Abstract
The state of the art of research on the environmental physiology of marine fishes is reviewed from the perspective of how it can contribute to conservation of biodiversity and fishery resources. A major constraint to application of physiological knowledge for conservation of marine fishes is the limited knowledge base; international collaboration is needed to study the environmental physiology of a wider range of species. Multifactorial field and laboratory studies on biomarkers hold promise to relate ecophysiology directly to habitat quality and population status. The ‘Fry paradigm’ could have broad applications for conservation physiology research if it provides a universal mechanism to link physiological function with ecological performance and population dynamics of fishes, through effects of abiotic conditions on aerobic metabolic scope. The available data indicate, however, that the paradigm is not universal, so further research is required on a wide diversity of species. Fish physiologists should interact closely with researchers developing ecological models, in order to investigate how integrating physiological information improves confidence in projecting effects of global change; for example, with mechanistic models that define habitat suitability based upon potential for aerobic scope or outputs of a dynamic energy budget. One major challenge to upscaling from physiology of individuals to the level of species and communities is incorporating intraspecific variation, which could be a crucial component of species’ resilience to global change. Understanding what fishes do in the wild is also a challenge, but techniques of biotelemetry and biologging are providing novel information towards effective conservation. Overall, fish physiologists must strive to render research outputs more applicable to management and decision-making. There are various potential avenues for information flow, in the shorter term directly through biomarker studies and in the longer term by collaborating with modellers and fishery biologists.
Keywords: Biomarkers, ecological models, fisheries, Fry paradigm, individual variation, telemetry
Introduction
Marine ecosystems provide essential resources and services, with fishes being of prime socio-economic importance. There are alarming global trends of excessive exploitation and habitat degradation of marine fishes, with most commercial stocks either overfished or nearing capacity (Pauly et al., 2002; FAO, 2014; Pauly and Zeller, 2016). Global climate change is also altering patterns of marine biodiversity, with more pronounced effects expected in the future (Perry et al., 2005; IPCC, 2014; McNeil and Sasse, 2016). The consequences of over-exploitation, habitat degradation and global climate change are unknown, but there is legitimate concern about irreversible loss of fisheries resources and biodiversity, leading to reduced food security and even direct threats to ecosystem integrity (Perry et al., 2005; Cheung et al., 2009; Sumaila et al., 2011; Duarte, 2014; Elliott et al., 2015; Pauly and Zeller, 2016). There is a need, therefore, to improve the scientific knowledge base underpinning advice on the sustainable management of marine fish biodiversity and fisheries resources.
There is a growing belief that physiological information, understanding how animals function, can contribute significantly to the resolution of management and conservation problems for marine fishes and to the ability accurately to project potential impacts of environmental pressures (Wang and Overgaard, 2007; Pörtner and Farrell, 2008; Rijnsdorp et al., 2009; Wilson et al., 2009; Pörtner and Peck, 2010; Jørgensen et al., 2012; Seebacher and Franklin, 2012; Wilson, 2014). Physiologists typically take a Darwinian view (Fig. 1), whereby the abiotic factors within a given habitat can define which animals survive and reproduce there, based upon their physiology. Over the course of generations, there is natural selection of physiological adaptations to prevailing conditions (Prosser, 1950; Schmidt-Neilsen, 1982; Garland and Carter, 1994), with the evolution of a functional niche for each species (Hutchinson, 1957). In each new generation, the physiology of the individuals contributes to their performance, behaviour and fitness in a realized niche (Arnold, 1983; Garland and Carter, 1994; Feder et al., 2000; Huey et al., 2012). This influences the abundance and distribution of their population and species (Buckley et al., 2012; Huey et al., 2012; Chave, 2013; Heffernan et al., 2014) and, by logical extension, the composition of communities and assemblages in the ecosystem (Buckley et al., 2012; Chave, 2013; Cooke et al., 2014; Heffernan et al., 2014).
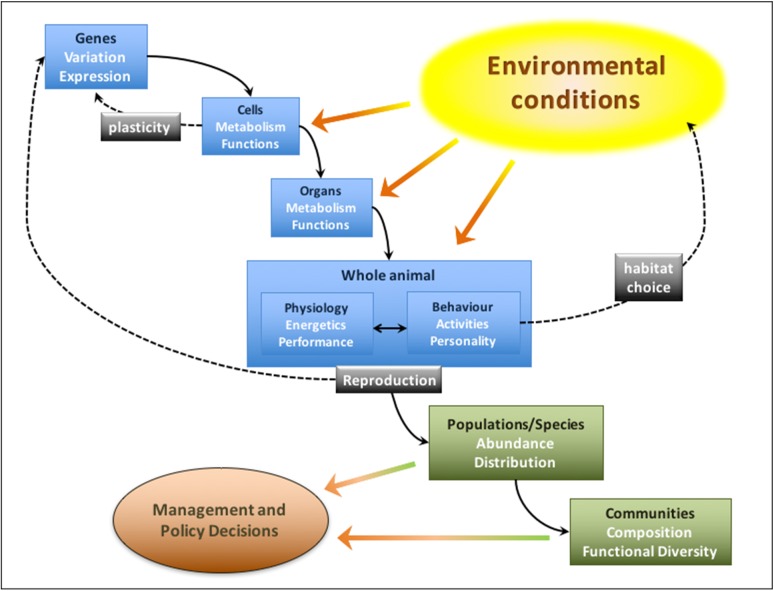
Figure 1:
How the physiology of an individual marine fish can influence species population dynamics and community biodiversity, through hierarchical levels of biological organization (inspired by Le Quesne and Pinnegar, 2012). Environmental conditions influence whole-animal physiology (energetics and performance) by influencing gene expression and the biochemistry and physiology (metabolism and function) of cells and organs. Physiology has a complex interplay with the behaviour (activities and personality) of the individual that, together, influence its ecological performance and reproductive output (fitness). This, then, influences the abundance and distribution of the species in the environment. This, in turn, influences the composition and functional diversity of communities and assemblages in the ecosystem. This is the scale at which management decisions are required and made. The dotted black lines show feedbacks. The effects of environmental conditions on cells feed back to influence gene expression, which then influences function by phenotypic plasticity/flexibility. Behavioural habitat choice feeds back to influence environmental conditions and, therefore, their effects on lower levels of organization. Reproduction each generation feeds back to influence genetic variation and drive the evolution of local adaptation in the species.
The vast majority of marine fishes are water-breathing ectotherms; therefore, physiological and behavioural responses to increasing temperatures, growing zones of hypoxia, ocean acidification, eutrophication and general habitat degradation are to be expected. This has obvious implications for conservation research because the prediction would be that, as environmental conditions change, so will the distribution of habitat that any given species chooses to, or is able to, occupy (Buckley et al., 2012; Huey et al., 2012; Le Quesne and Pinnegar, 2012; Cooke et al., 2014; Martin et al., 2015).
This overall premise was the impetus to establish a network of interested scientists, funded by the European Union's Cooperation in Science and Technology (COST) programme. The COST Action FA1004 ‘Conservation Physiology of Marine Fishes’ (2011–2015) provided a forum for dialogue on this topic, through a series of international conferences and workshops (http://fish-conservation.nu). This article reviews the main issues that were raised and discussed over COST FA1004’s lifetime, with general perspectives on a broad series of topics. These topics fall into four main themes: (i) the state of basic physiological knowledge for marine fishes and how this might be applied directly towards conservation goals; (ii) how physiological knowledge can be integrated into ecological models; (iii) how biotelemetry and biologging studies can contribute to conservation research; and (iv) how conservation physiology research might be rendered relevant and available to decision-makers. These perspectives set the stage for this Theme Section, which comprises reviews, perspectives and research articles that, together, provide an indication of the state of the art of thinking and research in the field.
The physiological knowledge base is limited
The restricted knowledge base is currently a major constraint to the use of physiology for conservation of marine fishes. Although there are >30 000 species of fishes, knowledge on marine fishes is confined to tens of species, which occur in countries with developed fish ecophysiology research communities. Within these countries, there is a focus on species that are economically or ecologically important and/or are relatively easy to obtain and maintain in captivity. These include temperate species, such as Atlantic cod (Gadus morhua), Atlantic salmon (Salmo salar), Dover sole (Solea solea), European sea bass (Dicentrarchus labrax), Pacific salmonids of the genus Oncorhynchus or turbot (Scopthalmus maximus), plus various tropical species from the Great Barrier Reef. Recent meta-analyses examining how the physiology of ectotherms might shape responses to global change all note the lack of physiological information on a vast majority of fish species and geographical areas (Sunday et al., 2012; Seebacher et al., 2015; Lefevre, 2016; Killen et al., 2016b).
It is essential that physiologists pursue cooperative research projects progressively to fill knowledge gaps (Cattano et al., 2016; Ferreira-Martins, 2016; di Santo et al., 2016), to investigate the ecophysiology of many more species, which may be harder to obtain and/or keep in captivity. Focus could be on key elements in food chains and species with specific or rare (Mouillot et al., 2013) ecological functions. Studies are needed to evaluate ranges for tolerance of major environmental factors, such as temperature, dissolved gases (hypoxia and hypercarbia), acidification and salinity, within which different marine fish species function effectively, and the thresholds beyond which performance is impaired and survival or reproduction is at risk.
Researchers must keep in mind the complexities inherent to the physiology of marine fishes. For example, the environmental physiology of populations can vary markedly across a species’ geographical range (Conover et al., 2006; Gardiner et al., 2010), and specific life stages can be critical bottlenecks for population or species persistence in the presence of ongoing global change (Petitgas et al., 2013; Ferreira-Martins, 2016). Physiological research on minute marine fish embryos and larvae is technically very challenging (Peck and Moyano, 2016), but these life stages may be the most sensitive to environmental stressors (Killen et al., 2007). This Theme Section has two research articles on the ecophysiology of early life stages, with a study of tolerance of little skate (Leucoraja erinacea) embryos to hypoxia (di Santo et al., 2016) and a study of tolerance of larvae of the ocellated wrasse (Symphodus ocellatus) to natural acidification at Mediterranean volcanic seeps (Cattano et al., 2016).
A database is currently being prepared for the public domain, as an output of Action FA1004. At present, it comprises effects of temperature on aerobic metabolic scope, digestive energetics (specific dynamic action) and growth, as well as an analysis of hypoxia tolerance, in marine and euryhaline fish species. In this Theme Section, Rogers et al. (2016) analyse the database of hypoxia tolerance, measured by respirometry as the critical oxygen partial pressure for regulation of aerobic metabolism (Pcrit). This revealed that Pcrit was, as expected, highly variable among species but was also influenced by temperature, CO2, acidification, toxic metals and feeding, as well as by the method used to measure it, especially if CO2 accumulated in the respirometry system. The database will provide an open repository for a progressive accumulation of physiological trait data, which can be used towards conservation objectives; for example, directly in terms of tolerance thresholds that can be ‘biomarkers’ of environmental stress or to parameterize ecological models, as described below.
Applications for physiological biomarkers
Biomarkers of environmental pressures hold promise for conservation research in marine fishes (Cooke and O'Connor, 2010); for example, to evaluate ecological quality of habitats within the context of the EU Water Framework Directive or to establish the ‘health’ of populations in particular habitats of interest. A prime example of an endocrine biomarker is plasma vitellogenin, which, in freshwater fishes, has been established as a key indicator of exposure to endocrine-disrupting chemicals (Sumpter and Jobling, 1995; Tyler et al., 1996).
If endocrine, cellular and molecular biomarkers are to be useful for conservation research, it is important to understand their limitations, which can include a lack of mechanistic basis for their interpretation, complicated response patterns in wild animals and unclear links to Darwinian fitness (Forbes et al., 2006; McKenzie et al., 2007; Dantzer et al., 2014). The most promising approaches are multifactorial and use a combination of indicators at different levels of biological organization. These allow relationships to be established among measures of functional integrity, such as condition factor or otolith growth rates, and the endocrine, cellular and molecular biomarkers. This, in turn, can then be related to differences in the biotic and abiotic quality of habitats (Cooke and Suski, 2008; Adams and Ham, 2011; Jeffrey et al., 2015; King et al., 2016; Madliger et al., 2016). In developing such suites of biomarkers for conservation research, the focus should be on reliable and user-friendly measures that combine field and experimental approaches and provide ecological relevance (Adams and Ham, 2011; Jeffrey et al., 2015; Madliger et al., 2016).
