Abstract
Objective To develop an efficient surveillance approach for childhood diabetes by type across 2 large US health care systems, using phenotyping algorithms derived from electronic health record (EHR) data.
Materials and Methods Presumptive diabetes cases <20 years of age from 2 large independent health care systems were identified as those having ≥1 of the 5 indicators in the past 3.5 years, including elevated HbA1c, elevated blood glucose, diabetes-related billing codes, patient problem list, and outpatient anti-diabetic medications. EHRs of all the presumptive cases were manually reviewed, and true diabetes status and diabetes type were determined. Algorithms for identifying diabetes cases overall and classifying diabetes type were either prespecified or derived from classification and regression tree analysis. Surveillance approach was developed based on the best algorithms identified.
Results We developed a stepwise surveillance approach using billing code–based prespecified algorithms and targeted manual EHR review, which efficiently and accurately ascertained and classified diabetes cases by type, in both health care systems. The sensitivity and positive predictive values in both systems were approximately ≥90% for ascertaining diabetes cases overall and classifying cases with type 1 or type 2 diabetes. About 80% of the cases with “other” type were also correctly classified. This stepwise surveillance approach resulted in a >70% reduction in the number of cases requiring manual validation compared to traditional surveillance methods.
Conclusion EHR data may be used to establish an efficient approach for large-scale surveillance for childhood diabetes by type, although some manual effort is still needed.
Keywords: automated algorithm, ascertainment and classification, childhood diabetes, electronic health records, surveillance
BACKGROUND AND SIGNIFICANCE
Large population-based registries, including the SEARCH for Diabetes in Youth (SEARCH) study1 and others,2,3 have provided critical data for surveillance of diabetes among US children and adolescents. Increasing trends in the prevalence of both type 1 and type 2 diabetes and in the incidence of type 1 diabetes have been documented.1,2,4–6 However, such surveillance systems are resource intensive and are associated with considerable delays in reporting results.7 A more efficient surveillance system is needed for sustained monitoring of the incidence and prevalence of childhood diabetes, so as to inform health care needs for this growing population.
Many US healthcare systems have transitioned to the use of electronic health records (EHRs).8 An EHR-based surveillance system has the potential to substantially increase the quantity, breadth, and timeliness of data available to health care systems and health departments, and could be more efficient than traditional surveillance methods.9 However, few US studies have reported EHR-derived algorithms and their performance in youth with diabetes.10,11 No validation study has been done to assess the generalizability of these algorithms. Little is known about the utility of automated algorithms derived from EHR data for surveillance of childhood diabetes within or across health care systems.
Previously, automated algorithms for surveillance were prespecified by manually manipulating the available EHR data elements.10,11 This approach does not maximize the potential of EHR data, and may be the reason that algorithms to ascertain type 2 diabetes in youth with reasonable sensitivity, specificity, and positive predictive value (PPV) have yet to be found.10,11 The value of classification and regression tree (CART) analysis in public health research has been recognized and emphasized, but infrequently used.12 The CART method makes no assumptions about the variable distributions or relationships and generates a graphical prediction tree of classification that is easy to visualize.13 Therefore, CART analysis may be a useful tool for electronically deriving algorithms for surveillance of diabetes in youth, in particular a type 2 diabetes algorithm.
OBJECTIVES
The objectives of this study were to: (i) determine if CART analysis yields EHR algorithms with reasonable sensitivity, specificity, and PPV for surveillance of childhood diabetes overall and by diabetes type compared to the prespecified simple algorithms; (ii) determine whether algorithms derived from one health care system have comparable performance in another independent health care system; (iii) assess whether algorithm performance differs by age or race; and (iv) develop an efficient approach for sustainable surveillance of childhood diabetes incorporating use of the derived EHR algorithms that would substantially reduce the need for review of individual medical records.
MATERIALS AND METHODS
We used EHR data from the Medical University of South Carolina (MUSC) and the University of North Carolina Health Care System (UNC). The current study was designed to specifically address surveillance of prevalent diabetes cases in youth receiving care within the healthcare systems. Preliminary results related to EHR data from UNC have been published.10
Description of 2 health care systems and data sources
MUSC and UNC are large not-for-profit integrated academic health care systems located in Charleston, South Carolina, and central North Carolina, respectively. They both provide health care for a broad range of patients, including those without insurance. MUSC is a 700-bed referral and teaching facility, providing care for over 250 000 patients annually. With a central 800-bed tertiary care center, and through its network of primary care and specialty physician practices located in 5 counties, UNC cares for over 800 000 patients annually. This study was approved by the Institutional Review Boards at MUSC and UNC.
During the data collection period, MUSC used the Epic EHR system and UNC used a locally developed EHR system. EHR data in both health care systems included demographics, outpatient medication lists for prescriptions, a variety of notes entered by health care professionals, and laboratory test results. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used at both locations for health services billing (ie, billing data). At MUSC, billing data were incorporated into the EHRs, patient problem lists were not available, and medication data were not available until May 2012. At UNC, billing data were maintained and updated separately from the EHRs. “Full data” in this study were the data elements available in both health care systems, including billing data, outpatient anti-diabetes medication data, laboratory test results, and demographics.
Case identification and validation
The study population at MUSC included all children <20 years of age as of December 31, 2012, who were seen by a health care provider at least once for any reason between July 1, 2012, and December 31, 2012. At UNC, we included all children <20 years of age as of December 31, 2011, who were seen by a health care provider at least once for any reason in 2011.
The initial criteria used to identify presumptive diabetes cases from the study population at both health care systems were: (i) ≥1 HbA1c ≥ 6.0% (42 mmol/mol); or (ii) ≥2 random blood glucose ≥200 mg/dL on different days or ≥1 fasting blood glucose ≥126 mg/dL; or (iii) ≥1 patient problem list diabetes-related ICD-9-CM codes; or (iv) ≥1 billing data diabetes-related ICD-9-CM codes; or (v) ≥1 outpatient anti-diabetes medications, including insulin, glucagon, metformin, sulfonylurea, glucagon-like peptide-1 receptor agonists, thiazolidinediones, and other hypoglycemic agents.10 The following diabetes-related ICD-9-CM codes were used: 250.xx (diabetes mellitus), 775.1 (neonatal diabetes), 648.0x (diabetes in pregnancy, non-gestational), 357.2 (diabetic neuropathy), 362.0x (diabetic retinopathy), and 366.41 (diabetic cataract). We searched EHR data from July 1, 2009, to December 31, 2012, at MUSC, and from July 1, 2008, to December 31, 2011, at UNC for these 5 diabetes criteria. Patients meeting any of the 5 criteria were identified as presumptive diabetes cases. The definition of presumptive diabetes cases was designed to be highly sensitive and thus children not identified as presumptive cases were assumed to not have diabetes (ie, true negatives).
