This article describes a simple tool to facilitate development of international standards that may systematize cellular products across boundaries and accelerate the delivery of safe and effective adipose stem cell (ASC)-based tools. This tool can discriminate between fibroblasts and ASCs and can be rapidly implemented before the release of ASC-based therapy.
Keywords: Adipose stem cells, Fibroblasts, Purity, Cell therapy, Tool
Abstract
It is important to standardize methods to quantify the purity of adipose tissue-derived cells for regenerative medicine. We developed a simple and robust tool to discriminate fibroblasts and adipose stem cells (ASCs) by testing release of specific growth factors. ASCs and dermal fibroblasts (DFs) were isolated from human donors (n = 8). At passage 4, cultures were prepared with progressive ASC/DF ratios of 100%/0%, 75%/25%, 50%/50%, 25%/75%, and 0%/100% for each donor and incubated in hypoxic chambers at 0.1% and 5% O2 and hyperglycemia at 1.0 and 4.5 g/l. After incubation for 24 hours, cell survival, proliferation, and growth factor release (vascular endothelial growth factor [VEGF], hepatocyte growth factor [HGF], insulin-like growth factor 1 [IGF-1], stromal cell-derived factor 1α [SDF-1α], and basic fibroblast growth factor [bFGF]) were assessed for each condition. The proliferation and viability of ASCs and DFs were not impacted by the oxygen tension conditions. No significant difference in HGF, IGF-1, bFGF, and keratinocyte growth factor secretome was found across the various ASC/DF ratios. Interestingly, a negative relation for VEGF secretion was found when ASCs were contaminated by fibroblasts, especially when cells were exposed to 4.5 g/l glucose and 0.1% O2 (R = −0.521; p < .001). In contrast, secretion of SDF-1α was positively correlated with the fibroblast ratio, more prominently in low glucose and low oxygen tension (r = .657; p < .001). Above and beyond these previously unreported metabolic features, these results (a) allow us to discriminate fibroblasts and ASCs specifically and (b) allow new tools be developed for the rapid testing (a response within 24 hours) for the release of ASC-based therapies.
Significance
In order to provide direction to academia, industry, and regulatory authorities regarding purity assessment for adipose tissue-derived cells, this report describes a simple tool to facilitate development of international standards based on reproducible parameters and endpoints that may systematize cellular products across boundaries and accelerate the delivery of safe and effective adipose stem cell (ASC)-based tools to the medical community and the patients it serves. This tool (a) can discriminate specifically fibroblasts and ASCs and (b) can be rapidly implemented and performed before the release of the ASC-based therapy (a response within 24 hours).
Introduction
Adipose tissue is a rich and very convenient source of mesenchymal-lineage stem cells for regenerative medicine [1–5]. However, the development of cellular therapies with the greatest clinical potential is fraught with safety concerns relating to product purity [6–8]. It is therefore necessary to find methods and refine reproducible assays to quantify the purity of adipose tissue-derived cells (adipose stem cells [ASCs]) with a view to advance this field of research with shared standard operating procedures [6–8]. We developed a simple, rapid, and robust tool to discriminate fibroblasts and adipose stem cells by testing specific growth factors’ release.
Materials and Methods
This study was performed according to the guidelines of the Belgian Ministry of Health. All procedures were approved by the Ethical Committee of the Medical Faculty (Université Catholique de Louvain) for tissue procurement (B40320108280). All materials were obtained from Lonza (Verviers, Switzerland, http://www.lonza.com), Sigma-Aldrich (St. Louis, MO, http://www.sigmaaldrich.com), or Thermo Fisher Scientific Life Sciences (Oakwood Village, OH, https://www.thermofisher.com), unless otherwise noted.
Harvesting of adipose tissue (mean: 7.4 g) was performed in eight nondiabetic patients, who were undergoing elective plastic surgery, after informed consent and serologic screening, by lipoaspiration using the Coleman technique [9, 10]. Adipose tissue was digested with collagenase (in a water bath at 37°C for 60 minutes) to isolate adipose stem cells. ASCs were then suspended in a proliferation medium consisting of Dulbecco's modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, l-glutamine (2 mM), and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, and 1 µl/ml amphotericin B).