Revealing generalized ‘stress’ in natural populations can be very informative. The stress hormones, glucocorticoids, measured in feathers, hair, moulted skin or scat, are widely used in conservation physiology research in wild tetrapods (Dantzer et al., 2014). There is evidence that scales can be used in this manner in fishes (Aerts et al., 2015), which opens up this practice for conservation research. Cortisol can be measured in fish eggs as a biomarker of maternal stress levels; in tropical reef fishes, increased egg cortisol was linked to poor reproductive success and reduced offspring size (Gagliano and McCormick, 2009). A blood sample can also provide a wealth of biomarker information in fishes. A major problem with wild marine fishes is accounting for the acute stress of capture, but various biomarkers are presumably not sensitive to this, such as some oxidative stress indicators, stress proteins and the expression of stress-related genes in nucleated teleost red blood cells (Beaulieu and Costantini, 2014; Chadwick et al., 2015; Jeffrey et al., 2015; Madliger et al., 2016).
Simple measures of condition factor and energy reserves are informative physiological biomarkers of population health (Claireaux et al., 2004; Jeffrey et al., 2015) that, in this Theme Section, are applied as biomarkers for effects of parasitism in a small pelagic species, the European anchovy (Engraulis encrasicolus), in the northwest Mediterranean (Ferrer-Maza et al., 2016). Some studies have measured physiological indicators of whole-animal performance, such as swimming ability, or hypoxia and thermal tolerance, including measurements on fishes in mesocosms or caged at specific sites (Claireaux et al., 2004; McKenzie et al., 2007; Roze et al., 2013). A multifactorial approach has a number of obvious applications in evaluating impacts of major environmental stressors on marine fishes, and to predict the relative sensitivity of different species.
The critical thermal maximum is a physiological ‘biomarker’ of incipient lethal thermal tolerance (Lutterschmidt and Hutchison, 1997), which has been related to ecological phenomena caused by global change (Pörtner and Peck, 2010; Sunday et al., 2011, 2012). In natural populations, habitat warming and extreme thermal events can generate sublethal molecular biomarker responses, notably heat shock proteins. Some freshwater Arctic charr (Salvelinus alpinus) populations inhabit water bodies that, as a result of global change, now exceed the fish's seasonal thermal optimum. These populations exhibit constitutive heat shock protein and glucose stress responses (Chadwick et al., 2015). A history of exposure to extreme warming events can affect glucocorticoid responsiveness to acute stress in coral reef fishes (Mills et al., 2015), and the thermal regime during development and incubation may have marked influences on offspring (Zambonino-Infante et al., 2013; Moyano et al., 2016). There is an opportunity, therefore, to develop databases on lethal thresholds, evaluated as the critical thermal maximum, but also to investigate how evidence of sublethal thermal stress in populations of interest might relate to functional indicators, such as indicators of bioenergetic or nutritional status. Beyond direct management applications, this sort of information would also inform projections of species sensitivity to predicted patterns of global warming.
Loss of equilibrium during progressive hypoxia has been used as an indicator of incipient lethal hypoxic threshold (Anttila et al., 2013; Roze et al., 2013; Claireaux and Chabot, 2016), and the Pcrit (or O2crit) is a physiological ‘biomarker’ of sublethal hypoxia tolerance (Claireaux and Chabot, 2016; Rogers et al., 2016). Above the O2crit, fishes can show reduced aerobic scope, which can be linked to impairments to physiological performance and reduced appetite. Although these physiological effects of hypoxia may be understood mechanistically, evaluating the impact of environmental hypoxia upon fish ecology and evolution remains difficult in practice. Overcoming this challenge is becoming increasingly important in the face of growing marine hypoxic zones. The constitution of a database gathering key information about species’ oxygen requirements and susceptibility to reduced oxygen availability (Rogers et al., 2016) is an important first step. Once again, a multifactorial approach holds promise. Knowledge of hypoxia thresholds, based upon laboratory experiments, could be compared with biochemical, physiological, bioenergetic, nutritional and behavioural indicators in populations of interest, in order to gain insight into the ecological consequences of prevailing hypoxic stress.
Noise pollution can be a major environmental stress for marine fishes, which has been studied for its effects on their behaviour (Slabbekoorn et al., 2010; Simpson et al., 2016) and their physiology (Nichols et al., 2015; Sierra-Flores et al., 2015; Celi et al., 2016; Simpson et al., 2016). In experimental conditions, noise can cause physiological stress responses and upregulation of stress proteins (Nichols et al., 2015; Celi et al., 2016). Research in aquaculture has shown that Atlantic cod exposed to daily, low-level noise pollution during the spawning window accumulated cortisol in their eggs and had lower egg production and fertilization rates (Sierra-Flores et al., 2015). It seems evident, therefore, that further research should be performed to investigate responses to noise pollution in natural populations, applying a multifactorial approach (Adams and Ham, 2011; Jeffrey et al., 2015) that could also include maternal effects, such as accumulation of cortisol in eggs and the potential downstream effects on larvae. Research should distinguish between the type and intensity of noise, on a continuum from not detectable to chronic but allowing habituation to acute and damaging.
In Europe, reforms to the Common Fisheries Policy have made discard of unwanted bycatch an important policy issue, and one where physiological biomarkers clearly have useful applications. This is a very active area of research, so this is restricted here to some generalizations about management needs that are useful to highlight for anyone who is starting out, as follows: (i) characterize the relative sensitivity of different species relative to gear types, environmental conditions and handling procedures; (ii) predict mortality (and sublethal fitness impacts) of discarded fishes (Davis, 2010); and (iii) identify strategies for reducing stress, injury and mortality and improving welfare (Metcalfe, 2009). Bearing in mind that all captured fishes will experience some level of stress and injury, these effects are related and, therefore, may be difficult to disentangle. It is difficult to generalize across species and capture method, and impacts may vary seasonally and ontogenetically. Even fishes that escape capture may suffer impacts of some kind.
Is there a universal paradigm linking physiological function to ecological performance?
Understanding the physiological mechanisms that determine how marine fishes perform, in relationship to environmental conditions, should contribute to conservation activities by providing insights into current and future species abundance and distribution (Pörtner and Farrell, 2008; Pörtner, 2010; Jørgensen et al., 2012; Teal et al., 2015; Marras et al., 2015a). One major hypothesis to define how environmental conditions affect performance, with implicit consequences for population dynamics and habitat selection, focuses on the ability of fishes to increase their rate of oxygen uptake to meet the metabolic demands of essential activities. First formulated by F. E. J. Fry, hence called the ‘Fry paradigm’ (Fry, 1971, 1947; Priede, 1985; Kerr, 1990), it is based upon scope for aerobic activity (Fig. 2). Aerobic scope is the integrated capacity of the cardiovascular and respiratory systems to provide oxygen for essential activities beyond vital basal metabolic processes, i.e. activities such as locomotion (e.g. evading predators, foraging, social interactions, migration), digestion and somatic and gonadal growth (Fry, 1971; Priede, 1985; Claireaux and Lefrançois, 2007; Pörtner and Farrell, 2008; Schulte, 2015; Baktoft et al., 2016). Thus, the hypothesis provides a mechanistic link from the structural, biochemical and physiological components of metabolism to ecologically relevant performance measures. There is evidence that large-scale failures in upriver spawning migrations of adult Pacific salmon (Oncorhynchus species) may have occurred because abnormally high summer temperatures impaired swimming performance through reduced aerobic scope, which is one of the most prominent examples of conservation physiology research for fishes (Eliason et al., 2011; Patterson et al., 2016).
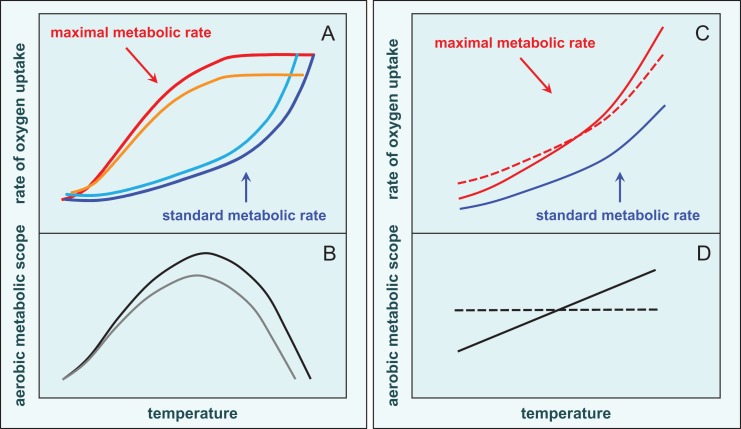
Figure 2:
(A) The Fry or oxygen- and capacity- limited thermal tolerance (OCLTT) paradigm, a conceptual model of how environmental factors influence aerobic metabolic scope (AS) of fishes (Fry, 1971; Pörtner, 2010). Acclimatization temperature, on the abscissa, is a factor that controls the rates of all metabolic processes in ectothermic marine fishes. The blue and red lines in (A) model how standard (minimal) metabolic rate (SMR) and maximal metabolic rate (MMR) vary as a function of temperature. The difference between SMR and MMR is AS, the capacity of the cardiorespiratory system to provide oxygen for all activities above maintenance, i.e. locomotion, digestion and tissue growth. The SMR, the cost of vital basal processes, increases exponentially with temperature, owing to direct acceleratory effects of heat. At low temperatures, MMR is low because of depressive effects of cold on biochemistry and physiology, including mitochondrial oxygen supply and ATP production. As a result, AS is small. As temperature rises, AS increases as biochemical and physiological rate capacity increase. At a certain temperature, however, MMR reaches an asymptote, attributable to intrinsic limitations to cardiorespiratory capacity. At temperatures above this, SMR rises inexorably until basic maintenance requires the entire cardiorespiratory capacity, so AS falls to zero. (B) The resultant relationship of AS with acclimatization temperature, with a clear bell-shaped performance curve and a temperature range where AS is maximal. The form of this curve is expected to be species (or even population) specific and to reflect evolutionary history. The other lines in (A) show effects of loading factors, such as stress (light blue), and limiting factors, such as hypoxia (orange), with the resultant reduction in AS shown in (B) in grey. Figure redrawn from McKenzie (2011). (C) The relationship between SMR, MMR and temperature that is shown by many species, with (D) showing the resultant relationship of AS with temperature (Lefevre et al., 2016). All fishes show a similar effect of temperature on SMR, but some show a parallel increase in MMR, such that scope is the same across all temperatures (dashed MMR and AS lines in C and D, respectively). Other species show a greater increase in MMR than in SMR, such that scope rises progressively with temperature (continuous MMR and AS lines in C and D, respectively).
An important potential strength of the Fry paradigm is that it can integrate effects of major environmental stressors (Fig. 2), notably hypoxia, ocean acidification and pollutants, because they can all constrain aerobic scope (Claireaux and Lagardère, 1999; Lefrançois and Claireaux, 2003; Claireaux and Lefrançois, 2007; Ishimatsu et al., 2008; Claireaux and Chabot, 2016). This focus on aerobic metabolism of fishes was the stimulus for a previous journal special issue by COST FA1004, which provided definitions and methods for measures of metabolic rate in fishes, and case studies illustrating the relevance of metabolic rate in management of fishing and environmental changes (Chabot et al., 2016).
The Fry paradigm was the basis for the more mechanistic oxygen- and capacity- limited thermal tolerance (OCLTT) hypothesis, which posits that the decline in aerobic scope outside an optimal temperature range is caused by an impaired capacity of mitochondria to use oxygen at low temperatures or of the cardiorespiratory system to supply oxygen at high temperatures (Pörtner and Knust, 2007; Pörtner and Farrell, 2008; Pörtner, 2010; Schulte, 2015). An influential study by Pörtner and Knust (2007) indicated a correlation across laboratory measurements of aerobic scope at various temperatures with thermal tolerance, growth rate and local abundance of a wild population of the eel pout (Zoarces viviparous). The Fry and OCLTT paradigms have attracted considerable research attention for fishes (Clark et al., 2013; Gräns et al., 2014; Norin et al., 2014; Wang et al., 2014; Brijs et al., 2015) and other ectotherms (Overgaard et al., 2012; Ern et al., 2014; Fobian et al., 2014; Verberk et al., 2016), especially to investigate the mechanistic tenets of the OCLTT hypothesis. In this Theme Section, Baktoft et al. (2016) found no phenotypic covariance between aerobic scope of European perch (Perca fluviatilis), as measured in the laboratory, and their spontaneous swimming activity in the wild, suggesting that other factors may override any links between scope and fish performance in routine ‘benign’ conditions (Killen et al., 2013). Careful laboratory studies on various fishes have failed to find evidence that aerobic scope declines when they are near their upper lethal temperature or that oxygen delivery is the factor defining tolerance of acute warming (Norin et al., 2014; Wang et al., 2014; Brijs et al., 2015). A study by Claësson et al. (2016) on European eel (Anguilla anguilla), in this Theme Section, reports that aerobic scope increases with acute warming, underpinned by increases in cardiac output, until temperatures that are almost lethal. Thus, the universality of the Fry and OCLTT paradigms has been questioned, and this remains an active debate (Clark et al., 2013; Schulte, 2015; Farrell, 2016).