True diabetes status and type were determined by the presence of a diabetes diagnosis in the EHR in 1 or more notes written by health care providers (gold standard), which is consistent with the SEARCH case validation protocol.14 Reviewers at each of the 2 health care systems were trained using the SEARCH standardized protocol by a member of the SEARCH team (J.T.), who has over 10 years of experience with the SEARCH case ascertainment protocol. More details of the case validation processes can be found elsewhere.10
Criteria for evaluating algorithms’ performance
Attributes critical for a disease surveillance system recommended by the Centers for Disease Control and Prevention include simplicity, timeliness, high sensitivity, and high PPV.15 High sensitivity is crucial for identifying most of the diabetes cases. High PPV is preferred in order to reduce the number of false positives. For surveillance of childhood diabetes, high specificity is important for differentiating between diabetes types and also critical to yield high PPV. A promising algorithm for surveillance should yield a sensitivity, specificity, and PPV ≥90%.10 In this report, specificity was only shown in the results when evaluating the type 1 and type 2 algorithms in true diabetes cases, because we aimed to conduct population surveillance so specificity would be consistently >90% due to the low prevalence of diabetes in children.
Statistical Analysis
The characteristics of the study population, presumptive cases and true cases, were described. We evaluated the performance of the prespecified algorithms for identifying diabetes cases regardless of type, then by type 1 or type 2 diabetes cases in both health care systems. Those pre-specified algorithms were created based on clinical knowledge and were previously published.10 Next, we assessed the performance of CART algorithms within and between health care systems. Specifically, we performed CART analysis on the data from MUSC (training dataset) and then applied the derived algorithms from the training dataset to UNC data (validation dataset) and vice versa. The CART analysis recursively identified classifiers that efficiently segmented the sample into mutually exclusive subgroups (eg, diabetes and not diabetes, or type 1 and type 2 diabetes).13 There was a complexity parameter tuned by a 10-fold cross-validation procedure to control the depth of the generated tree (ie, to avoid over-fitting). We searched over a prespecified finite set of complexity levels to select the best complexity parameter that minimized the cross-validation error. This parameter was used to replicate the same prediction tree (ie, derived algorithm) from the training dataset in the validation dataset. The variables used for CART analysis were coded and processed identically between the 2 health care systems (Appendix, Table 1).
The performance of the algorithms for identifying diabetes cases regardless of type was evaluated within the total study population. The performance of type 1 or type 2 algorithms was evaluated within the true cases.10,11 We also evaluated whether algorithm performance differed by age (<10 years versus 10–19 years) or race (white versus non-white). Only 2.3% of the total type 2 cases were <10 years of age, so the performance was not calculated for this younger group.
Finally, a stepwise surveillance approach was developed with the goal of improving efficiency of the process by employing the top-performing algorithms in order to minimize the manual validation efforts and maintain accuracy. The top-performing diabetes type–specific algorithms used for this approach should have ≥90% sensitivity, specificity, and PPV.10 In practice, our approach would assume that the individuals ascertained by the best top-performing type 1 or type 2 algorithm were true type 1 or type 2 cases, respectively, without manual validation of each case against medical records. For the remaining cases who were not selected by the best algorithms, manual medical record review would be conducted. To evaluate this approach, we considered sensitivity and PPV based on the gold standard. Also, the number and percentage of medical records that required manual review were calculated as a marker of efficiency.
CART analysis was performed using the rpart package and R statistical software package, version 3.0.2.16 Other analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
At MUSC, we identified 1055 presumptive cases from 43 511 children <20 years of age with at least 1 health care visit between July 1 and December 31, 2012 (Table 1). Based on the manual medical record review of presumptive cases (gold standard), 660 (62.6%) true cases (483 type 1, 129 type 2, and 48 other types) were ascertained. At UNC, the initial algorithm identified 1289 presumptive diabetes cases from 57 767 children <20 years of age with at least 1 health care visit in 2011.10 Of those, 537 (41.7%) were true cases (405 type 1, 86 type 2, and 46 other diabetes types).
