement of fracture healing [60, 61]. More importantly, it has been reported that ectopic bone formation in the nude mouse can be achieved upon implantation of bone-like nodules formed via in vitro culture of MSCs or osteoblastic cells in bioreactors [62, 63], which proves the concept that bone-like nodules can support in vivo osteogenic differentiation and highlights its translational potentials. It is expected that knowledge of stem cell differentiation into the osteoblastic lineage through bone-like nodule formation can be translated into novel bone tissue engineering tools to enhance bone regenerative therapies.
Author Contributions
S.M.A.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.C.G.: administrative support, final approval of manuscript; D.L.-M.: administrative support, final approval of manuscript; Y.W.: conception/design, manuscript writing, final approval of manuscript; H.K.: conception/design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Aubin JE, Heersche JNM. Osteoprogenitor cell differentiation to mature bone-forming osteoblasts. Drug Dev Res. 2000;49:206–215. [Google Scholar]
- 2.Boyan BD, Schwartz Z, Boskey AL. The importance of mineral in bone and mineral research. Bone. 2000;27:341–342. doi: 10.1016/s8756-3282(00)00347-1. [DOI] [PubMed] [Google Scholar]
- 3.Grayson WL, Bunnell BA, Martin E, et al. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11:140–150. doi: 10.1038/nrendo.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackie EJ. Osteoblasts: Novel roles in orchestration of skeletal architecture. Int J Biochem Cell Biol. 2003;35:1301–1305. doi: 10.1016/s1357-2725(03)00107-9. [DOI] [PubMed] [Google Scholar]
- 5.Malaval L, Liu F, Roche P, et al. Kinetics of osteoprogenitor proliferation and osteoblast differentiation in vitro. J Cell Biochem. 1999;74:616–627. [PubMed] [Google Scholar]
- 6.Bhargava U, Bar-Lev M, Bellows CG, et al. Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone. 1988;9:155–163. doi: 10.1016/8756-3282(88)90005-1. [DOI] [PubMed] [Google Scholar]
- 7.Ghita A, Pascut FC, Sottile V, et al. Monitoring the mineralisation of bone nodules in vitro by space- and time-resolved Raman micro-spectroscopy. Analyst (Lond) 2014;139:55–58. doi: 10.1039/c3an01716h. [DOI] [PubMed] [Google Scholar]
- 8.Ecarot-Charrier B, Shepard N, Charette G, et al. Mineralization in osteoblast cultures: A light and electron microscopic study. Bone. 1988;9:147–154. doi: 10.1016/8756-3282(88)90004-x. [DOI] [PubMed] [Google Scholar]
- 9.Nefussi JR, Boy-Lefevre ML, Boulekbache H, et al. Mineralization in vitro of matrix formed by osteoblasts isolated by collagenase digestion. Differentiation. 1985;29:160–168. doi: 10.1111/j.1432-0436.1985.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 10.Boonrungsiman S, Gentleman E, Carzaniga R, et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci USA. 2012;109:14170–14175. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellows CG, Aubin JE, Heersche JN, et al. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986;38:143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- 12.Erdmann J, Kögler C, Diel I, et al. Age-associated changes in the stimulatory effect of transforming growth factor beta on human osteogenic colony formation. Mech Ageing Dev. 1999;110:73–85. doi: 10.1016/s0047-6374(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Liao L, Wang S, et al. Cell therapy with autologous mesenchymal stem cells: How the disease process impacts clinical considerations. Cytotherapy. 2013;15:893–904. doi: 10.1016/j.jcyt.2013.01.218. [DOI] [PubMed] [Google Scholar]
- 14.Hong L, Colpan A, Peptan IA. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng. 2006;12:2747–2753. doi: 10.1089/ten.2006.12.2747. [DOI] [PubMed] [Google Scholar]
- 15.Henrich D, Nau C, Kraft SB, et al. Effect of the harvest procedure and tissue site on the osteogenic function of and gene expression in human mesenchymal stem cells. Int J Mol Med. 2016;37:976–988. doi: 10.3892/ijmm.2016.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montjovent MO, Burri N, Mark S, et al. Fetal bone cells for tissue engineering. Bone. 2004;35:1323–1333. doi: 10.1016/j.bone.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Chang PL, Blair HC, Zhao X, et al. Comparison of fetal and adult marrow stromal cells in osteogenesis with and without glucocorticoids. Connect Tissue Res. 2006;47:67–76. doi: 10.1080/03008200600584074. [DOI] [PubMed] [Google Scholar]