Skin biopsies (mean: 1.5 cm2) were procured from the same nondiabetic patients (n = 8). Dermal fibroblasts (DFs) were isolated by explant technique from de-epidermized dermal biopsies, cut in 4-mm2 fragments, and placed in plastic wells. The initial passage of the primary cells is referred to as passage 0. Dermal pieces were removed from the culture dish when adherent cells were visible on the plastic surface surrounding tissue fragments. Cells were maintained in proliferation medium (changed two times per week) up to passage 4, after sequential trypsinizations. At passage 4, ASCs and DFs were characterized for standard cell surface markers (anti-CD44, -CD45, -CD73, -CD90, -CD105, -Stro1, -CD106, -CD146, -CD166, -CD19, -CD31, -CD11b, -CD79α, -CD13, -human leukocyte antigen [HLA]-DR, -CD14, and -CD34) by fluorescence-activated cell sorting (FACScan; BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) as previously described [10]. ASCs’ capacity of differentiation toward osteogenic lineage (Alizarin red and osteocalcin staining) was also tested at passage 4.
After passage 4 trypsinization, cells were counted, and five progressive dilutions were performed in ratios of 100%/0%, 75%/25%, 50%/50%, 25%/75%, and 0%/100% for ASCs/DFs, respectively, and seeded in 12-well culture plates in triplicate for incubation in hypoxic chambers (Modular Incubator Chamber MIC-101; Billups-Rothenberg, Del Mar, CA, http://www.brincubator.com) at 0.1% and 5% O2, corresponding to a highly hypoxic environment and tissue oxygen tension, respectively. Cells were exposed (for each dilution and oxygen tension) to normoglycemic (1.0 g/l) or hyperglycemic (4.5g/l) proliferation medium without fetal bovine serum (to avoid interferences in growth factor detection). After incubation for 24 hours in these conditions, cell culture supernatants were harvested individually and stored at −20°C for further growth factor quantification by enzyme-linked immunosorbent assay (vascular endothelial growth factor [VEGF], hepatocyte growth factor [HGF], insulin-like growth factor 1 [IGF-1], stromal cell-derived factor 1α [SDF-1α], and basic fibroblast growth factor [bFGF] by Quantikine ELISA kit; R&D Systems, Minneapolis, MN, USA, https://www.rndsystems.com). Cellular viability was assessed immediately after the hypoxic stress by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium solution assay (Promega, Leiden, the Netherlands, http://www.promega.com). Testing of hypoxic and glycemic conditions and growth factor quantifications was performed in triplicate and duplicate, respectively. Results are expressed in picograms per millimeter.
One-sample Kolmogorov test and Q-Q plots were used to assess the normal distribution of values. Statistically significant differences between groups (with normal distribution) were tested by paired t test and one-way analysis of variance with the Bonferroni post hoc test. Principal components analysis was used to extract the best linear combinations of variables, (i.e., to identify a set of variables loading on the same component that could then be considered as a valid measure of ASC/DF purity). Criteria for determining the number of components were Kaiser criterion and screen plot. Internal consistency was tested with item-score correlations and Cronbach’s α. Statistical tests were performed with PASW 18 (SPSS; IBM, New York, NY, http://www.ibm.com); p < .05 was considered significant.