Table 1.
Demographic and clinical characteristics of the total study population, presumptive diabetes cases, and true diabetes cases at MUSC in 2012 and at UNC in 2011
| Characteristics | Study population | Presumptive casesa | True diabetes cases | Type 1 cases | Type 2 cases | Other casesb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MUSC | UNCc | MUSC | UNC c | MUSC | UNC c | MUSC | UNC c | MUSC | UNC c | MUSC | UNCc | |
| All, n | 43 511 | 57 767 | 1055 | 1289 | 660 | 537 | 483 | 405 | 129 | 86 | 48 | 46 |
| Female, % | 51.5 | 48.1 | 52.3 | 52.7 | 52.7 | 55.7 | 48.0 | 54.8 | 72.9 | 60.5 | 45.8 | 54.3 |
| Age (years)d, % | ||||||||||||
| 0–4.9 | 35.3 | 36.3 | 11.4 | 26.9 | 2.3 | 2.8 | 2.9 | 3.2 | 0 | 0 | 2.1 | 4.3 |
| 5.0–9.9 | 24.8 | 22.2 | 13.7 | 12.1 | 13.5 | 12.5 | 16.6 | 15.6 | 2.3 | 2.3 | 12.5 | 4.3 |
| 10.0–14.9 | 20.8 | 19.8 | 34.1 | 24.0 | 37.3 | 32.6 | 39.5 | 34.6 | 32.6 | 34.9 | 27.1 | 10.9 |
| 15.0–17.9 | 12.1 | 12.7 | 26.1 | 22.0 | 31.2 | 30.2 | 27.7 | 26.4 | 41.1 | 37.2 | 39.6 | 50.0 |
| 18.0–19.9 | 7.1 | 9.0 | 14.7 | 15.0 | 15.8 | 22.0 | 13.3 | 20.3 | 24.0 | 25.6 | 18.8 | 30.4 |
| Mean age, years (SD) | 8.0 (5.9) | 8.6 (6.2) | 12.3 (5.1) | 11.0 (6.5) | 13.6 (3.9) | 14.4 (4.1) | 13.1 (4.0) | 13.9 (4.3) | 15.2 (2.7) | 15.8 (2.6) | 14.1 (3.8) | 16.0 (3.7) |
| Age at diagnosis, years (SD) | 9.3 (4.3) | 8.8 (4.6) | 8.0 (4.0) | 7.9 (4.3) | 13.0 (2.7) | 12.9 (2.3) | 12.2 (3.9) | 12.8 (5.3) | ||||
| Racee, % | ||||||||||||
| White | 49.3 | 48.5 | 51.7 | 53.0 | 59.6 | 61.6 | 72.7 | 67.9 | 13.2 | 32.6 | 52.1 | 60.9 |
| Black | 37.0 | 21.0 | 42.7 | 29.0 | 35.8 | 25.5 | 22.2 | 19.8 | 83.7 | 50.0 | 43.8 | 30.4 |
| Other/unknown/missing | 13.7 | 30.5 | 5.7 | 18.0 | 4.7 | 12.9 | 5.2 | 12.3 | 3.1 | 17.4 | 4.2 | 8.7 |
| Health insurance type, % | ||||||||||||
| Private | 39.2 | 43.2 | 40.0 | 40.1 | 48.2 | 50.8 | 57.8 | 55.3 | 21.7 | 27.9 | 22.9 | 54.3 |
| Government | 52.5 | 42.4 | 48.0 | 47.6 | 38.8 | 34.1 | 29.4 | 29.1 | 66.7 | 57.0 | 58.3 | 34.8 |
| Tricaref | 5.0 | 8.3 | 4.1 | 9.1 | 4.9 | 11.0 | 5.4 | 12.1 | 1.6 | 5.8 | 8.3 | 10.9 |
| Not insured/missing/other | 3.4 | 6.2 | 8.0 | 3.3 | 8.2 | 4.1 | 7.5 | 3.5 | 10.1 | 9.3 | 10.4 | 0 |
Abbreviations: MUSC: Medical University of South Carolina; SD: standard deviation; UNC: University of North Carolina.
aPresumptive cases were individuals with ≥1 of these criteria: ≥1 HbA1c ≥ 6.0% (42 mmol/mol); or ≥2 random blood glucose ≥ 200 mg/dL on different days; or ≥1 fasting blood glucose ≥126 mg/dL; or ≥1 patient problem list diabetes-related ICD-9-CM codes; or ≥1 billing data diabetes-related ICD-9-CM codes; or ≥1 diabetes-related outpatient medications, including insulin, glucagon, metformin, sulfonylurea, glucagon-like peptide-1 receptor agonists, thiazolidinediones, and other hypoglycemic agents.
bAt MUSC: “Other” cases included 6 cases of maturity onset diabetes of the young (MODY) and 42 secondary diabetes cases, mostly steroid induced or CF-related. At UNC: “Other” cases included 25 secondary diabetes cases, 2 MODY cases, and 19 diabetes type-unspecified cases.
cThe UNC data were previously published,10 but the presumptive cases here did not include those captured by inpatient medications.
dAt MUSC: Age was calculated as of December 31, 2012. At UNC: Age was calculated as of December 31, 2011.|
eEthnicity data were missing for about 98% of the patients at MUSC, so we were only able to categorize race.
fMilitary health insurance plan.
Performance of the prespecified algorithms in 2 health care systems
Identifying diabetes cases regardless of diabetes type
Billing data had similar performance overall and within age or racial subgroups between the 2 health care systems (Appendix, Tables 2 and 3). The sensitivity and PPV of billing data were 97.9% and 81.6%, respectively, in MUSC and were 97.0% and 82.2%, respectively, in UNC.10 Outpatient medications at MUSC had a low sensitivity of 12.7%, compared to a sensitivity of 88.5% at UNC. The algorithm in which ≥2 criteria were met approximately satisfied our prespecified 90% criteria in both health care systems. The algorithm in which ≥1 criteria were met had poor PPV in both systems (62.6% and 41.7% at MUSC and UNC, respectively). Among age and racial subgroups, in both systems, the PPV of the glucose criterion or billing data was much lower in youth <10 years of age than in the older youth. HbA1c had higher PPV among white youth than youth with non-white racial backgrounds.
Type 1 algorithms
All type 1 algorithms performed very similarly between the 2 health care systems (Table 2). The 2 best type 1 algorithms were the ratio of type 1 codes to the sum of type 1 and type 2 codes ≥0.5 or 0.6. When the ratio was ≥0.6, the sensitivity, specificity, and PPV were 97.7%, 94.9%, and 98.1%, respectively, at MUSC, and 93.3%, 96.2%, and 98.7%, respectively, at UNC. Age and racial differences were small, except that the specificity of these best 2 algorithms was <90% in youth <10 years of age and in white youth (Table 3).
Table 2.