Also in this Theme Section, Lefevre (2016) presents a comprehensive review and analysis of the effects of temperature and mild hypercarbia (reflecting projected increases in water CO2), alone and in combination, on aerobic scope in fishes and other marine ectotherms. This revealed more variation in the response of aerobic scope to elevated temperature and CO2 than would be predicted by the Fry and OCLTT paradigms. Although some species exhibited an aerobic performance curve that rose and then declined as a function of increasing temperature, a considerable number of species did not. Some exhibited no change or a decrease in aerobic scope, whereas many exhibited a constant increase, without any mortality, as they were warmed towards their lethal threshold (Fig. 2; Lefevre, 2016). The effects of elevated CO2 also varied among species, often being without effect or sometimes increasing aerobic scope. In cases where hypercarbia suppressed aerobic scope, high temperature sometimes had a synergistic effect, but a simple additive effect was the most common (Lefevre, 2016).
Overall, although it is intuitive that physiological energetics will be of ecological significance for aquatic ectotherms (Fry, 1947, 1971; Ware, 1982; Priede, 1985; Kerr, 1990; Jørgensen et al., 2012), it would also be unwise blindly to assume that the Fry and OCLTT paradigms hold for all marine fish species (Lefevre, 2016). The effects of temperature on aerobic scope may depend upon a species’ ecology and history of exposure to diurnal or seasonal temperature variations (Norin et al. 2014; Lefevre, 2016). A lasting impact of the paradigms, whatever might be learned about their universality, is that they have focused attention on how thermal performance curves can provide a mechanistic link between physiology and ecology for fishes (Schulte, 2015). In particular, they can be integrated into ecological models to provide insights for management and conservation.
Integrating physiology into ecological models
Models are now important tools for projecting the impact of global change on abundance and distribution of marine fishes. The ability to transfer knowledge of historical observations and make robust projections of future distributions is essential to provide sound advice for management decisions (Jørgensen et al., 2012; Cooke et al., 2014; Peck et al., 2016). This is an area where physiology is perceived to hold great promise for conservation research, through integration into mechanistically based models of habitat suitability, which should provide increased confidence in projections (Hollowed et al., 2011; Jørgensen et al., 2012, 2016; Teal et al., 2012, 2015; Cooke et al., 2013, 2014; Deutsch et al., 2015; Marras et al., 2015a; Peck et al., 2016).
Species distribution models are a common ecologically based approach, which use associations between aspects of habitat and known occurrences of species in order to define sets of conditions in which species are likely to occur (Ben Rais Lasram et al., 2010; Albouy et al., 2012, 2013). The correlative approach has contributed significantly to projections of the potential effects of climate change on marine fish distributions. Its practical advantages are simplicity and flexibility in data requirements, and the range of biotic/abiotic interactions that can be incorporated (Kearney and Porter, 2009). Such correlative approaches are not, however, underpinned by mechanistic causalities, which is a prerequisite for confident projections of species range shifts (Jørgensen et al., 2012; Teal et al., 2015).
Physiology-based models should be able to deal with these issues of extrapolation because the organismal response is measured in the laboratory in controlled environmental conditions. Furthermore, physiology-based models overcome the circularity of predicting species response to climate change using range filling of potential distributional areas (Teal et al., 2015; Peck et al., 2016). Models that incorporate physiology typically focus on energetics because of the intuitive link to ecological performance (Jørgensen et al., 2012). These models vary in the assumptions and structure of the physiology that is included, from the empirically driven Wisconsin school of bioenergetics modelling (Hewett and Johnson, 1987; Hanson et al., 1997) to dynamic energy budget (DEB) models that strive for a universal description of organismal energetics derived from first principles (Kooijman, 1993, 2010). That being said, the integration of physiology into models is an area where further research and input are vitally needed. There is a need for reliable knowledge about how fishes function, in order to ensure that ‘universal’ traits of energetics are valid and are correctly represented in model parameterizations. This is essential to improve confidence in predictions about effects of climate change (Brander, 2015; Peck et al., 2016). The debate surrounding the Fry and OCLTT paradigms’ performance curves has already been mentioned, and the physiological principles underlying some other influential model projections (Pauly, 1981; Cheung et al., 2011, 2012) have also been questioned (Brander, 2015).
Aerobic scope can be a useful physiological parameter for models that link individual energetics to processes at higher biological levels (Fig. 3), and which can incorporate interactions among stressors (Jørgensen et al., 2012). One approach has used aerobic scope to define habitat suitability, based on laboratory measurements of it as a function of acclimatization temperature in target species, coupled with oceanographic modelling. The model outputs include ‘metabolic maps’ (Del Raye and Weng, 2015; Deutsch et al., 2015; Marras et al., 2015a,b; see also Martin et al., 2015) based on the hypothesis that scope is an indicator of relative fitness potential (Fig. 4). Models based on aerobic scope can be useful in studies of invasive species, by projecting the relative performance of a native species and its competitor counterpart, thus estimating the ‘winners’ and ‘losers’ under climate-driven change for various locations and at different times (Marras et al., 2015a). Other applications could include studies on key predators or prey species, in order to evaluate possible effects of global change on trophic relationships and food webs.
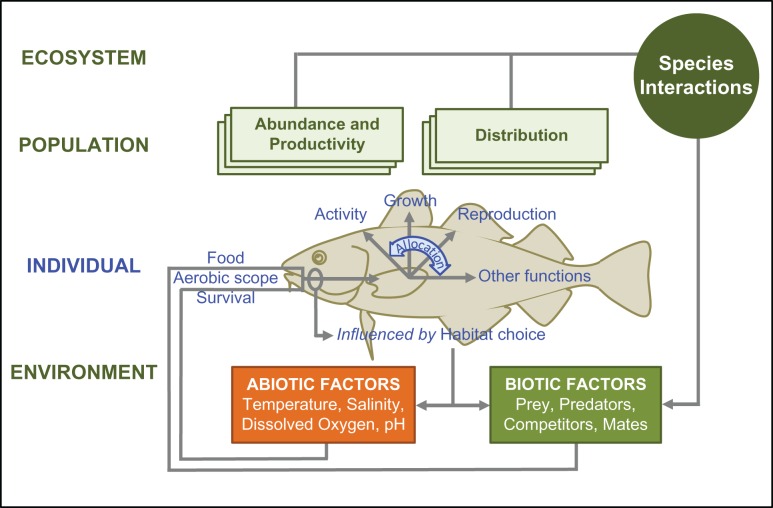
Figure 3:
A schematic diagram of how aerobic scope can be integrated into mechanistically based models. It can be used to form a performance curve with temperature, to describe habitat suitability (metabolic maps, e.g. Marras et al., 2015a) and it can be a constraint for oxygen allocation to competing activities in life-history models (Holt and Jørgensen, 2014, 2015). See Jørgensen et al. (2012) for more details.
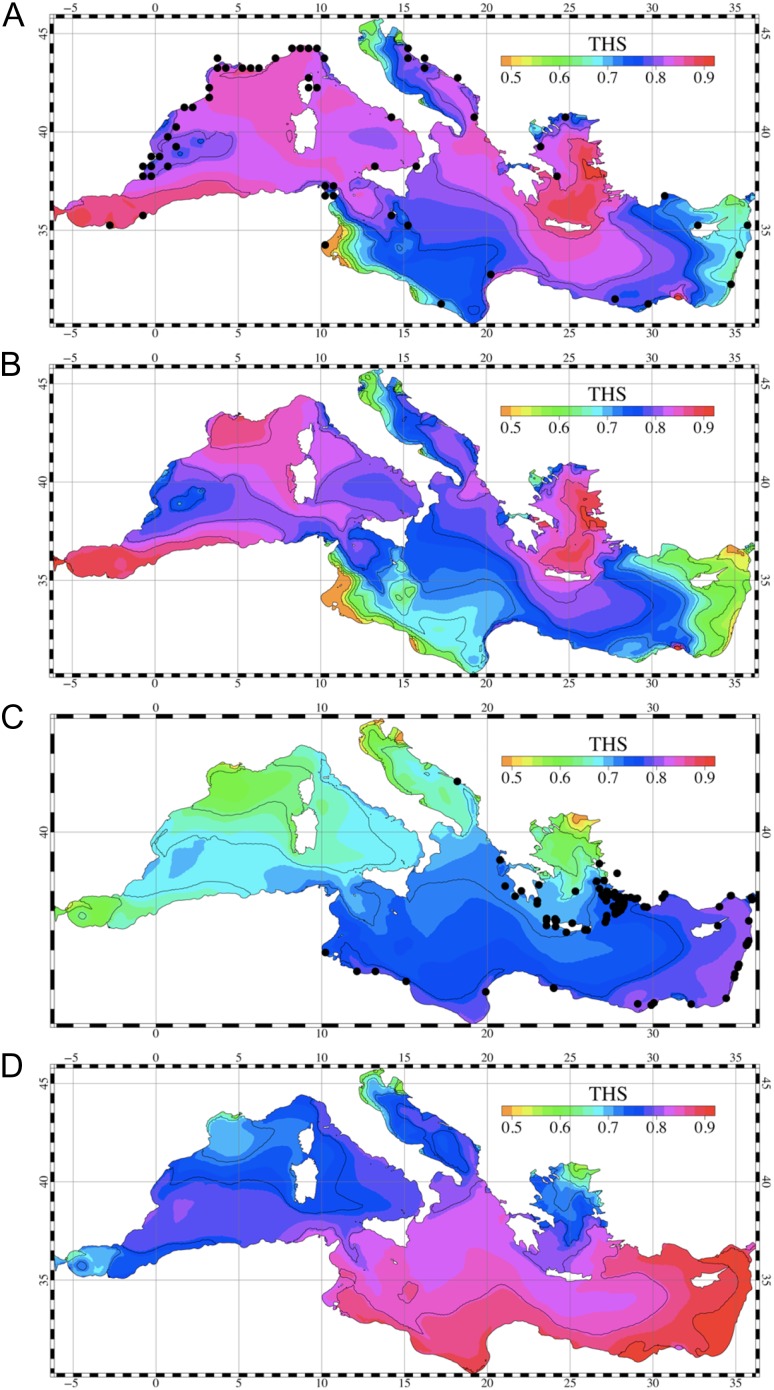
Figure 4:
An example of metabolic maps for two herbivorous fishes in the Mediterranean, developed by combining an aerobic scope performance curve with oceanographic data (for details, see Marras et al., 2015a). Thermal habitat suitability (THS) was computed for the whole Mediterranean Sea from the basin-scale model results. (A) Thermal habitat suitability of a native species, salema (Salpa salpa), based on present-day simulation results. (B) Thermal habitat suitability of the salema projected for a future scenario. (C) Thermal habitat suitability of an invasive lessepsien species, the marbled spinefoot (Siganus rivulatus), based on present-day simulation. (D) Thermal habitat suitability of the marbled spinefoot projected for the future scenario. Black dots represent the sites where the fish species have been observed.
There are also life-history models that incorporate aerobic scope as a constraint in life-history evolution, in order to explore its links to fitness (Holt and Jørgensen, 2014, 2015; Jørgensen et al., 2016). They integrate the physiology of oxygen uptake and use with foraging and digestion and with life-history traits, such as growth, survival and reproduction (Holt and Jørgensen, 2014, 2015). When these characteristics of an individual were optimized together in a model for Atlantic cod, simulations suggested that fitness would rapidly decline at high temperatures as a result of energy-budgeting conflicts (Holt and Jørgensen, 2014, 2015; Jørgensen et al., 2016), driven in part by increased food requirements (Johansen et al., 2015). These models are interesting because their projections appear to be relatively robust to the shape of the aerobic scope performance curve near the lethal limit, because fitness peaked at cooler temperatures (Holt and Jørgensen, 2014, 2015; Jørgensen et al., 2016). Thus, irrespective of any doubts about the universality of the Fry or OCLTT paradigms, these life-history models suggest that oxygen budgets may well define a main constraint for future projections of marine fishes under environmental change (Holt and Jørgensen, 2014, 2015; Johansen et al., 2014, 2016).