Results
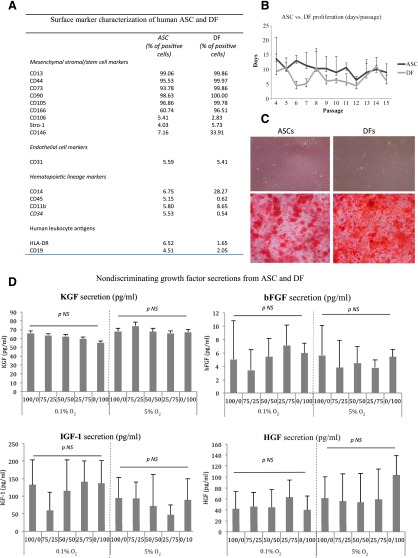
As expected and described by others [6–8], no distinguishing features were observed that consistently and unequivocally distinguished ex vivo culture-expanded ASCs from fibroblasts (Fig. 1A), including (a) culture-derivation methodology (the proliferation and viability of ASCs and DFs were not impacted by different oxygen tension until passage 15); (b) morphology (ASCs showed typical fibroblast-like morphology and did not display any particular morphological differences when compared with fibroblasts); (c) differentiation potential (osteogenic differentiation capacity was shown for both ASCs and DFs by Alizarin red staining and osteocalcin immunohistochemistry); and (d) cell-surface marker expression pattern (both ASCs and DFs share similar negative endothelial cell markers, hematopoietic markers, and HLA-DR, CD79α, and CD19). The majority of mesenchymal stem cell (MSC) markers, identified for bone marrow-MSCs (BM-MSCs), were expressed by both ASCs and DFs, except for CD106, Stro-1, and CD146. ASCs, as well as DFs, expressed a lower CD106 in comparison with BM-MSCs, as reported by Bourin et al. [7]. Stro-1 was also found with a lower expression for ASCs and DFs in comparison with BM-MSCs, as confirmed by Lv et al. [11]. However, in contrast to Lv et al., who postulated that CD146 may be the most appropriate “stemness” marker (because it is universally detected in the MSC population isolated from various tissues), we found that CD146 was not expressed by ASCs and DFs (culture up to passage 4) [11]. To date, it is not clear that specific markers of BM-MSCs can be applied to other sources of MSCs such as adipose stem cells. Moreover, it is not entirely clear that this exact in situ identity of MSCs can discriminate between MSCs and fibroblasts. Currently, questions have been raised about whether MSCs may derive from fibroblasts or pericytes [6, 8, 11]. Hence, our results are in contrast with Wagner et al. but support the finding of Lorenz et al. [12, 13].
Figure 1.
Similarities between ASCs and DFs. (A): Table shows the phenotype characterization of ASCs and DFs in culture. Fluorescence-activated cell sorting analysis was performed when all in vitro cultures were at passage 4. Data represent the percentage of positive cells for each marker analyzed on ASCs and DFs and are means. Variability of each marker tested among the different cell preparations was ≤10%. Note that some markers, such as CD146, CD166, and CD40, are more highly expressed in DFs when compared with ASCs. (B): ASCs were isolated from subcutaneous fat specimens obtained from eight patients (19–62 years old) after abdominal and breast surgery. ASCs and DFs were cultured by using Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Successful isolation and culture expansion of ASCs and DFs were obtained from all eight specimens processed. DF cell lines were maintained in vitro under the same culture conditions of ASCs up to passage 4. Cells were usually plated in T25 culture flasks at a density of 20,000 cells per cm2. Soon after seeding, ASCs showed typical fibroblast-like morphology and did not display any particular morphological differences when compared with DFs, even after passage 15 in vitro. (C): ASCs and DFs display similar capacity to differentiate into osteogenic cells. The figure shows the capacity of ASCs and DFs to differentiate toward the osteogenic lineages in the presence of lineage-specific induction factors. Magnification, ×20. (D): The sequential dilution of ASCs (100/0) by DFs (up to 0/100) did not demonstrate an impact on HGF, IGF-1, bFGF, and KGF secretion at 0.1% versus 5% O2 and 1.0 versus 4.5g/l glucose. The KGF secretion from ASC was not impacted in hypoxia/hyperglycemia, whereas DF secretion was altered (p < .05). Abbreviations: ASC, adipose stem cell; bFGF, basic fibroblast growth factor; DF, dermal fibroblast; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor 1; KGF, keratinocyte growth factor; NS, not significant.