Performance of the prespecified type 1 and type 2 algorithms by age applied to all true cases at MUSC and UNCa
| Non-CART Diabetes Algorithms | System | Met Criteria (N) | Sensitivity (%) | Specificity (%) | PPV (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | <10 y | ≥10 y | Total | <10 y | ≥10 y | Total | <10 y | ≥10 y | Total | <10 y | ≥10 y | ||
| Type 1 Algorithms | |||||||||||||
| ≥1 type 1 codes | MUSC | 570 | 97 | 473 | 99.0 | 100.0 | 98.7 | 48.0 | 70.0 | 46.7 | 83.9 | 96.9 | 81.2 |
| UNC | 443 | 76 | 367 | 97.0 | 98.7 | 96.7 | 62.1 | 83.3 | 61.1 | 88.7 | 98.7 | 86.6 | |
| ≥2 type 1 codes | MUSC | 537 | 94 | 443 | 96.7 | 97.9 | 96.4 | 60.5 | 80.0 | 59.3 | 87.0 | 97.9 | 84.7 |
| UNC | 415 | 76 | 339 | 93.6 | 98.7 | 92.4 | 72.7 | 83.3 | 72.2 | 91.3 | 98.7 | 89.7 | |
| 0 type 2 codes and ≥1 type 1 codes | MUSC | 195 | 50 | 145 | 39.8 | 53.2 | 36.5 | 98.3 | 100.0 | 98.2 | 98.5 | 100.0 | 97.9 |
| UNC | 141 | 18 | 123 | 34.6 | 23.7 | 37.1 | 99.2 | 100.0 | 99.2 | 99.3 | 100.0 | 99.2 | |
| Ratio of type 1 codes to the sum of type 1 and type 2 codes ≥ 0.3 | MUSC | 510 | 96 | 414 | 98.8 | 98.9 | 98.7 | 81.4 | 70.0 | 82.0 | 93.5 | 96.9 | 92.8 |
| UNC | 413 | 76 | 337 | 96.8 | 98.7 | 96.4 | 84.1 | 83.3 | 84.1 | 94.9 | 98.7 | 94.1 | |
| Ratio of type 1 codes to the sum of type 1 and type 2 codes ≥ 0.4 | MUSC | 496 | 95 | 401 | 98.6 | 98.9 | 98.5 | 88.7 | 80.0 | 89.2 | 96.0 | 97.9 | 95.5 |
| UNC | 402 | 76 | 326 | 96.3 | 98.7 | 95.7 | 90.9 | 83.3 | 91.3 | 97.0 | 98.7 | 96.6 | |
| Ratio of type 1 codes to the sum of type 1 and type 2 codes ≥ 0.5 | MUSC | 490 | 95 | 395 | 98.6 | 98.9 | 98.5 | 92.1 | 80.0 | 92.8 | 97.1 | 97.9 | 97.0 |
| UNC | 397 | 76 | 321 | 95.6 | 98.7 | 94.8 | 92.4 | 83.3 | 92.9 | 97.5 | 98.7 | 97.2 | |
| Ratio of type 1 codes to the sum of type 1 and type 2 codes ≥ 0.6 | MUSC | 481 | 95 | 386 | 97.7 | 98.9 | 97.4 | 94.9 | 80.0 | 95.8 | 98.1 | 97.9 | 98.2 |
| UNC | 383 | 75 | 308 | 93.3 | 97.4 | 92.4 | 96.2 | 83.3 | 96.8 | 98.7 | 98.7 | 98.7 | |
| Type 2 Algorithms | |||||||||||||
| ≥1 type 2 codes | MUSC | 451 | 50 | 401 | 93.0 | N/A | 94.4 | 37.7 | N/A | 34.4 | 26.6 | N/A | 29.7 |
| UNC | 375 | 62 | 313 | 91.9 | N/A | 92.9 | 34.4 | N/A | 36.7 | 21.1 | N/A | 24.9 | |
| ≥2 type 2 codes | MUSC | 316 | 33 | 283 | 83.7 | N/A | 85.7 | 60.8 | N/A | 59.3 | 34.2 | N/A | 38.2 |
| UNC | 256 | 31 | 225 | 82.6 | N/A | 83.3 | 59.0 | N/A | 58.2 | 27.7 | N/A | 31.0 | |
| ≥1 type 2 codes and 0 type 1 codes | MUSC | 76 | 3 | 73 | 41.9 | N/A | 42.1 | 95.9 | N/A | 95.3 | 71.1 | N/A | 72.6 |
| UNC | 73 | 4 | 69 | 54.7 | N/A | 54.8 | 94.2 | N/A | 93.8 | 64.4 | N/A | 66.7 | |
| Ratio of type 2 codes to the sum of type 1 and type 2 codes ≥ 0.3 | MUSC | 181 | 8 | 173 | 93.0 | N/A | 94.4 | 88.5 | N/A | 87.4 | 66.3 | N/A | 68.8 |
| UNC | 151 | 10 | 141 | 91.9 | N/A | 92.9 | 84.0 | N/A | 83.0 | 52.3 | N/A | 55.3 | |
| Ratio of type 2 codes to the sum of type 1 and type 2 codes ≥ 0.4 | MUSC | 168 | 7 | 161 | 91.5 | N/A | 92.9 | 90.6 | N/A | 89.8 | 70.2 | N/A | 72.7 |
| UNC | 138 | 6 | 132 | 91.9 | N/A | 92.9 | 86.9 | N/A | 85.4 | 57.2 | N/A | 59.1 | |
| Ratio of type 2 codes to the sum of type 1 and type 2 codes ≥ 0.5 | MUSC | 164 | 5 | 159 | 91.5 | N/A | 92.9 | 91.3 | N/A | 90.2 | 72.0 | N/A | 73.6 |
| UNC | 128 | 4 | 124 | 88.4 | N/A | 89.3 | 88.5 | N/A | 86.8 | 59.4 | N/A | 60.5 | |
| Ratio of type 2 codes to the sum of type 1 and type 2 codes ≥ 0.6 | MUSC | 151 | 5 | 146 | 88.4 | N/A | 89.7 | 93.0 | N/A | 92.3 | 75.5 | N/A | 77.4 |
| UNC | 116 | 4 | 112 | 84.9 | N/A | 85.7 | 90.5 | N/A | 89.2 | 62.9 | N/A | 64.3 | |
True diabetes cases (N = 660 at MUSC and N = 537 at UNC) confirmed by medical record review established our “gold standard” for evaluation of the algorithms’ performance.
Abbreviations: MUSC: Medical University of South Carolina; PPV: positive predictive value; UNC: University of North Carolina; y, years.aResults from UNC were previously published.10
Table 3.