Dynamic energy budget theory has also been used as a mechanistic basis to model habitat suitability (Teal et al., 2012, 2016; Raab et al., 2013). The theory is grounded in the idea that metabolism is organized in the same way within all organisms, including fishes (Fig. 5). It derives from a number of assumptions that can describe empirical patterns, such as the van Bertlanffy growth curve or Kleiber's rule (for a list, see Sousa et al., 2008), which are consistent throughout the animal kingdom. The advantage is that the standard DEB model can be applied to all organisms and therefore all fish species, with each described by a set of species-specific parameters. Although parameterization requires empirical data, if data are lacking the model can still provide useful insights with data from related species for which more is known. The potential applications of the DEB-based models are similar to the aerobic scope models, but their particular value is that they provide outputs of growth and fecundity in relationship to environmental conditions, such as temperature or food availability. Dynamic energy budget modelling, in combination with ecosystem models that provide spatial and temporal data on environmental conditions, has been used to develop maps of optimal habitats for growth of marine flatfishes and to project these under climate-driven warming (Teal et al., 2012). The DEB theory can also be used to investigate effects on energetic pathways of other stressors, such as hypoxia, acidification or pollutants, if data are available.
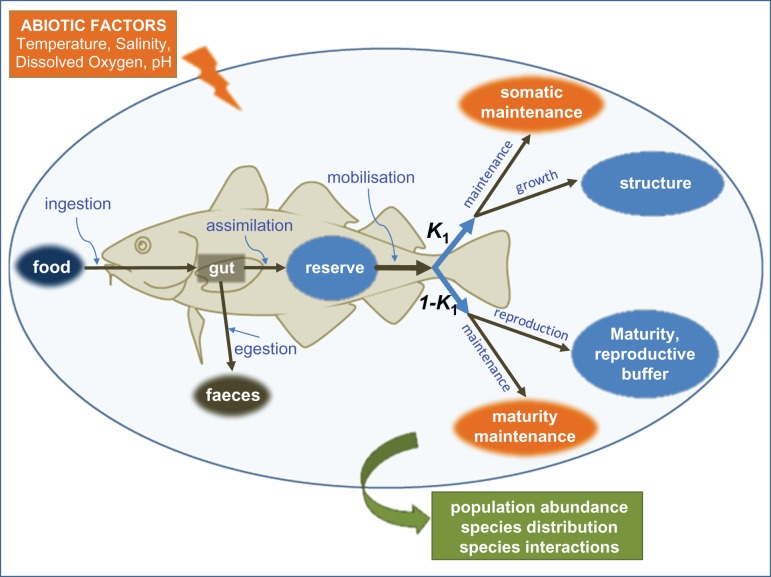
Figure 5:
Schematic representation of the standard dynamic energy budget (DEB) model showing the paths of energy flow through a fish (or any organism). Sources or sinks of energy are shown as green, brown and orange ovals; the blue ovals are the three state variables describing the organism. Processes affecting energy flows are indicated by black arrows. A defining feature of DEB models is the existence of reserves, from which allocation rules (proportion K1) define the partition of energy among processes such as maintenance (somatic or gonadal), somatic growth and reproduction. Dynamic energy budget models can be parameterized to account for effects of abiotic variables, and their universal principles allow for interspecific comparisons of parameter estimates. See Teal et al. (2012) for details of an application to evaluate and project marine fish habitat suitability.
Physiological models based on aerobic scope or DEB need to be integrated with physical ocean models and validated against population- and community-level data, so that they can achieve their promise. This would allow them to contribute to, for example, the Intergovenmental Panel on Climate Change (IPCC) predictions for effects of warming on global marine fisheries (Cheung et al., 2009, 2013; Sumaila et al., 2011). The ability to investigate how other processes, such as hypoxia, ocean acidification and trophic disruption, will interact with warming is now recognized as a research priority (Gunderson et al., 2016) and is a major strength of the models that incorporate aerobic scope or DEB. This has been highlighted as an area of great uncertainty in other physiology-based models (Brander, 2015). Once again, however, application of physiology in models requires more information on many more marine fish species. Parameterizing any mechanistically based model with valid physiological data could be a major undertaking, requiring significant long-term studies, use of facilities and personnel. International collaboration and funding are therefore required to coordinate development of laboratory and field measurements of physiology and physiologically based models.
Embedding physiological knowledge of species within models representing the spatial dynamics of marine food webs can provide concrete advice for fish conservation. This is especially true in light of the current emphasis on ecosystem-based fisheries and ecosystem management in Europe and elsewhere. Ecophysiologists and modellers can collaborate to create new tools, beyond well-established models, such as the Ecopath with Ecosim (Christensen and Walters, 2004) or species distribution models based on bioclimate envelopes (Peck et al., 2016), to enhance understanding of fishes and their responses to global change and to provide knowledge and tools to support adaptive management (Williams, 2011; Elliott et al., 2015; Queirós et al., 2016).
The significance of individual variation
A major challenge for conservation of biodiversity is to understand the capacity of species to acclimatize and, ultimately, to adapt genetically to ongoing global change (Seebacher and Franklin, 2012; Crozier and Hutchings, 2014; Seebacher et al., 2015). A core issue is to understand the different facets of intraspecific diversity, i.e. the differences among individuals within a population or species that are the substrate for evolution by natural selection. Of particular interest, from a conservation perspective, is the individual variation in physiological sensitivity to environmental conditions that exists within a given species, including how this may vary among populations across their geographical range, as a result of local adaptation. This variation needs to be understood in itself, as an indication of the potential resilience of a given population or species to environmental change and habitat modification. Ultimately, the goal is to understand how such variation links to life-history variation, to adaptation and evolution of the population or species, and so to underlying heritable genetic variation. Such associations are far from being understood in marine fishes, even for the most intensively studied species, such as Atlantic cod or Atlantic salmon.
The causes and consequences of individual variation in physiology are currently major areas of research, and there are many fundamental and ‘mechanistic’ physiological questions with conservation implications, such as the significance for an ecosystem approach to fisheries (Killen et al., 2015; Ward et al., 2016). Intraspecific diversity can have a genetic basis but it can also vary with life stages and sex and be affected by transgenerational maternal effects and early life experience (Gore and Burggren, 2012; Ho and Burggren, 2012; Miller et al., 2012; Zambonino-Infante et al., 2013). One area of individual physiological variation that is of major interest is ‘metabolic phenotypes’, meaning animals with different metabolic rates and aerobic scopes, and the ecological and evolutionary significance of this (Metcalfe et al., 2016b). An associated core issue, which transcends physiology, is to understand how major physiological, behavioural and life-history traits might co-vary, whether they might associate into syndromes, and how these might be maintained by ecological trade-offs (Réale et al., 2010; Careau and Garland, 2012; Killen et al., 2013). These various questions are far too broad and complex to be reviewed adequately here, but they are very poorly understood in marine fishes as a whole. A perspectives paper in this Theme Section considers these issues and, therefore, the reasons why individual variation should be taken into account in the ecosystem approach to fisheries (Ward et al., 2016).
Although physiological traits are often attributed ecological and evolutionary significance, there is a need to investigate trait repeatability in wild fish populations and whether the temporal stability of traits may be affected by changing environmental conditions. Temporal stability of physiological traits, plus a genetic component to the observed intraspecific variation, is a prerequisite for a trait to be a target for natural selection. This would influence the ability of species to evolve the trait in response to environmental conditions. Changing environments may erode or enhance trait repeatability, possibly changing which traits are under direct and correlated selection. At present, investigation of potential effects of climate change in marine fishes have primarily examined how warming or ocean acidification can influence population means for variables such as locomotory capacity, metabolism or behaviour (Seebacher et al., 2015; Lefevre, 2016; Nagelkerken and Munday, 2016). The current lack of information about how such environmental disturbances affect trait repeatability is a crucial gap that hinders the ability to predict how populations can cope through evolutionary responses. Ongoing advances in respirometry and biotelemetry/biologging, in particular, should increase understanding of trait repeatability in marine fishes and its response to changing environments. The repeatability of traits, and the extent to which this is context dependent, is the topic of a review by Killen et al. (2016a) in this Theme Section, with consideration of the implications for management and conservation of fish populations.
It is worthwhile to consider whether ecological models can incorporate individual variation, how these might be parameterized, and whether new models might be needed. Unstructured population models, such as the Lotka–Volterra competition or predator–prey model, assume that all individuals are equal. Age-structured models, such as the well-known Leslie matrix model (Caswell, 2000), take ontogenetic stage into account but ignore other potential sources of variation, assuming a constant and similar environment for all individuals and that animals of the same age remain exactly the same across time. Physiologically structured population models consider the potential for a variable environment to introduce variation, such that animals of a similar age may differ significantly, allowing for richer ecological interactions (de Roos, 1997; de Roos and Persson, 2013). The basic physiology and behaviour of each individual in these models is characterized by a parameter vector, which thus represents the genotype, and the vector of state variables represents the phenotype. The DEB model of the individual can serve as a building block for these physiologically structured models, with an example provided by van der Meer (2016) in this Theme Section. All individuals usually have the same parameter vector and thus the same genetic constitution in physiologically structured population models (Kooi and van der Meer, 2010). Studies to investigate the evolutionary stability of populations require genetic variability. Adaptive dynamics models (Dieckmann and Law, 1996), for example, aim to find a population where mutants (with a slightly different vector to residents) can no longer invade (Metz et al., 1992). The approach normally assumes clonal reproduction, but sexual reproduction can be incorporated, such that diversity among sexes can be considered. In other models (e.g. Giske et al., 2014), the genotype is explicitly modelled, which allows for emergent genetic variation and coexistence of different genotypes and phenotypes (e.g. behavioural strategies).
Many current population and community models ignore all potential sources of individual variation. Given that there is a huge difference between an Atlantic cod larva and full-grown adult, and a huge difference in growth rate between a well-fed or starved cod, ontogenetic stage and environmental history should, at least, be incorporated into population models. Beyond that, certain phenomena may not be understood and may not be predicted well if such variation is not considered (Ward et al., 2016). Furthermore, the variation may itself be of interest and provide insights into the underlying biological processes that have produced and maintained it. Given that parameterizing models to account for the various different potential facets of individual variation is a major long-term undertaking, in terms of facilities and personnel, pragmatic alternatives are worthy of careful investigation. One such alternative is pattern-oriented modelling (Grimm et al., 2005), which focuses on empirically quantifying processes and key trade-offs that cause or constrain variation, then using a model to predict individual variation and compare it with observed variation.
Understanding what fishes do in nature
It remains a central problem to relate the physiology of fishes, measured in the laboratory, to the habitats and conditions they experience (and will select) in their natural environment. There are immense technical difficulties in following fishes in the vast three-dimensional marine realm, let alone in measuring physiological variables or estimating their physiological state and whether they occupy habitats that optimize some element of their physiology (Freitas et al., 2016).
Active and passive acoustic tracking is already widely used in marine fishes, in order to follow them and estimate variables such as swimming speed and distance, plus two- and three-dimensional positioning. The acoustic signal carries over only relatively limited spatial scales, but the ongoing development of networks of acoustic receivers along coastlines, such as the Ocean Tracking Network (http://oceantrackingnetwork.org/research/canadian-projects/), will provide extremely valuable information about, for example, habitat use or migration patterns of marine fishes, that has major applications for conservation research and policy. Rapidly evolving techniques of measurement of physiological variables from free-living animals, including fishes, have been suggested to provide ‘answers to questions that we did not know we should ask’.