- 18.Yen ML, Chien CC, Chiu IM, et al. Multilineage differentiation and characterization of the human fetal osteoblastic 1.19 cell line: A possible in vitro model of human mesenchymal progenitors. Stem Cells. 2007;25:125–131. doi: 10.1634/stemcells.2006-0295. [DOI] [PubMed] [Google Scholar]
- 19.Foster LJ, Zeemann PA, Li C, et al. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005;23:1367–1377. doi: 10.1634/stemcells.2004-0372. [DOI] [PubMed] [Google Scholar]
- 20.Declercq H, Van den Vreken N, De Maeyer E, et al. Isolation, proliferation and differentiation of osteoblastic cells to study cell/biomaterial interactions: Comparison of different isolation techniques and source. Biomaterials. 2004;25:757–768. doi: 10.1016/s0142-9612(03)00580-5. [DOI] [PubMed] [Google Scholar]
- 21.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacQueen L, Sun Y, Simmons CA. Mesenchymal stem cell mechanobiology and emerging experimental platforms. J R Soc Interface. 2013;10:20130179. doi: 10.1098/rsif.2013.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schipani E, Maes C, Carmeliet G, et al. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janardhanan S, Wang MO, Fisher JP. Coculture strategies in bone tissue engineering: The impact of culture conditions on pluripotent stem cell populations. Tissue Eng Part B Rev. 2012;18:312–321. doi: 10.1089/ten.teb.2011.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lian JB, Javed A, Zaidi SK, et al. Regulatory controls for osteoblast growth and differentiation: Role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- 26.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto N, Furuya K, Hanada K. Progressive development of the osteoblast phenotype during differentiation of osteoprogenitor cells derived from fetal rat calvaria: Model for in vitro bone formation. Biol Pharm Bull. 2002;25:509–515. doi: 10.1248/bpb.25.509. [DOI] [PubMed] [Google Scholar]
- 28.Malaval L, Modrowski D, Gupta AK, et al. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994;158:555–572. doi: 10.1002/jcp.1041580322. [DOI] [PubMed] [Google Scholar]
- 29.Whitson SW, Harrison W, Dunlap MK, et al. Fetal bovine bone cells synthesize bone-specific matrix proteins. J Cell Biol. 1984;99:607–614. doi: 10.1083/jcb.99.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith E, Frenkel B, Schlegel R, et al. Expression of cell cycle regulatory factors in differentiating osteoblasts: Postproliferative up-regulation of cyclins B and E. Cancer Res. 1995;55:5019–5024. [PubMed] [Google Scholar]
- 31.Purpura KA, Zandstra PW, Aubin JE. Fluorescence activated cell sorting reveals heterogeneous and cell non-autonomous osteoprogenitor differentiation in fetal rat calvaria cell populations. J Cell Biochem. 2003;90:109–120. doi: 10.1002/jcb.10596. [DOI] [PubMed] [Google Scholar]
- 32.Kondo H, Ohyama T, Ohya K, et al. Temporal changes of mRNA expression of matrix proteins and parathyroid hormone and parathyroid hormone-related protein (PTH/PTHrP) receptor in bone development. J Bone Miner Res. 1997;12:2089–2097. doi: 10.1359/jbmr.1997.12.12.2089. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Malaval L, Aubin JE. Global amplification polymerase chain reaction reveals novel transitional stages during osteoprogenitor differentiation. J Cell Sci. 2003;116:1787–1796. doi: 10.1242/jcs.00376. [DOI] [PubMed] [Google Scholar]
- 34.Yasutomo Y, Ishikawa N, Nagata N, Kimura N. Nucleoside diphosphate kinase in fetal rat bone tissue: Increased expression in osteoblasts and localization in the matrix vesicle fraction. J Bone Miner Metab. 1998;16:215–226. [Google Scholar]
- 35.Bellows CG, Aubin JE, Heersche JN. Initiation and progression of mineralization of bone nodules formed in vitro: The role of alkaline phosphatase and organic phosphate. Bone Miner. 1991;14:27–40. doi: 10.1016/0169-6009(91)90100-e. [DOI] [PubMed] [Google Scholar]