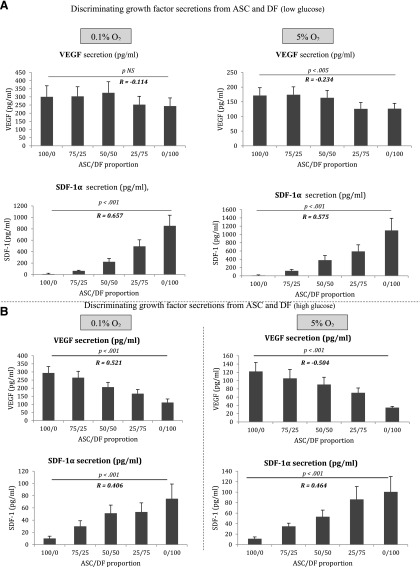
Therefore, we postulate that it may be important to test the secretion of specific and defined sets of growth factors by ASCs as a quantifiable potency test [14]. The study of HGF, IGF-1, bFGF, and keratinocyte growth factor (KGF) secretion (at 0.1% and 5% O2 and 1.0 and 4.5 g/l glucose) by sequential dilutions of ASCs and DFs did not reveal a relationship between these growth factors in culture supernatant and the purity of an ASC relative to fibroblast contamination (Fig. 1). Interestingly, we found two opposite correlations between the levels of VEGF and SDF-1α secretions and the concentration of ASCs and DFs. A negative relation for VEGF secretion was found when ASCs were contaminated by fibroblasts, especially when cells were exposed to high glucose concentration and low oxygen tension (0.1% O2, R = −0.521; p < .001; Fig. 2). These data are consistent with previous reports indicating the strong angiogenic potential of ASCs and their potential use for the therapy of ischemic diseases [10, 15–17]. Although it is still under debate, fibroblasts can be considered more mature cells and appear less angiogenic [14]. By allowing an increase in the sensibility of the testing for the assessment of the ASCs’ purity, the secretion of SDF-1α was found to be positively correlated to the degree of fibroblast contamination mostly in low glucose and low oxygen tension (r = .657, p < .001; Fig. 2). Interestingly, these findings demonstrate that low oxygen tension exposure results in the release of selective growth factors by ASCs and DFs with the secretion of VEGF and SDF-1α, respectively [18]. The study also validated the concept that ASCs secrete a lower amount of SDF-1α in comparison with DFs (in each oxygen and glucose condition). The chemokine SDF-1α (also known as CXCL12) attracts cells expressing C-X-C chemokine receptor-4 (CXCR4) as MSC and participates in numerous tissue regenerations [19, 20]. Recently, Zwingenberger et al. demonstrated that MSCs overexpressing the CXCR4 receptor (by lentiviral transduction) were highly attracted by medium obtained from SDF-1α adenoviral-transduced fat tissue [21]. As a consequence, ASCs could be considered as a target of SDF-1α (explaining the low secretion rate of this growth factor) to participate in the tissue regeneration, whereas DFs recruit MSCs by the SFD-1α release.
Figure 2.
The secretion of VEGF and SDF-1α to discriminate ASCs and DFs in function of the glucose concentration and the oxygen level. (A): At physiological concentration of glucose (at 1.0 g/l), a significant positive correlation was found between the ratio of ASCs/DFs and SDF-1α at both 0.1% and 5% O2 (p < .001). In contrast, the VEGF secretion was negatively correlated to the ratio of ASCs/DFs at only 5% oxygen (p < .005). VEGF secretion was significantly stimulated by hypoxia (vs. tissue normoxia) for ASCs (p < .005) and DFs (p < .001). SDF-1α was mainly secreted by DFs (p < .001 when compared with ASCs) and was not significantly impacted by oxygen tension. ASC did not release SDF-1α. (B): The negative correlation between VEGF secretion and the degree of ASC purity was significantly improved at 4.5 g/l for both 0.1% and 5% oxygen tension (p < .001) in comparison with the conditions at 1.0 g/l. The significant correlation between SDF-1α secretion and DF contamination was maintained at 4.5 g/l glucose at both 0.1% and 5% O2. DF function was altered in hyperglycemia for VEGF and SDF-1α secretions (p = .001), whereas ASCs were not affected. VEGF secretion by ASCs was higher than by DFs in both glucose concentrations (p < .001 in normoglycemia; p < .05 in hyperglycemia). Abbreviations: ASC, adipose stem cell; DF, dermal fibroblast; SDF-1α, stromal cell-derived factor 1α; VEGF, vascular endothelial growth factor.