The first biotelemetric measurement was probably performed by Marey (1896). Biotelemetry and biologging (Fig. 6) are now starting to provide information on the physiology of animals in the field. Together with tracking data, they are providing a better picture of the life cycles of some economically important species, plus information about the structure of their populations (Metcalfe, 2006; Rutz and Hays, 2009; Block et al., 2011; Metcalfe et al., 2012; Whitlock et al., 2015). These tools can record an animal's physiology while simultaneously recording environmental conditions around it, in order to investigate assumptions based on laboratory experiments. For example, some acoustic telemetry tags can measure oxygen content in the water surrounding a fish and transmit this in real time (Svendsen et al., 2006). There are emerging techniques to collect physiological information on free-swimming fishes, which can then be used to estimate energetics as a function of prevailing environmental conditions (Gräns et al., 2010; Wright et al., 2014; Metcalfe et al., 2016a). Third-generation biotelemetry systems are being developed for simultaneous measurement of multiple physiological variables; for example, blood flow, blood pressure, electrocardiograms, electromyograms, three-dimensional acceleration and temperature. These can have a bidirectional radio frequency link that allows the implant to send data and accept commands to perform tasks. The signal from the implant can be viewed online, with a transmission range of ~10 m in air. This is, however, reduced in water, especially sea water, where alternative strategies are required, such as acoustic signalling or biologging.
Figure 6:

An Atlantic cod (Gadus morhua) carrying a data storage tag that records pressure, temperature and salinity. Photograph: Stefan Neuenfeldt, DTU Aqua.
Biologging, where the physiological data are stored in the tag/implant and then recovered, can be used on fishes released into open water (Fig. 6). Recovery of biologging tags remains a constraint, in particular for species that are not fished commercially or are under a fishing moratorium. A low recovery rate can make this method very costly, not only for the initial investment in tags but for the effort to implant them. Biologgers can collect and store both physiological variables (e.g. electrocardiogram, acceleration) and environmental parameters (e.g. pressure, temperature) that can be used to reconstruct migration pathways (Metcalfe and Arnold, 1997; Hunter et al., 2004), link behaviour to environmental conditions (Righton et al., 2001, 2010; Sims et al., 2003), characterize population structure (Metcalfe, 2006) and estimate energetic costs of different behaviours or interactions with humans (reviewed by Cooke et al. 2016). Pop-up tags that store data are also now widely used in studies on marine fishes, with the major advantage that data can be recovered via satellite. There are limitations to the size of fish that can carry the tags, and the tags can be expensive. Thus, most research has been on large and economically valuable pelagic or demersal fishes where, however, pop-ups have provided valuable knowledge for management and conservation (e.g. Block et al., 2011; Whitlock et al., 2015). In most cases, these tags are not ‘biologgers’; they store data only on environmental parameters, such as temperature and pressure.
An exciting development is the application of three-axis accelerometer tags (both in telemetry and biotelemetry/logger platforms) to monitor energy expenditure (Metcalfe et al., 2016a), activity and state. Movement is one of the four main bodily functions that incur energetic costs in animals. The energy expenditure is governed by muscle contractions and is typified by variable acceleration of the body (Gleiss et al., 2010), so records of the tri-axial acceleration of fishes should provide a useful proxy for activity-specific energy costs. Recent studies have correlated dynamic tri-axial body acceleration with rates of oxygen uptake in various aquatic species, including hammerhead sharks (Sphyrna lewini; Gleiss et al. 2010) and European sea bass (Dicentrarchrus labrax; Wright et al., 2014). Bi-axial and tri-axial acceleration, root mean square acceleration and acoustically transmitted acceleration data have also provided some exciting insights into fish behaviour and physiology (Clark et al., 2010; Wilson et al., 2013; Marras et al., 2015b). High-frequency accelerometry can be used to distinguish among various behaviours, such as feeding strikes and anti-predator escapes (Broell et al., 2013).
There are potential applications for biotelemetry, especially biologging, that can be highlighted for marine fish conservation research (Metcalfe et al., 2012). Tracking can improve our understanding of seasonal movements and space use and would be invaluable for evaluating the design and effectiveness of marine protected areas and to identify potential spawning aggregations. The addition of measurements of pressure, temperature and acceleration to the tracking devices can enable investigation of ontogenetic changes in the behaviour of pelagic species and evaluation of options for selective fishing strategies. Biotelemetric/logging data can also be combined with other assays, in particular of blood chemistry, to determine post-release survival of bycaptured (or sport-fished) animals. Environmental data collected by tracking or biotelemetric/logger tags can be used to define behavioural thresholds for critical habitat parameters, such as temperature (Neat and Righton, 2007; Righton et al., 2010), oxygen concentration (Prince et al., 2010) and salinity. This remains, therefore, a very exciting area of research and technological development.
Making physiology relevant to decision-making
The value of mechanistic physiological information is currently not widely appreciated by resource managers and policymakers, not least because physiologists have not made a consistent effort to promote their science in this regard (Cooke and O'Connor, 2010; Horodysky et al., 2015, 2016; Patterson et al., 2016). There is a real opportunity to develop fish environmental physiology as a discipline, by contributing to conservation research (Cooke et al., 2013; Madliger et al., 2016). Physiology can reveal mechanisms, which can be used to explain ecological patterns, which may then support evidence-based predictions and management decisions (Cooke et al., 2013; Madliger et al., 2016). Physiological tools and knowledge have already contributed to conservation goals for marine fishes; for example, to the management of migrating Pacific salmon, to improving survival from bycatch in specific fisheries, or to reducing the impact of tourism on some natural fish populations (Madliger et al., 2016; Patterson et al., 2016). Physiology should also be able to inform policy decisions about the following: limiting mortalities from discards from many further fisheries; the design of marine protected zones; adaptation to global change; predicting potential for invasive species; and many other things.
In this Theme Section, Horodysky et al. (2016) and Patterson et al. (2016) provide thoughtful analyses of how physiological research and the research process relate to the needs of resource managers and their decision-making process. Physiology must contribute to a broader toolbox or conceptual framework within which policy operates. Although mechanistic insight can be very useful for managers and physiology can provide a component of this, it cannot be the only source of information; it must be considered alongside genetics, behavioural ecology, trophic webs, physical oceanography, and so on (Horodysky et al., 2015, 2016). Patterson et al. (2016) synthesize the reasons why physiological research on sockeye salmon (Oncorhynchus nerka) migration contributed successfully to management decisions in British Columbia (Canada). A main driver was an existing political motivation, based on observations of reduced salmon runs that seemed linked to rising river temperatures, which then funded targeted research; that is, there was a direct connection between a management problem and funding of physiologically based solutions. The collaboration was then successful because of accountability, legal clarity, effective institutional environments, good personal relationships and peer acceptance. Interactions between researchers and stake-holders were crucial, so that the people most affected by decisions were familiar with the research and so that personal relationships improved overall trust. Patterson et al. (2016) urge researchers to be aware of the need to provide confident predictions regarding future outcomes, which are tailored to specific management objectives; in particular, to be able to quantify uncertainty to the level desired by managers or other knowledge practitioners.
Fish physiologists generally lack contact with key policymakers and do not have direct information channels to attune and balance their research with policy decisions. Thus, a pervasive challenge is to integrate with other disciplines and scale up from physiology to decision-making. Fishery biologists, by the nature of their work, do have direct contacts and therefore represent an important link to policy for physiologists. Figure 7 is a flow diagram of how physiological research can inform management and policy decisions, and the feedbacks to research that can be used for adaptive management strategies (Williams, 2011). Monitoring of biomarkers, at immediate time scales, can provide advice for specific local conservation decisions; for example, to assess ecological status of coastal zones or for early warning of impacts of global change. This can feed back to elicit more focused biomarker research and monitoring at the local scale, but also feed forward to national and international monitoring; for example, in the context of the EU Water Framework Directive. As mentioned, however, another route to influence policy is through increased interactions with fishery biologists. Information about biomarkers of survival after discard can be provided to fishery biologists, in support of advice they might provide at a national level (Fig. 7). Information from field and laboratory physiology studies can populate databases, in order to then parameterize models to project ecological consequences at the level of populations, species and assemblages. These can influence wider-scale policy decisions, over longer time scales (Cooke et al., 2014). This flow of information may reveal knowledge gaps and longer-term policy priorities that, in turn, can feed back to drive more research; for example, in large international collaborative projects.
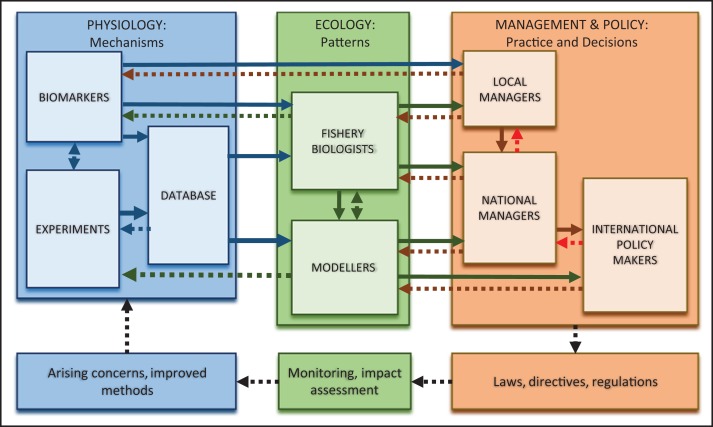
Figure 7:
Flow diagram of how physiological information can inform management and/or policy decisions (continuous lines) for marine fishes, and how analysis of the information can be fed back to develop targeted research activities (dotted lines). Red dotted arrows show flow of policy decisions (in the European Union). Biomarker information can be used directly for local management (in particular, early warning and evaluation of ecological status). Physiological information can also influence national and international management/policy indirectly, by interactions with ecologists; for example, biomarkers of bycatch survival to inform fishery biologists, or physiological databases for use in modelling of population dynamics or effects of global change. The number of dotted lines feeding back to physiology reveal the many contributions that physiological research could make to adaptive management programmes, including large-scale and long-term research in response to, for example, EU or Intergovenmental Panel on Climate Change recommendations.
It is worthwhile to consider how detailed knowledge of the physiology, ecology and life history of marine fishes might be distilled for easy application in wide-scale decision-making on sensitivity of communities and assemblages to environmental change. Biological traits analysis has often been applied to assess the impact of environmental change on terrestrial and freshwater communities but, so far, has relatively few applications to marine fishes (Elleouet et al., 2014). Biological traits analysis holds promise because physiological, ecological and life-history trait data exist for many marine fish species, and an analysis of available information is the first step towards constructing a trait-based index of climate change sensitivity, in order to identify which aspects would be needed to develop the index as a tool. This would then be assessed against existing community-level data from observational studies, at sites that have been subject to recent and documented environmental change. Ideally, in the future, such functional sensitivity indices could be used to construct simple models of species extinctions and predict the likely impact of climate change on biodiversity and community function.
Initial attempts at bridging gaps among marine fish physiologists, ecologists, modellers and policymakers have been made, which bear reporting here. Roundtable discussions at a conference, funded by Action FA1004, were aimed at understanding some of the barriers to knowledge exchange between physiological and advisory processes, how to refine policy-management issues so they can be reflected better in conservation research, and whether fish physiologists have sufficiently considered the impact of their research on stakeholder and policy advice. A diversity of views was expressed, and the discussions are best summarized as the following general themes.
The need for commonality of language. Dialogue is needed to achieve common understanding among physiologists, modellers and policy advisors. The simplest of terms can have a different meaning among scientists from different fields, and among stakeholders. This needs to be overcome without diminishing the autonomy of the various disciplines. A glossary of common terms, linked to the database of physiological information, would be useful.
Temporal scales are often different for research and policy. Physiology may not be able to provide rapid advice to support a pressing policy decision. Fishery discards are a prime example, where policy changes resulted from societal pressure and not scientific understanding. Robust scientific underpinning would have required detailed and complex studies, achieved too slowly for policymakers. Such policy can, of course, then fund post hoc research to investigate physiological impacts of discarding and the likelihood of surviving it for the relevant species. A troubling example of temporal asynchrony is the lack of immediate concrete policy responses to evidence of profound effects on marine ecosystems of gradual ongoing climate change (Elliott et al., 2015).
Don't give me the details, just the summary. Physiologists are interested in mechanistic detail, the responses of individuals and populations to changes in environmental conditions, typically of one particular model species. Such details often, however, run counter to effective stakeholder engagement and/or the advice needed for policy, which require synthesis of information into tangible effects. For example, the implications for fishing and fishermen, for future scenarios on fishing areas and species (in the context of climate change) or for the number and size of marine protected areas. Physiologists must learn to present their information in a holistic and understandable manner, including presentation to others in the scientific community, such as ecologists and modellers, who could translate, interpret and summarize the consequences for policy advice.