Performance of the prespecified type 1 and type 2 algorithms by race applied to all true cases at MUSC and UNCa
| Non-CART Diabetes Algorithms | System | Met Criteria (N) | Sensitivity (%) | Specificity (%) | PPV (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | white | Other | Total | White | Other | Total | white | Other | Total | white | Other | ||
| Type 1 Algorithms | |||||||||||||
| ≥1 type 1 codes | MUSC | 570 | 366 | 204 | 99.0 | 99.4 | 97.7 | 48.0 | 59.5 | 44.4 | 83.9 | 95.4 | 63.2 |
| UNC | 443 | 288 | 155 | 97.0 | 97.1 | 96.9 | 62.1 | 62.5 | 61.8 | 88.7 | 92.7 | 81.3 | |
| ≥2 type 1 codes | MUSC | 537 | 355 | 182 | 96.7 | 96.6 | 97.0 | 60.5 | 61.9 | 60.0 | 87.0 | 95.5 | 70.3 |
| UNC | 415 | 276 | 139 | 93.6 | 94.2 | 92.3 | 72.7 | 69.6 | 75.0 | 91.3 | 93.8 | 86.3 | |
| 0 type 2 codes and ≥1 type 1 codes | MUSC | 195 | 152 | 4 | 39.8 | 43.0 | 31.1 | 98.3 | 97.6 | 98.5 | 98.5 | 99.3 | 95.3 |
| UNC | 141 | 103 | 38 | 34.6 | 37.1 | 29.2 | 99.2 | 98.2 | 100.0 | 99.3 | 99.0 | 100.0 | |
| Ratio of type 1 codes to the sum of type 1 and type 2 codes ≥ 0.3 | MUSC | 510 | 358 | 152 | 98.8 | 99.1 | 97.7 | 81.4 | 76.2 | 83.0 | 93.5 | 97.2 | 84.9 |
| UNC | 413 | 277 | 136 | 96.8 | 96.7 | 96.9 | 84.1 | 80.4 | 86.8 | 94.9 | 96.0 | 92.6 | |
| Ratio of type 1 codes to the sum of type 1 and type 2 codes ≥ 0.4 | MUSC | 496 | 357 | 139 | 98.6 | 99.1 | 97.0 | 88.7 | 78.6 | 91.9 | 96.0 | 97.5 | 92.1 |
| UNC | 402 | 273 | 129 | 96.3 | 96.7 | 95.4 | 90.9 | 87.5 | 93.4 | 97.0 | 97.4 | 96.1 | |
| Ratio of type 1 codes to the sum of type 1 and type 2 codes ≥ 0.5 | MUSC | 490 | 355 | 135 | 98.6 | 99.1 | 97.0 | 92.1 | 83.3 | 94.8 | 97.1 | 98.0 | 94.8 |
| UNC | 397 | 269 | 128 | 95.6 | 96.0 | 94.6 | 92.4 | 91.1 | 93.4 | 97.5 | 98.1 | 96.1 | |
| Ratio of type 1 codes to the sum of type 1 and type 2 codes ≥ 0.6 | MUSC | 481 | 350 | 131 | 97.7 | 98.3 | 96.2 | 94.9 | 88.1 | 97.0 | 98.1 | 98.6 | 96.9 |
| UNC | 383 | 262 | 121 | 93.3 | 93.8 | 92.3 | 96.2 | 92.9 | 98.7 | 98.7 | 98.5 | 99.2 | |
| Type 2 Algorithms | |||||||||||||
| ≥1 type 2 codes | MUSC | 451 | 237 | 214 | 93.0 | 94.1 | 92.9 | 37.7 | 41.2 | 29.0 | 26.6 | 6.8 | 48.6 |
| UNC | 375 | 219 | 156 | 91.9 | 89.3 | 93.1 | 34.4 | 36.0 | 31.1 | 21.1 | 11.4 | 34.6 | |
| ≥2 type 2 codes | MUSC | 316 | 140 | 176 | 83.7 | 64.7 | 86.6 | 60.8 | 65.7 | 49.0 | 34.2 | 7.9 | 55.1 |
| UNC | 256 | 133 | 123 | 82.6 | 82.1 | 82.8 | 59.0 | 63.7 | 49.3 | 27.7 | 17.3 | 39.0 | |
| ≥1 type 2 codes and 0 type 1 codes | MUSC | 76 | 23 | 53 | 41.9 | 58.8 | 39.3 | 95.9 | 96.5 | 94.2 | 71.1 | 43.5 | 83.0 |
| UNC | 73 | 34 | 39 | 54.7 | 46.4 | 58.6 | 94.2 | 93.1 | 96.6 | 64.4 | 38.2 | 87.2 | |
| Ratio of type 2 codes to the sum of type 1 and type 2 codes ≥ 0.3 | MUSC | 181 | 50 | 131 | 93.0 | 94.1 | 92.9 | 88.5 | 91.0 | 82.6 | 66.3 | 32.0 | 79.4 |
| UNC | 151 | 75 | 76 | 91.9 | 89.3 | 93.1 | 84.0 | 83.5 | 85.1 | 52.3 | 33.3 | 71.1 | |
| Ratio of type 2 codes to the sum of type 1 and type 2 codes ≥ 0.4 | MUSC | 168 | 42 | 126 | 91.5 | 94.1 | 91.1 | 90.6 | 93.1 | 84.5 | 70.2 | 38.1 | 81.0 |
| UNC | 138 | 65 | 73 | 91.9 | 89.3 | 93.1 | 86.9 | 86.8 | 87.2 | 57.2 | 38.5 | 74.0 | |
| Ratio of type 2 codes to the sum of type 1 and type 2 codes ≥ 0.5 | MUSC | 164 | 39 | 125 | 91.5 | 94.1 | 91.1 | 91.3 | 93.9 | 85.2 | 72.0 | 41.0 | 81.6 |
| UNC | 128 | 58 | 70 | 88.4 | 82.1 | 91.4 | 88.5 | 88.4 | 88.5 | 59.4 | 39.7 | 75.7 | |
| Ratio of type 2 codes to the sum of type 1 and type 2 codes ≥ 0.6 | MUSC | 151 | 32 | 119 | 88.4 | 82.4 | 89.3 | 93.0 | 95.2 | 87.7 | 75.5 | 43.8 | 84.0 |
| UNC | 116 | 51 | 65 | 84.9 | 75.0 | 89.7 | 90.5 | 90.1 | 91.2 | 62.9 | 41.2 | 80.0 | |
True diabetes cases (N = 660 at MUSC and N = 537 at UNC) confirmed by medical record review established our “gold standard” for evaluation of the algorithms’ performance.
Abbreviations: MUSC: Medical University of South Carolina; PPV: positive predictive value; UNC: University of North Carolina. aResults from UNC were previously published.10
Type 2 algorithms
For both health care systems, none of the type 2 algorithms evaluated had a sensitivity, specificity, and PPV approaching 90%; the highest PPV was 75.5% (Table 2). The ratio of type 2 codes to the sum of type 1 and type 2 codes ≥0.4 or 0.5 or 0.6 had similar performance within the health care systems, although the PPV was higher at MUSC than at UNC. The PPV was considerably higher among youth with non-white racial backgrounds in both health care systems (Table 3). Among the non-white subgroup, the sensitivity, specificity, and PPV of the ratio of type 2 codes to the sum of type 1 and type 2 codes ≥0.6 were 89.3%, 87.7%, and 84.0%, respectively, at MUSC, and 89.7%, 91.2%, and 80.0%, respectively, at UNC.