Discussion
In our view, this study disclosed important new insights into functional differences between ASCs and fibroblasts. The functional diversity and positional identity of ASCs and fibroblasts may act to regulate local parenchymal cells in several ways.
This report addresses the concerns of regulatory authorities about the negative impact of fibroblasts in stem cell therapy products. Indeed, advanced cell therapy products are drugs and therefore are required to demonstrate safety and efficacy—and therefore purity—before marketing authorization is given for widespread use in patients.
Conclusion
In order to provide direction to academia, industry, and regulatory authorities regarding purity assessment for adipose tissue-derived cells, we described in this article a simple tool to facilitate development of international standards based on reproducible parameters and endpoints that will possibly systematize cellular products across boundaries and accelerate the delivery of safe and effective an ASC-based tool to the medical community and the patients it serves. This tool (a) can discriminate specifically between fibroblasts and ASCs and (b) can be rapidly implemented before the release of the ASC-based therapy (a response within 24 hours).
Acknowledgments
We thank Université Catholique de Louvain for the technical environment.
Author Contributions
D.D.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; A.L.: provision of study materials, collection and/or assembly of data, manuscript writing.
Disclosure of Potential Conflicts of Interest
D.D. has compensated employment and uncompensated intellectual property rights for Novadip Biosciences. The other author indicated no potential conflicts of interest.
References
- 1.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuk PA. The adipose-derived stem cell: Looking back and looking ahead. Mol Biol Cell. 2010;21:1783–1787. doi: 10.1091/mbc.E09-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser JK, Wulur I, Alfonso Z, et al. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Haniffa MA, Collin MP, Buckley CD, et al. Mesenchymal stem cells: The fibroblasts’ new clothes? Haematologica. 2009;94:258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hematti P. Mesenchymal stromal cells and fibroblasts: A case of mistaken identity? Cytotherapy. 2012;14:516–521. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- 9.Coleman SR. Structural fat grafts: The ideal filler? Clin Plast Surg. 2001;28:111–119. [PubMed] [Google Scholar]
- 10.Lafosse A, Desmet C, Aouassar N, et al. Autologous adipose stromal cells seeded onto a human collagen matrix for dermal regeneration in chronic wounds: Clinical proof of concept. Plast Reconstr Surg. 2015;136:279–295. doi: 10.1097/PRS.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 11.Lv FJ, Tuan RS, Cheung KM, et al. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 12.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz K, Sicker M, Schmelzer E, et al. Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp Dermatol. 2008;17:925–932. doi: 10.1111/j.1600-0625.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- 14.Blasi A, Martino C, Balducci L, et al. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell. 2011;3:5. doi: 10.1186/2045-824X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 16.Schubert T, Xhema D, Vériter S, et al. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials. 2011;32:8880–8891. doi: 10.1016/j.biomaterials.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Vériter S, Aouassar N, Adnet PY, et al. The impact of hyperglycemia and the presence of encapsulated islets on oxygenation within a bioartificial pancreas in the presence of mesenchymal stem cells in a diabetic Wistar rat model. Biomaterials. 2011;32:5945–5956. doi: 10.1016/j.biomaterials.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 18.Frazier TP, Gimble JM, Kheterpal I, et al. Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie. 2013;95:2286–2296. doi: 10.1016/j.biochi.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 19.McGrath KE, Koniski AD, Maltby KM, et al. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 20.Henderson PW, Singh SP, Krijgh DD, et al. Stromal-derived factor-1 delivered via hydrogel drug-delivery vehicle accelerates wound healing in vivo. Wound Repair Regen. 2011;19:420–425. doi: 10.1111/j.1524-475X.2011.00687.x. [DOI] [PubMed] [Google Scholar]
- 21.Zwingenberger S, Yao Z, Jacobi A, et al. Stem cell attraction via SDF-1α expressing fat tissue grafts. J Biomed Mater Res A. 2013;101:2067–2074. doi: 10.1002/jbm.a.34512. [DOI] [PMC free article] [PubMed] [Google Scholar]