- 36.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong D, Chen HX, Yu HQ, et al. Morphological and proteomic analysis of early stage of osteoblast differentiation in osteoblastic progenitor cells. Exp Cell Res. 2010;316:2291–2300. doi: 10.1016/j.yexcr.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 2007;53:219–228. doi: 10.1097/MAT.0b013e31802deb2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J Cell Sci. 1997;110:2187–2196. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 40.Xiao G, Wang D, Benson MD, et al. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 41.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13:558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 42.Sisti KE, de Andrés MC, Johnston D, et al. Skeletal stem cell and bone implant interactions are enhanced by LASER titanium modification. Biochem Biophys Res Commun. 2016;473:719–725. doi: 10.1016/j.bbrc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Batra N, Kar R, Jiang JX. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochem Biophys Acta. 2012;1818:1909–1918. doi: 10.1016/j.bbamem.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiba H, Sawada N, Oyamada M, et al. Relationship between the expression of the gap junction protein and osteoblast phenotype in a human osteoblastic cell line during cell proliferation. Cell Struct Funct. 1993;18:419–426. doi: 10.1247/csf.18.419. [DOI] [PubMed] [Google Scholar]
- 45.Schiller PC, D’Ippolito G, Balkan W, et al. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone. 2001;28:362–369. doi: 10.1016/s8756-3282(00)00458-0. [DOI] [PubMed] [Google Scholar]
- 46.Johnson K, Jung A, Murphy A, et al. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000;43:1560–1570. doi: 10.1002/1529-0131(200007)43:7<1560::AID-ANR21>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 47.Bordat C, Guerquin-Kern JL, Lieberherr M, et al. Direct visualization of intracellular calcium in rat osteoblasts by energy-filtering transmission electron microscopy. Histochem Cell Biol. 2004;121:31–38. doi: 10.1007/s00418-003-0601-9. [DOI] [PubMed] [Google Scholar]
- 48.Rohde M, Mayer H. Exocytotic process as a novel model for mineralization by osteoblasts in vitro and in vivo determined by electron microscopic analysis. Calcif Tissue Int. 2007;80:323–336. doi: 10.1007/s00223-007-9013-5. [DOI] [PubMed] [Google Scholar]
- 49.Wuthier RE, Lipscomb GF. Matrix vesicles: Structure, composition, formation and function in calcification. Front Biosci (Landmark Ed) 2011;16:2812–2902. doi: 10.2741/3887. [DOI] [PubMed] [Google Scholar]
- 50.Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 51.Kihara T, Hirose M, Oshima A, et al. Exogenous type I collagen facilitates osteogenic differentiation and acts as a substrate for mineralization of rat marrow mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2006;341:1029–1035. doi: 10.1016/j.bbrc.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 52.Herbertson A, Aubin JE. Cell sorting enriches osteogenic populations in rat bone marrow stromal cell cultures. Bone. 1997;21:491–500. doi: 10.1016/s8756-3282(97)00197-x. [DOI] [PubMed] [Google Scholar]
- 53.Candeliere GA, Liu F, Aubin JE. Individual osteoblasts in the developing calvaria express different gene repertoires. Bone. 2001;28:351–361. doi: 10.1016/s8756-3282(01)00410-0. [DOI] [PubMed] [Google Scholar]
- 54.Yoon K, Buenaga R, Rodan GA. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987;148:1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- 55.Gentleman E, Swain RJ, Evans ND, et al. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat Mater. 2009;8:763–770. doi: 10.1038/nmat2505. [DOI] [PubMed] [Google Scholar]
- 56.Morriss-Kay GM, Wilkie AOM. Growth of the normal skull vault and its alteration in craniosynostosis: Insights from human genetics and experimental studies. J Anat. 2005;207:637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozawa H, Hoshi K, Amizuka N. Current concepts of bone biomineralization. J Oral Biosci. 2008;50:1–14. [Google Scholar]
- 58.Mahamid J, Sharir A, Gur D, et al. Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: A cryo-electron microscopy study. J Struct Biol. 2011;174:527–535. doi: 10.1016/j.jsb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 59.Black CRM, Goriainov V, Gibbs D, et al. Bone tissue engineering. Curr Mol Biol Rep. 2015;1:132–140. doi: 10.1007/s40610-015-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 61.Kovach TK, Dighe AS, Lobo PI, et al. Interactions between MSCs and immune cells: Implications for bone healing. J Immunol Res. 2015;2015:1–17. doi: 10.1155/2015/752510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang ZY, Teoh SH, Teo EY, et al. A comparison of bioreactors for culture of fetal mesenchymal stem cells for bone tissue engineering. Biomaterials. 2010;31:8684–8695. doi: 10.1016/j.biomaterials.2010.07.097. [DOI] [PubMed] [Google Scholar]
- 63.Janssen FW, Oostra J, Oorschot A, et al. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: In vivo bone formation showing proof of concept. Biomaterials. 2006;27:315–323. doi: 10.1016/j.biomaterials.2005.07.044. [DOI] [PubMed] [Google Scholar]