Performance of CART algorithms in 2 health care systems
Using billing data only
For algorithms used for identifying diabetes cases regardless of type, most CART algorithms were close to the prespecified 90% criteria and the algorithms had similar performance between health care systems. Only small differences were observed between age and racial subgroups (Appendix, Table 4). Type 1 algorithms derived from CART analysis were not superior to the prespecified type 1 algorithms. The performance of type 2 algorithms was also not improved by using the CART method relative to the prespecified method.
Using full data
The billing data only based algorithms for identifying diabetes cases without regard to type, and type 1 algorithms were not improved upon by adding additional data elements. For type 2 algorithms, use of full data compared to use of billing data alone improved PPV from 58.3% to 80.6% and decreased sensitivity from 93.1% to 80.1% at UNC; use of full data improved PPV from 70.3% to 81.9% and decreased sensitivity from 96.0% to 91.4% at MUSC. The type 2 algorithm had a sensitivity of 91.4%, specificity of 95.1%, and PPV of 81.9% at MUSC (Appendix, Figure 1; results in Appendix, Table 4) and had a sensitivity of 80.1%, specificity of 96.3%, and PPV of 80.6% at UNC (Appendix, Figure 2; results in Appendix, Table 4).
A stepwise surveillance approach
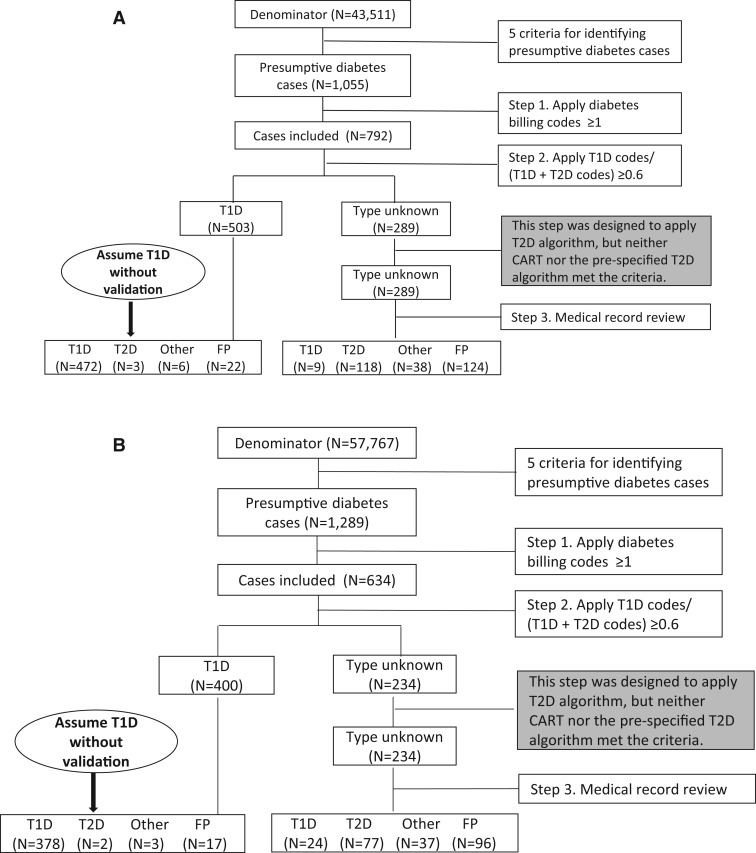
An identical stepwise surveillance approach was used in both health care systems, which began with identifying presumptive cases (Figures 1A and 1B). The steps were: (i) applying ≥1 billing codes; (ii) applying the best prespecified type 1 algorithm (ie, the ratio of type 1 codes to the sum of type 1 and type 2 codes ≥0.6); the following step was originally designed to apply the best type 2 algorithm with ≥90% sensitivity, specificity, and PPV, but neither CART nor the prespecified type 2 algorithm met the 90% criterion; (iii) reviewing the medical records of the remaining cases that were not classified by the previous steps; and (iv) evaluating the performance of the stepwise surveillance approach.
Figure 1:
(A) The stepwise surveillance approach at MUSC. (B) The stepwise surveillance approach at UNC.Abbreviations: MUSC: Medical University of South Carolina; FP: false positive; T1D: type 1. diabetes; T2D: type 2 diabetes; UNC: University of North Carolina.
Using this approach, 289 (27.4%) of the 1055 medical records at MUSC (Table 4) and 234 (18.2%) of the 1289 records at UNC required manual review. The sensitivity and PPV were close to or greater than 95% for identifying total diabetes cases and type 1 cases in both health care systems. For type 2 diabetes, the sensitivity was approximately 90% in both systems and PPV was 100.0%. This approach also classified cases of other diabetes types with a sensitivity of nearly 80% and PPV of 100%.
Table 4.
The performance of the stepwise surveillance approach
| Diabetes Type | MUSC | UNC | ||
|---|---|---|---|---|
| Review 289 (27.4%) of 1055 records | Review 234 (18.2%) of 1289 records | |||
| Sensitivity | Positive predictive value | Sensitivity | Positive predictive value | |
| Total | 97.9 | 96.7 | 97.0 | 96.8 |
| Type 1 | 99.6 | 93.9 | 99.3 | 94.8 |
| Type 2 | 91.5 | 100.0 | 89.5 | 100 |
| Othera | 79.2 | 100 | 80.4 | 100 |
Abbreviations: MUSC: Medical University of South Carolina; UNC: University of North Carolina.
aIncluded cases with diabetes other than type 1 or type 2.
DISCUSSION
In both health care systems, we found that using billing data alone in the prespecified algorithms was the most efficient for diabetes case identification overall and for type 1 case classification. Billing data–based prespecified type 1 algorithms met our criteria for surveillance, and additional data including medication and laboratory data did not improve the prespecified type 1 algorithms. CART type 1 algorithms were not superior to the prespecified type 1 algorithms. For type 2 diabetes identification, the additional EHR data provided only a small improvement. However, neither CART nor the prespecified type 2 algorithms reached our 90% criteria for surveillance. We developed a stepwise surveillance approach using a combination of billing data–based prespecified algorithms and targeted manual review of EHRs that ascertained and classified diabetes cases efficiently and accurately in both health care systems. This stepwise approach required much less manual effort (>70% reduction in EHR review) compared to traditional methods. The performance of the stepwise surveillance approach, billing data, and prespecified type 1 algorithms was similar between the 2 health care systems.