Physiologists need to champion their cause. Physiologists must recognize that their research has impact, particularly through interaction with other related disciplines. It is not sufficient for physiologists to provide the data to parameterize models, then to dissociate themselves from the modelling outcomes. They must contribute to interpretation of results, in order to influence policy decisions. Physiologists need to understand the realms of policy work and policy decisions better, and the linkages from physiology to ecology then policy, in order to influence outcomes through co-production of knowledge and transdisciplinary research.
Conclusions
There is much potential for physiological research to contribute to conservation of marine fish biodiversity and fisheries, which strengthens fish environmental physiology as a discipline. There is a clear need to increase the overall knowledge base about marine fish environmental physiology, especially tolerance thresholds for major environmental stressors and how such stressors affect performance within their tolerated range. Physiologists should explore avenues for international collaborative research, in order to avoid duplication of effort and cover as broad a range of species as possible. A particular application of such data would be to improve the reliability of models in order to gain a better understanding of what defines current fish distribution and abundance and, therefore, to increase confidence in projections of the effects of ongoing global change. Increased interaction with researchers using other tools, notably fishery biologists, ecologists and modellers, will provide a very fruitful avenue to increase the scope and impact of marine fish conservation physiology research and to make such research relevant to policy decisions.
Acknowledgements
The authors are grateful to the Society for Experimental Biology and Oxford University Press for publishing the Theme Section and for waiving publication fees. They are also grateful to the COST Office Scientific Officer of Action FA1004, Dr Ioanna Stavridou, to the Action Financial Officer, Dr Susana Moreira, and to the Action Grant Holder, CIIMAR Porto. The COST Action FA1004 Final Conference and associated workshop on linking marine fish conservation physiology to policy was supported in part by the Région Languedoc Roussillon and by the Centre for Marine Biodiversity Exploitation and Conservation (UMR9190 Marbec) in Montpellier, France.
Funding
This publication arose from conferences and workshops funded by EU COST Action FA1004 Conservation Physiology of Marine Fishes.
References
- Adams SM, Ham KD (2011) Application of biochemical and physiological indicators for assessing recovery of fish populations in a disturbed stream. Environ Manag 47: 1047–1063. [DOI] [PubMed] [Google Scholar]
- Aerts J, Metz JR, Ampe B, Decostere A, Flik G, De Saeger S (2015) Scales tell a story on the stress history of a fish. PLoS One 10: e0123411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy C, Guilhaumon F, Araújo MB, Mouillot D, Leprieur F (2012) Combining projected changes in species richness and composition reveals climate change impacts on coastal Mediterranean fish assemblages. Glob Chang Biol 18: 2995–3003. [DOI] [PubMed] [Google Scholar]
- Albouy C, Guilhaumon F, Leprieur F, Ben Rais Lasram F, Somot S, Aznar R, Velez L, Le Loc'h F, Mouillot D (2013) Projected climate change and the changing biogeography of coastal Mediterranean fishes. J Biogeogr 40: 534–547. [Google Scholar]
- Anttila K, Dhillon RS, Boulding EG, Farrell AP, Glebe BD, Elliott JAK, Wolters WR, Schulte PM (2013) Variation in temperature tolerance among families of Atlantic salmon (Salmo salar) is associated with hypoxia tolerance, ventricle size and myoglobin level. J Exp Biol 216: 1183–1190. [DOI] [PubMed] [Google Scholar]
- Arnold SJ. (1983) Morphology, performance and fitness. Am Zool 23: 347–361. [Google Scholar]
- Baktoft H, Jacobsen L, Skov C, Koed A, Jepsen N, Berg S, Boel M, Aarestrup K, Svendsen JC (2016) Phenotypic variation in metabolism and morphology correlating with animal swimming activity in the wild: relevance for the OCLTT (oxygen- and capacity-limitation of thermal tolerance), allocation and performance models. Conserv Physiol 4: cov055; doi:10.1093/conphys/cov055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu M, Costantini D (2014) Biomarkers of oxidative status : missing tools in conservation physiology. Conserv Physiol 2: cou014; doi:10.1093/conphys/cou014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Rais Lasram F, Guilhaumon F, Albouy C, Somot S, Thuiller W, Mouillot D (2010) The Mediterranean Sea as a ‘cul-de-sac’ for endemic fishes facing climate change. Glob Chang Biol 16: 3233–3245. [Google Scholar]
- Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, Bograd SJ, Hazen EL, Foley DG, Breed GA, Harrison A-L et al. (2011) Tracking apex marine predator movements in a dynamic ocean. Nature 475: 86–90. [DOI] [PubMed] [Google Scholar]
- Brander K. (2015) Improving the reliability of fishery predictions under climate change. Curr Clim Chang Rep 1: 40–48. [Google Scholar]
- Brijs J, Jutfelt F, Clark TD, Gräns A, Ekström A, Sandblom E (2015) Experimental manipulations of tissue oxygen supply do not affect warming tolerance of European perch. J Exp Biol 218: 2448–2454. [DOI] [PubMed] [Google Scholar]
- Broell F, Noda T, Wright S, Domenici P, Steffensen JF, Auclair J-P, Taggart CT (2013) Accelerometer tags: detecting and identifying activities in fish and the effect of sampling frequency. J Exp Biol 216: 1255–1264. [DOI] [PubMed] [Google Scholar]
- Buckley LB, Hurlbert AH, Jetz W (2012) Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob Ecol Biogeogr 21: 873–885. [Google Scholar]
- Careau V, Garland T (2012) Performance, personality, and energetics: correlation, causation, and mechanism. Physiol Biochem Zool 85: 543–571. [DOI] [PubMed] [Google Scholar]
- Caswell H. (2000) Prospective and retrospective perturbation analyses: their roles in conservation biology. Ecology 81: 619–627. [Google Scholar]
- Cattano C, Giomi F, Milazzo M (2016) Effects of ocean acidification on embryonic respiration and development of a temperate wrasse living along a natural CO2 gradient. Conserv Physiol 4: cov073; doi:10.1093/conphys/cov073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celi M, Filiciotto F, Maricchiolo G, Genovese L, Quinci EM, Maccarrone V, Mazzola S, Vazzana M, Buscaino G (2016) Vessel noise pollution as a human threat to fish: assessment of the stress response in gilthead sea bream (Sparus aurata, Linnaeus 1758). Fish Physiol Biochem 42: 631–641. [DOI] [PubMed] [Google Scholar]
- Chabot D, McKenzie DJ, Craig JF (2016) Metabolic rate in fishes: definitions, methods and significance for conservation physiology. J Fish Biol 88: 1–9. [DOI] [PubMed] [Google Scholar]
- Chadwick JG, Nislow KH, McCormick SD (2015) Thermal onset of cellular and endocrine stress responses correspond to ecological limits in brook trout, an iconic cold-water fish. Conserv Physiol 3: cov017; doi:10.1093/conphys/cov017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chave J. (2013) The problem of pattern and scale in ecology: what have we learned in 20 years. Ecol Lett 16: 4–16. [DOI] [PubMed] [Google Scholar]
- 3.Cheung WWL, Lam VWY, Sarmiento JL, Kearney K, Watson R, Pauly D (2009) Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish 10: 235–251. [Google Scholar]
- Cheung WWL, Dunne J, Sarmiento JL, Pauly D (2011) Integrating ecophysiology and plankton dynamics into projected maximum fisheries catch potential under climate change in the Northeast Atlantic. ICES J Mar Sci 68: 1008–1018. [Google Scholar]
- Cheung WWL, Sarmiento JL, Dunne J, Frölicher TL, Lam VWY, Deng Palomares ML, Watson R, Pauly D (2012) Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat Clim Chang 2: 1–5. [Google Scholar]
- Cheung WWL, Pauly D, Sarmiento JL (2013) How to make progresss in projecting climate change impacts. ICES J Mar Sci 70: 1069–1074. [Google Scholar]
- Christensen V, Walters CJ (2004)Ecopath with Ecosim: methods, capabilities and limitations. Ecol Model 172: 109–139. [Google Scholar]
- Claësson D, Wang T, Malte H. (2016. ) Maximal oxygen consumption increases with temperature in the European eel (Anguilla anguilla) through increased heart rate and arteriovenous extraction. Conserv Physiol 4 doi:10.1093/conphys/cow027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claireaux G, Chabot D (2016) Responses by fishes to environmental hypoxia: integration through Fry's concept of aerobic metabolic scope. J Fish Biol 88: 232–251. [DOI] [PubMed] [Google Scholar]
- Claireaux G, Lagardère JP (1999) Influence of temperature, oxygen and salinity on the metabolism of the European sea bass. J Sea Res 42: 157–168. [Google Scholar]
- Claireaux G, Lefrançois C (2007) Linking environmental variability and fish performance: integration through the concept of scope for activity. Philos Trans R Soc Lond B Biol Sci 362: 2031–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claireaux G, Désaunay Y, Akcha F, Aupérin B, Bocquené G, Budzinski H, Cravedi J-P, Davoodi F, Galois R, Gilliers C et al. (2004) Influence of oil exposure on the physiology and ecology of the common sole Solea solea: experimental and field approaches. Aquat Living Resour 17: 335–351. [Google Scholar]
- Clark TD, Sandblom E, Hinch SG, Patterson DA, Frappell PB, Farrell AP (2010) Simultaneous biologging of heart rate and acceleration, and their relationships with energy expenditure in free-swimming sockeye salmon (Oncorhynchus nerka). J Comp Physiol B Biochem Syst Environ Physiol 180: 673–684. [DOI] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216: 2771–2782. [DOI] [PubMed] [Google Scholar]
- Conover DO, Clarke LM, Munch SB, Wagner GN (2006) Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. J Fish Biol 69: 21–47. [Google Scholar]
- Cooke SJ, O'Connor CM (2010) Making conservation physiology relevant to policy makers and conservation practitioners. Conserv Lett 3: 159–166. [Google Scholar]
- Cooke SJ, Suski CD (2008) Ecological restoration and physiology : an overdue integration. Bioscience 58: 957–968. [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: cot001;doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Killen SS, Metcalfe JD, McKenzie DJ, Mouillot D, Jørgensen C, Peck MA (2014) Conservation physiology across scales : insights from the marine realm. Conserv Physiol 2: cou024; doi:10.1093/conphys/cou024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Brownscombe JW, Raby GD, Broell F, Hinch SG, Clark TD, Semmens JM (2016). Remote bioenergetics measurements in wild fish: opportunities and challenges. Comp Biochem Physiol A Mol Integr Physiol. In press. doi: 10.1016/j.cbpa.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Crozier LG, Hutchings JA (2014) Plastic and evolutionary responses to climate change in fish. Evol Appl 7: 68–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ (2014) Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species. Conserv Physiol 2: cou023; doi:10.1093/conphys/cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MW. (2010) Fish stress and mortality can be predicted using reflex impairment. Fish Fish 11: 1–11. [Google Scholar]
- De Roos AM. (1997) A gentle introduction to physiologically structured population models In: Structured-population Models in Marine, Terrestrial, and Freshwater Systems (Tuljapurkar S. & Caswell H., eds), pp. 119–204. New York, USA: Chapman & Hall. [Google Scholar]
- De Roos AM, Persson L (2013) Population and community ecology of ontogenetic development. Princeton University Press; 538pp [Google Scholar]
- Del Raye G, Weng KC (2015) An aerobic scope-based habitat suitability index for predicting the effects of multi-dimensional climate change stressors on marine teleosts. Deep Res Part II Top Stud Oceanogr 113: 280–290. [Google Scholar]
- Deutsch C, Ferrel A, Seibel B, Pörtner HO, Huey RB (2015) Ecophysiology. Climate change tightens a metabolic constraint on marine habitats. Science 348: 1132–1135. [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Law R (1996) The dynamical theory of coevolution: a derivation from stochastic ecological processes. J Math Biol 34: 579–612. [DOI] [PubMed] [Google Scholar]
- di Santo V, Tran AH, Svendsen JC (2016) Progressive hypoxia decouples activity and aerobic performance of skate embryos. Conserv Physiol 4: cov067; doi:10.1093/conphys/cov067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte C. (2014) Global change and the future ocean: a grand challenge for marine sciences. Front Mar Sci 1: 1–16. [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. [DOI] [PubMed] [Google Scholar]
- Elleouet J, Albouy C, Ben Rais Lasram F, Mouillot D, Leprieur F (2014) A trait-based approach for assessing and mapping niche overlap between native and exotic species: the Mediterranean coastal fish fauna as a case study. Divers Distrib 20: 1333–1344. [Google Scholar]
- Elliott M, Borja A, McQuatters-Gollop A, Mazik K, Birchenough S, Andersen JH, Painting S, Peck M (2015) Force majeure: will climate change affect our ability to attain good environmental status for marine biodiversity. Mar Pollut Bull 95: 7–27. [DOI] [PubMed] [Google Scholar]
- Ern R, Huong DTT, Phuong NT, Wang T, Bayley M (2014) Oxygen delivery does not limit thermal tolerance in a tropical eurythermal crustacean. J Exp Biol 217: 809–814. [DOI] [PubMed] [Google Scholar]
- FAO (2014) The State of World Fisheries and Aquaculture. Food and Agriculture Oraganization of the United Nations. [Google Scholar]
- Farrell AP. (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88: 322–343. [DOI] [PubMed] [Google Scholar]
- Feder ME, Bennett AF, Huey RB (2000) Evolutionary physiology. Annu Rev Ecol Syst 31: 315–341. [Google Scholar]
- Ferreira-Martins D, Coimbra J, Antunes C, Wilson JM (2016) Effects of salinity on upstream-migrating , spawning sea lamprey, Petromyzon marinus. Conserv Physiol 4: cov064; doi:10.1093/conphys/cov064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Maza D, Lloret J, Faliex E, Sasal P (2016) Links between parasitism, energy reserves and fecundity of European anchovy, Engraulis encrasicolus, in the northwestern Mediterranean Sea. Conserv Physiol 4: cov069; doi:10.1093/conphys/cov069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fobian D, Overgaard J, Wang T (2014) Oxygen transport is not compromised at high temperature in pythons. J Exp Biol 217: 3958–3961. [DOI] [PubMed] [Google Scholar]
- Forbes VE, Palmqvist A, Bach L (2006) The use and misuse of biomarkers in ecotoxicology. Environ Toxicol Chem 25: 272–280. [DOI] [PubMed] [Google Scholar]
- Freitas C, Olsen EM, Knutsen H, Albretsen J, Moland E (2016) Temperature-associated habitat selection in a cold-water marine fish. J Anim Ecol 85: 628–637. [DOI] [PubMed] [Google Scholar]
- Fry FEJ. (1947) The effects of the environment on animal activity. Univ Toronto Stud, Biol Ser 55: 1–62. [Google Scholar]
- Fry FEJ. (1971) The effect of environmental factors on the physiology of fish In: Hoar WS, Randall DJ, eds, Fish Physiology, Vol 6 Academic Press, New York, pp 1–98. [Google Scholar]
- Gagliano M, McCormick MI (2009) Hormonally mediated maternal effects shape offspring survival potential in stressful environments. Oecologia 160: 657–665. [DOI] [PubMed] [Google Scholar]
- Gardiner NM, Munday PL, Nilsson GE (2010) Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS One 5: e13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Carter PA (1994) Evolutionary physiology. Annu Rev Physiol 56: 579–621. [DOI] [PubMed] [Google Scholar]
- Giske J, Eliassen S, Fiksen Ø, Jakobsen PJ, Aksnes DL, Mangel M, Jørgensen C (2014) The emotion system promotes diversity and evolvability . Proc Biol Sci 281: 20141096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiss AC, Dale JJ, Holland KN, Wilson RP (2010) Accelerating estimates of activity-specific metabolic rate in fishes: testing the applicability of acceleration data-loggers. J Exp Mar Biol Ecol 385: 85–91. [Google Scholar]
- Gore M, Burggren WW (2012) Cardiac and metabolic physiology of early larval zebrafish (Danio rerio) reflects parental swimming stamina. Front Physiol 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräns A, Olsson C, Pitsillides K, Nelson HE, Cech JJ Jr, Axelsson M (2010) Effects of feeding on thermoregulatory behaviours and gut blood flow in white sturgeon (Acipenser transmontanus) using biotelemetry in combination with standard techniques. J Exp Biol 213: 3198–3206. [DOI] [PubMed] [Google Scholar]
- Gräns A, Jutfelt F, Sandblom E, Jönsson E, Wiklander K, Seth H, Olsson C, Dupont S, Ortega-Martinez O, Einarsdottir I et al. (2014) Aerobic scope fails to explain the detrimental effects on growth resulting from warming and elevated CO2 in Atlantic halibut. J Exp Biol 217: 711–717. [DOI] [PubMed] [Google Scholar]
- Grimm V, Revilla E, Berger U, Jeltsch F, Mooij WM, Railsback SF, Thulke H-H, Weiner J, Wiegand T, DeAngelis DL (2005) Pattern-Oriented Modeling of Agent-Based Complex Systems: Lessons from Ecology. Science 310: 987–991. [DOI] [PubMed] [Google Scholar]
- Gunderson AR, Armstrong EJ, Stillman JH (2016) Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Ann Rev Mar Sci 8: 357–378. [DOI] [PubMed] [Google Scholar]
- Hanson PC, Johnson TB, Schindler DE, Kitchell JF (1997) Fish bioenergetics 3.0. University of Wisconsin, Sea Grant Institute. Sea Grant Tech Report, WISCUT-97-001, Madison, Wisconsin.
- Heffernan JB, Soranno PA, Angilletta MJ, Buckley LB, Gruner DS, Keitt TH, Kellner JR, Kominoski JS, Rocha AV, Xiao J et al. (2014) Macrosystems ecology: understanding ecological patterns and processes at continental scales. Front Ecol Environ 12: 5–14. [Google Scholar]
- Hewett SW, Johnson BL (1987) A Generalized Bioenergetics Model of Fish Growth for Microcomputers. University of Wisconsin, Sea Grant Institute, Madison, Wisconsin. [Google Scholar]
- Ho DH, Burggren WW (2012) Parental hypoxic exposure confers offspring hypoxia resistance in zebrafish (Danio rerio). J Exp Biol 215: 4208–4216. [DOI] [PubMed] [Google Scholar]
- Hollowed AB, Barange M, Ito SI, Kim S, Loeng H, Peck MA (2011) Effects of climate change on fish and fisheries: forecasting impacts, assessing ecosystem responses, and evaluating management strategies. ICES J Mar Sci 68: 984–985. [Google Scholar]
- Holt RE, Jørgensen C (2014) Climate warming causes life-history evolution in a model for Atlantic cod (Gadus morhua). Conserv Physiol 2: cou050; doi:10.1093/conphys/cou050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RE, Jørgensen C (2015) Climate change in fish : effects of respiratory constraints on optimal life history and behaviour. Biol Lett 11: 20141032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodysky AZ, Cooke SJ, Brill RW (2015) Physiology in the service of fisheries science: why thinking mechanistically matters. Rev Fish Biol Fish 25: 425–447. [Google Scholar]
- Horodysky AZ, Cooke SJ, Graves JE, Brill RW (2016) Fisheries conservation on the high seas: linking conservation physiology and fisheries ecology for the management of large pelagic fishes. Conserv Physiol 4: cov059; doi:10.1093/conphys/cov059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE (2012) Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos Trans R Soc Lond B Biol Sci 367: 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E, Metcalfe JD, O'Brien CM, Arnold GP, Reynolds JD (2004) Vertical activity patterns of free-swimming adult plaice in the southern North Sea. Mar Ecol Prog Ser 279: 261–273. [Google Scholar]
- Hutchinson GE. (1957) Concluding remarks. Cold Spring Harb Lab Press 22: 415–427. [Google Scholar]
- IPCC (2014) Part A: Global and Sectoral Aspects. (Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change). Clim Chang 2014 Impacts, Adapt Vulnerability 1132.
- Ishimatsu A, Hayashi M, Kikkawa T (2008) Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser 373: 295–302. [Google Scholar]
- Jeffrey JD, Hasler CT, Chapman JM, Cooke SJ, Suski CD (2015) Linking landscape-scale disturbances to stress and condition of fish: implications for restoration and conservation. Integr Comp Biol 55: 618–630. [DOI] [PubMed] [Google Scholar]
- Johansen JL, Messmer V, Coker DJ, Hoey AS, Pratchett MS (2014) Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob Chang Biol 20: 1067–1074. [DOI] [PubMed] [Google Scholar]
- Johansen JL, Pratchett MS, Messmer V, Coker DJ, Tobin AJ, Hoey AS (2015) Large predatory coral trout species unlikely to meet increasing energetic demands in a warming ocean. Sci Rep 5: 13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen C, Peck MA, Antognarelli F, Azzurro E, Burrows MT, Cheung WWL, Cucco A, Holt RE, Huebert KB, Marras S et al. (2012) Conservation physiology of marine fishes: advancing the predictive capacity of models. Biol Lett 8: 900–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen C, Enberg K, Mangel M (2016) Modelling and interpreting fish bioenergetics: a role for behaviour, life-history traits and survival trade-offs. J Fish Biol 88: 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M, Porter W (2009) Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett 12: 334–350. [DOI] [PubMed] [Google Scholar]
- Kerr SR. (1990) The Fry paradigm: its significance for contemporary ecology. Trans Am Fish Soc 119: 779–785. [Google Scholar]
- Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc Biol Sci 274: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen SS, Marras S, Metcalfe NB, McKenzie DJ, Domenici P (2013) Environmental stressors alter relationships between physiology and behaviour. Trends Ecol Evol 28: 651–658. [DOI] [PubMed] [Google Scholar]
- Killen SS, Nati JJH, Suski CD (2015) Vulnerability of individual fish to capture by trawling is influenced by capacity for anaerobic metabolism. Proc Biol Sci 282: 20150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen SS, Adriaenssens B, Marras S, Claireaux G, Cooke SJ (2016. a) Context dependency of trait repeatability and its relevance for management and conservation of fish populations. Conserv Physiol 4: cow007; doi:10.1093/conphys/cow007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen SS, Glazier DS, Rezende EL, Clark TD, Atkinson D, Willener AST, Halsey LG (2016. b) Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am Nat 187: 592–606. [DOI] [PubMed] [Google Scholar]
- King GD, Chapman JM, Cooke SJ, Suski CD (2016) Stress in the neighborhood: tissue glucocorticoids relative to stream quality for five species of fish. Sci Total Environ 547: 87–94. [DOI] [PubMed] [Google Scholar]
- Kooi BW, van der Meer J (2010) Bifurcation theory, adaptive dynamics and dynamic energy budget-structured populations of iteroparous species. Philos Trans R Soc Lond B Biol Sci 365: 3579–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman SALM (1993) Dynamic Energy Budgets in Biological Systems: Theory and Applications in Ecotoxicology. New York, USA: Cambridge University Press. [Google Scholar]
- Kooijman SALM (2010) Dynamic Energy Budget Theory for Metabolic Organization, 3rd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Lefevre S. (2016) Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv Physiol 4: cow009; doi:10.1093/conphys/cow009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançois C, Claireaux G (2003) Influence of ambient oxygenation and temperature on metabolic scope and scope for heart rate in the common sole Solea solea. Mar Ecol Prog Ser 259: 273–284. [Google Scholar]
- Le Quesne WJF, Pinnegar JK (2012) The potential impacts of ocean acidification: scaling from physiology to fisheries. Fish Fish 13: 333–344. [Google Scholar]
- Lutterschmidt WI, Hutchison VH (1997) The critical thermal maximum: history and critique. Can J Zool 75: 1561–1574. [Google Scholar]
- McKenzie DJ. (2011) Swimming and Other Activities | Energetics of Fish Swimming. Encyclopedia of Fish Physiology. Elsevier Inc, London, United Kingdom. [Google Scholar]
- McKenzie DJ, Garofalo E, Winter MJ, Ceradini S, Verweij F, Day N, Hayes R, van der Oost R, Butler PJ, Chipman JK et al. (2007) Complex physiological traits as biomarkers of the sub-lethal toxicological effects of pollutant exposure in fishes. Philos Trans R Soc Lond B Biol Sci 362: 2043–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil BI, Sasse TP (2016) Future ocean hypercapnia driven by anthropogenic amplification of the natural CO2 cycle. Nature 529: 383–386. [DOI] [PubMed] [Google Scholar]
- Madliger CL, Cooke SJ, Crespi EJ, Funk JL, Hultine KR, Hunt KE, Rohr JR, Sinclair BJ, Suski CD, Willis CKR et al. (2016) Success stories and emerging themes in conservation physiology. Conserv Physiol 4: cov057; doi:10.1093/conphys/cov057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marey M. (1896) Phenomenon of flight in the animal kingdom. In: Smithsonian Annual Report. US Government Print Office, Washington, DC, pp 226–285.