The stepwise surveillance approach proposed in this study ascertained and classified diabetes cases in youth accurately, with approximately 90% or higher sensitivity and PPV for total diabetes cases, type 1 and type 2 cases; and efficiently, with a reduction of >70% in the number of cases requiring manual medical record review. Recent reports suggest that other types of diabetes, including drug-induced diabetes and monogenic diabetes, are important components of childhood diabetes.17,18 Together with the increasing prevalence of type 2 diabetes,1 they have altered the pediatric diabetes landscape. The stepwise surveillance approach also correctly classified most cases with other diabetes types (∼80%) in both health care systems.
Billing data was the most important source of data for surveillance with little gain from using additional EHR data. The sensitivity of billing data for ascertaining diabetes overall was ≥97%. Also, billing data–based prespecified type 1 algorithms performed very well in both health care systems. For type 2 case classification, we previously found that adding medication data improved the PPV of the prespecified type 2 algorithm, but the overall performance was not greatly improved.10 Dart et al.19 also reported that the addition of medication data did not substantially improve diabetes case identification, but their study was unable to differentiate diabetes types. In the current study, we employed CART analysis in an attempt to improve type 2 case classification. Within the health care systems, among total true cases, the type 2 algorithm was improved slightly, but it was still not sufficient for surveillance purposes.
Previous studies have identified the greater difficulty in classifying type 2 cases in youth.10,11,20,21 At the current state of EHR development and use of the ICD-9-CM coding system, together with the very low prevalence of type 2 diabetes in youth,1 relying solely on an automated algorithm for type 2 case classification may not be possible. First, ICD-9-CM type 2 codes include “unspecified” diabetes (250.X0 or 250.X2 with X = 0–9), which has very poor accuracy (<20%) for classifying type 2 cases in children.21 A study found that few “unspecified” diabetes codes were assigned by use of ICD-10-CM.22 However, more investigation is needed to determine the utility of ICD-10-CM codes for surveillance. Second, patients without diabetes may still be incorrectly assigned a type 2 code if they have cystic fibrosis–related diabetes, diabetes insipidus, or diabetes risk factors including insulin resistance, obesity, impaired glucose tolerance, etc.21 Third, type 2 codes may be misused for other forms of diabetes with less familiar codes, for example, steroid-induced diabetes (251.8 and E932.0). In our study, the type 2 algorithms which identified most type 2 cases also identified most cases with drug-induced diabetes cases (data not shown). Fourth, in some patients, a clinical differentiation between type 1 and type 2 diabetes is difficult at presentation with diabetes in children,23 if auto-antibodies and/or insulin sensitivity are not measured.24 Fifth, insulin is commonly used in children with type 2 diabetes,25 which limits the use of medication as an efficient type classifier, though we did see small improvement with the inclusion of medication data for type 2 algorithms in CART analysis. Finally, coders at hospitals or practices may use “unspecified” type 2 codes if diagnoses are indicated as “diabetes” on the billing form.21 Consequently, manual review of medical records may be necessary to classify type 2 cases accurately.It is critical to establish generalizability before a surveillance approach can be reliably implemented across health care systems. Our analyses indicated quite good agreement between 2 large independent health care systems. First, the proposed surveillance approach resulted in similar performance for estimating the number of total diabetes cases, type 1, type 2, and other types of diabetes cases, in both health care systems using identical prespecified algorithms in each step. Second, billing data had comparable performance in both health care systems and billing data alone may help to establish an efficient surveillance system. Additionally, billing data are commonly available in health care systems. However, the 2 health care systems compared here covered similar youth populations in terms of age, gender, and insurance status, and were also similar in terms of type and amount of data captured and organization of health care delivery. It is not clear whether our stepwise approach will perform similarly in different types of health care systems, such as integrated managed health care systems or the Indian Health Service.
Algorithms derived from CART analysis did not outperform the prespecified algorithms. This does not imply that CART analysis is not a useful tool. CART analysis has value in classifying other health problems.26–28 In the context of childhood diabetes, limitations were encountered in the application of CART analysis, including limitations of the ICD-9-CM coding system, miscoding issues, overlap in treatment as described above, and incomplete data in the EHRs. Of note, CART analysis yielded more complicated algorithms and captured interactions that were hard to explain, leading to poor generalizability.
Limitations of this study should be noted. First, both health care systems are public academic health care delivery systems. In both systems, the EHR systems are evolving and imperfect. This characteristic is, in a sense, also a strength of our study in that we sought to evaluate our approach in the real world. Second, we assumed that individuals who were not captured by the initial 5 criteria for identifying presumptive cases were true negatives, which may have missed a small number of true cases. However, among those presumptive cases that had only 1 indicator of diabetes, the false positive rate was about 90% or higher; the false positive rate was further increased to 95% or higher among those who had 1 indicator other than billing codes (data not shown). Therefore, it is highly unlikely that our findings would be biased by the false negatives that we failed to find, given the low prevalence of diabetes in children. Finally, we ascertained and classified prevalent cases, not incident cases. In SEARCH, natural language processing is being evaluated to extract diagnosis dates from medical records with the aim of identifying incident cases, which was not in the scope of this analysis.
CONCLUSION
We used billing data and targeted manual medical record review to develop a stepwise surveillance approach which efficiently and accurately ascertained and classified prevalent diabetes cases in 2 large, independent US public health care delivery systems. This approach relied largely on automated algorithms and required only a small amount (<30%) of manual validation efforts compared to traditional surveillance methods. Further validation of the stepwise surveillance approach in other health care systems or in the same health care system at different time points may provide additional information before it can be widely implemented across the United States for surveillance of childhood diabetes.
Acknowledgments
The SEARCH for Diabetes in Youth study is indebted to the many youth and their families and their health care providers, whose participation made this study possible. We sincerely thank Xin Zhou for providing us with R codes to perform CART analysis.
SUPPLEMENTARY MATERIAL
Supplementary material is available online at http://jamia.oxfordjournals.org/.
FUNDING
Grant Support: SEARCH for Diabetes in Youth Study is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Contract numbers: Kaiser Permanente Southern California (U48/CCU919219, U01-DP-000246, and U18-DP-002714), University of Colorado Denver (U48/CCU819241-3, U01-DP-000247, and U18-DP-000247-06A1), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01-DP-000248, and 1U18-DP-002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01-DP-000254, and U18-DP-002708-01), University of Washington School of Medicine (U58/CCU019235-4, U01-DP-000244, and U18-DP-002710-01), and Wake Forest School of Medicine (U48/CCU919219, U01-DP-000250, and 200-2010-35171). The authors wish to acknowledge the involvement of Seattle Children’s Hospital (National Institutes of Health Clinical and Translational Science Awards grant UL1 TR00423 of the University of Washington); University of Colorado Pediatric Clinical and Translational Research Center (grant number UL1 TR000154); the Barbara Davis Center at the University of Colorado Denver (DERC NIH P30 DK57516); the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 8 UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health. The MUSC effort was supported by the NIH – National Center for Advancing Translational Sciences: Grant Number UL1 TR000062.
COMPETING INTERESTS
V.W.Z. received financial support from the Sanofi Global Scholars Program. Other authors have no competing interests to declare.
CONTRIBUTORS
V.W.Z. participated in study design and coordination, reviewed medical records, analyzed data. and wrote the manuscript. E.J.M.-D. and J.S.O. conceived the study, participated in study design, reviewed and edited the manuscript, and contributed to discussion. J.B.C. and E.R.P. extracted and formatted the raw data from the data warehouse, reviewed and edited the manuscript, and contributed to discussion. J.T. participated in study design and coordination, trained medical record reviewers, validated the record review work, and reviewed and edited the manuscript. L.M.J. participated in study design and coordination, reviewed medical records, reviewed and edited the manuscript, and contributed to discussion. D.P.B., T.S.C., J.M.L., D.D., R.F.H., D.A.B., C.P., and S.H.S. reviewed and edited the manuscript and contributed to discussion. E.J.M.-D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipman TH, Levitt Katz LE, Ratcliffe SJ, et al. Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia Pediatric Diabetes Registry. Diabetes Care. 2013;36:1597–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith TL, Drum ML, Lipton RB. Incidence of childhood type I and non-type 1 diabetes mellitus in a diverse population: the Chicago Childhood Diabetes Registry, 1994 to 2003. J Pediatr Endocrinol Metab. 2007;20:1093–1107. [DOI] [PubMed] [Google Scholar]
- 4.Bobo N, Evert A, Gallivan J, et al. An update on type 2 diabetes in youth from the National Diabetes Education Program. Pediatrics. 2004;114:259–263. [DOI] [PubMed] [Google Scholar]
- 5.Writing Group for the SEARCH for Diabetes in Youth Study Group, Dabelea D, Bell RA, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence JM, Imperatore G, Dabelea D, et al. Trends in incidence of type 1 diabetes among non-Hispanic white youth in the U.S., 2002-2009. Diabetes. 2014;63:3938–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crume TL, Hamman RF, Isom S, et al. Factors influencing time to case ascertainment in youth with type 1 and type 2 diabetes in the SEARCH for Diabetes in Youth Study [Abstract]. Diabetes. 2013;62(Suppl 1): A412. [Google Scholar]
- 8.DesRoches CM, Charles D, Furukawa MF, et al. Adoption of electronic health records grows rapidly, but fewer than half of US hospitals had at least a basic system in 2012. Health.Aff. (Millwood) 2013;32:1478–1485. [DOI] [PubMed] [Google Scholar]
- 9.Klompas M, McVetta J, Lazarus R, et al. Integrating clinical practice and public health surveillance using electronic medical record systems. Am J Prev Med. 2012;42:S154–S162. [DOI] [PubMed] [Google Scholar]
- 10.Zhong VW, Pfaff ER, Beavers DP, et al. Use of administrative and electronic health record data for development of automated algorithms for childhood diabetes case ascertainment and type classification: the SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 2014;15:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence JM, Black MH, Zhang JL, et al. Validation of pediatric diabetes case identification approaches for diagnosed cases by using information in the electronic health records of a large integrated managed health care organization. Am J Epidemiol. 2014;179:27–38. [DOI] [PubMed] [Google Scholar]
- 12.Lemon SC, Roy J, Clark MA, et al. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–181. [DOI] [PubMed] [Google Scholar]
- 13.Therneau T, Atkinson E, Foundation M. An Introduction to Recursive Partitioning Using the RPART Routines. http://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf. Accessed June 26, 2015. [Google Scholar]
- 14.SEARCH Study Group. SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. [DOI] [PubMed] [Google Scholar]
- 15.German RR, Lee LM, Horan JM, et al. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50:1–35; quiz CE1-7. [PubMed] [Google Scholar]
- 16.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.r-project.org.libproxy.lib.unc.edu/. Accessed June 26, 2015. [Google Scholar]
- 17.Pettitt DJ, Talton J, Dabelea D, et al. Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care. 2014;37:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amed S, Dean HJ, Panagiotopoulos C, et al. Type 2 diabetes, medication-induced diabetes, and monogenic diabetes in Canadian children: a prospective national surveillance study. Diabetes Care. 2010;33:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dart AB, Martens PJ, Sellers EA, et al. Validation of a pediatric diabetes case definition using administrative health data in Manitoba, Canada. Diabetes Care. 2011;34:898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderloo SE, Johnson JA, Reimer K, et al. Validation of classification algorithms for childhood diabetes identified from administrative data. Pediatr.Diabetes 2012;13:229–234. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes ET, Laffel LM, Gonzalez TV, et al. Accuracy of administrative coding for type 2 diabetes in children, adolescents, and young adults. Diabetes Care. 2007;30:141–143. [DOI] [PubMed] [Google Scholar]
- 22.Moczygemba J, Fenton SH. Lessons learned from an ICD-10-CM clinical documentation pilot study. Perspect Health Inf Manag. 2012;9:1c. [PMC free article] [PubMed] [Google Scholar]
- 23.Reinehr T, Schober E, Wiegand S, et al. Beta-cell autoantibodies in children with type 2 diabetes mellitus: subgroup or misclassification? Arch Dis Child. 2006;91:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabelea D, Pihoker C, Talton JW, et al. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell RA, Mayer-Davis EJ, Beyer JW, et al. Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S102–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonarow GC, Adams KF, Jr, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. [DOI] [PubMed] [Google Scholar]
- 27.Schneider DF, Dobrowolsky A, Shakir IA, et al. Predicting acute kidney injury among burn patients in the 21st century: a classification and regression tree analysis. J Burn Care Res. 2012;33:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess KR, Abbruzzese MC, Lenzi R, et al. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5:3403–3410. [PubMed] [Google Scholar]