- Marras S, Cucco A, Antognarelli F, Azzurro E, Milazzo M, Bariche M, Butenschön M, Kay S, Di Bitetto M, Quattrocchi G et al. (2015. a) Predicting future thermal habitat suitability of competing native and invasive fish species: from metabolic scope to oceanographic modelling. Conserv Physiol 3: cou059; doi:10.1093/conphys/cou059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras S, Noda T, Steffensen JF, Svendsen MBS, Krause J, Wilson ADM, Kurvers RHJM, Herbert-Read J, Boswell KM, Domenici P (2015. b) Not so fast: swimming behavior of sailfish during predator–prey interactions using high-speed video and accelerometry. Integr Comp Biol 55: 719–727. [DOI] [PubMed] [Google Scholar]
- Martin BT, Nisbet RM, Pike A, Michel CJ, Danner EM (2015) Sport science for salmon and other species: ecological consequences of metabolic power constraints. Ecol Lett 18: 535–544. [DOI] [PubMed] [Google Scholar]
- Metcalfe JD. (2006) Fish population structuring in the North Sea: understanding processes and mechanisms from studies of the movements of adults. J Fish Biol 69: 48–65. [Google Scholar]
- Metcalfe JD. (2009) Welfare in wild-capture marine fisheries. J Fish Biol 75: 2855–2861. [DOI] [PubMed] [Google Scholar]
- Metcalfe JD, Arnold G (1997) Tracking fish with electronic tags. Nature 387: 665–666. [Google Scholar]
- Metcalfe JD, Le Quesne WJF, Cheung WWL, Righton DA (2012) Conservation physiology for applied management of marine fish: an overview with perspectives on the role and value of telemetry. Philos Trans R Soc Lond B Biol Sci 367: 1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe JD, Wright S, Tudorache C, Wilson RP (2016. a) Recent advances in telemetry for estimating the energy metabolism of wild fishes. J Fish Biol 88: 284–297. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Van Leeuwen TE, Killen SS (2016. b) Does individual variation in metabolic phenotype predict fish behaviour and performance. J Fish Biol 88: 298–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz JA, Nisbet RM, Geritz SA (1992) How should we define ‘fitness’ for general ecological scenarios. Trends Ecol Evol 7: 198–202. [DOI] [PubMed] [Google Scholar]
- Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL (2012) Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat Clim Chang 2: 858–861. [Google Scholar]
- Mills SC, Beldade R, Chabanet P, Bigot L, O'Donnell JL, Bernardi G (2015) Ghosts of thermal past: reef fish exposed to historic high temperatures have heightened stress response to further stressors. Coral Reefs 34: 1255–1260. [Google Scholar]
- Mouillot D, Bellwood DR, Baraloto C, Chave J, Galzin R, Harmelin-Vivien M, Kulbicki M, Lavergne S, Lavorel S, Mouquet N et al. (2013) Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol 11: e1001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano M, Illing B, Peschutter P, Huebert KB, Peck MA (2016) Thermal impacts on the growth, development and ontogeny of critical swimming speed in Atlantic herring larvae. Comp Biochem Physiol A Mol Integr Physiol 197: 23–34. [DOI] [PubMed] [Google Scholar]
- Nagelkerken I, Munday PL (2016) Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob Chang Biol 22: 974–989. [DOI] [PubMed] [Google Scholar]
- Neat F, Righton D (2007) Warm water occupancy by North Sea cod. Proc Biol Sci 274: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TA, Anderson TW, Širović A (2015) Intermittent noise induces physiological stress in a coastal marine fish. PLoS One 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norin T, Malte H, Clark TD (2014) Aerobic scope does not predict the performance of a tropical eurythermal fish at elevated temperatures. J Exp Biol 217:244–251. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Andersen JL, Findsen A, Pedersen PBM, Hansen K, Ozolina K, Wang T (2012) Aerobic scope and cardiovascular oxygen transport is not compromised at high temperatures in the toad Rhinella marina. J Exp Biol 215: 3519–3526. [DOI] [PubMed] [Google Scholar]
- Patterson DA, Cooke SJ, Hinch SG, Robinson KA, Young N, Farrell AP, Miller KM (2016). A perspective on physiological studies supporting the provision of scientific advice for the management of Fraser River sockeye salmon (Oncorhynchus nerka). Conserv Physiol 4: cow026; doi:10.1093/conphys/cow026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly D. (1981) The relationships between gill surface area and growth performance in fish : a generalization of von Bertalanffy's theory of growth. Berichte der Dtsch Wissenchaftlichen Kommission fur Meeresforsch 28: 251–282. [Google Scholar]
- Pauly D, Zeller D (2016) Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat Commun 7: 10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly D, Christensen V, Guenétte S, Pitcher TJ, Sumaila UR, Walters CJ, Watson R, Zeller D (2002) Towards sustainability in world fisheries. Nature 418: 689–695. [DOI] [PubMed] [Google Scholar]
- Persson L, De Roos AM (2006) Food-dependent individual growth and population dynamics in fishes. J Fish Biol 69: 1–20. [Google Scholar]
- Peck MA, Moyano M (2016) Measuring respiration rates in marine fish larvae: challenges and advances. J Fish Biol 88: 173–205. [DOI] [PubMed] [Google Scholar]
- Peck MA, Arvanitidis C, Butenschön M, Canu DM, Chatzinikolaou E, Cucco A, Domenici P, Fernandes JA, Gasche L, Huebert KB et al. (2016) Projecting changes in the distribution and productivity of living marine resources: a critical review of the suite of modelling approaches used in the large European project VECTORS. Estuar Coast Shelf Sci. doi:10.1016/j.ecss.2016.05.019. [Google Scholar]
- Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308: 1912–1915. [DOI] [PubMed] [Google Scholar]
- Petitgas P, Rijnsdorp AD, Dickey-Collas M, Engelhard GH, Peck MA, Pinnegar JK, Drinkwater K, Huret M, Nash RDM (2013) Impacts of climate change on the complex life cycles of fish. Fish Oceanogr 22: 121–139. [Google Scholar]
- Pörtner H-O. (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Farrell AP (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97. [DOI] [PubMed] [Google Scholar]
- Pörtner H-O, Peck MA (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77: 1745–1779. [DOI] [PubMed] [Google Scholar]
- Priede IG. (1985) Metabolic scope in fishes In: Tytler P, Calow P, eds, Fish Energetics, New Perspectives. Croom-Helm, London, pp 33–64. [Google Scholar]
- Prince ED, Luo J, Goodyear CP, Hoolihan JP, Snodgrass D, Orbesen ES, Serafy JE, Ortiz M, Schirripa MJ (2010) Ocean scale hypoxia-based habitat compression of Atlantic istiophorid billfishes. Fish Oceanogr 19:448–462. [Google Scholar]
- Prosser CL. (1950) Comparative Animal Physiology. Saunders, Philadelphia. [Google Scholar]
- Queirós A, Huebert K, Keyl F, Fernandes J, Stolte W, Maar M, Kay S, Jones M, Teal L, Hamon K et al. (2016). Solutions for ecosystem-level protection of ocean systems against climate change. Glob Chang Biol. In press. doi: 10.1111/gcb.13423. [DOI] [PubMed] [Google Scholar]
- Raab K, Llope M, Nagelkerke LAJ, Rijnsdorp AD, Teal LR, Licandro P, Ruardij P, Dickey-Collas M (2013) Influence of temperature and food availability on juvenile European anchovy Engraulis encrasicolus at its northern boundary. Mar Ecol Prog Ser 488: 233–245. [Google Scholar]
- Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos Trans R Soc Lond B Biol Sci 365: 4051–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righton D, Metcalfe JD, Connolly P (2001) Fisheries. Different behaviour of North and Irish Sea cod. Nature 411: 156. [DOI] [PubMed] [Google Scholar]
- Righton D, Andersen K, Neat F, Thorsteinsson V, Steingrund P, Svedäng H, Michalsen K, Hinrichsen H, Bendall V, Neuenfeldt S et al. (2010) Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima. Mar Ecol Prog Ser 420: 1–13. [Google Scholar]
- Rijnsdorp AD, Peck MA, Engelhard GH, Mo C, Pinnegar JK (2009) Resolving the effect of climate change on fish populations. ICES J Mar Sci 66: 1570–1583. [Google Scholar]
- Rogers NJ, Urbina MA, Reardon EE, McKenzie DJ, Wilson RW (2016) A new analysis of hypoxia tolerance in fishes using a database of critical oxygen level (Pcrit). Conserv Physiol 4: cow012; doi:10.1093/conphys/cow012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze T, Christen F, Amerand A, Claireaux G (2013) Trade-off between thermal sensitivity, hypoxia tolerance and growth in fish. J Therm Biol 38: 98–106. [Google Scholar]
- Rutz C, Hays GC (2009) New frontiers in biologging science. Biol Lett 5: 289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Neilsen K. (1982) Animal Physiology. Adaptation and Environment. Cambridge University Press, New York. [Google Scholar]
- Schulte PM. (2015) The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218: 1856–1866. [DOI] [PubMed] [Google Scholar]
- Seebacher F, Franklin CE (2012) Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Philos Trans R Soc Lond B Biol Sci 367: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F, White CR, Franklin CE (2015) Physiological plasticity increases resilience of ectothermic animals to climate change. Nat Clim Chang 5: 61–66. [Google Scholar]
- Sierra-Flores R, Atack T, Migaud H, Davie A (2015) Stress response to anthropogenic noise in Atlantic cod Gadus morhua L. Aquac Eng 67: 67–76. [Google Scholar]
- Simpson SD, Radford AN, Nedelec SL, Ferrari MCO, Chivers DP, McCormick MI, Meekan MG (2016) Anthropogenic noise increases fish mortality by predation. Nat Commun 7: 10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DW, Southall EJ, Richardson AJ, Reid PC, Metcalfe JD (2003) Seasonal movements and behaviour of basking sharks from archival tagging: no evidence of winter hibernation. Mar Ecol Prog Ser 248: 187–196. [Google Scholar]
- Slabbekoorn H, Bouton N, Van Opzeeland I, Coers A, Cate C, Popper AN (2010) A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol Evol 25: 419–427. [DOI] [PubMed] [Google Scholar]
- Sousa T, Domingos T, Kooijman SALM (2008) From empirical patterns to theory: a formal metabolic theory of life. Philos Trans R Soc Lond B Biol Sci 363: 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaila UR, Cheung WWL, Lam VWY, Pauly D, Herrick S (2011) Climate change impacts on the biophysics and economics of world fisheries. Nat Clim Chang 1: 449–456. [Google Scholar]
- Sumpter JP, Jobling S (1995) Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect 103: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday JM, Bates AE, Dulvy NK (2011) Global analysis of thermal tolerance and latitude in ectotherms. Proc Biol Sci 278: 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nat Clim Chang 2: 686–690. [Google Scholar]
- Svendsen JC, Aarestrup K, Steffensen JF, Herskin J (2006) A Novel Acoustic Dissolved Oxygen Transmitter for Fish Telemetry. Mar Technol Soc J 40: 103–108 [Google Scholar]
- Teal LR, van Hal R, van Kooten T, Ruardij P, Rijnsdorp AD (2012) Bio-energetics underpins the spatial response of North Sea plaice (Pleuronectes platessa L.) and sole (Solea solea L.) to climate change. Glob Chang Biol 18: 3291–3305. [Google Scholar]
- Teal LR, Marras S, Peck MA, Domenici P (2016) Physiology-based modelling approaches to characterize fish habitat suitability: their usefulness and limitations. Estuar Coast Shelf Sci. doi:10.1016/j.ecss.2015.11.014. [Google Scholar]
- Tyler CR, Van Der Eerden B, Jobling S, Panter G, Sumpter JP (1996) Measurement of vitellogenin, a biomarker for exposure to oestrogenic chemicals, in a wide variety of cyprinid fish. J Comp Physiol B Biochem Syst Environ Physiol 166: 418–426. [Google Scholar]
- Van Der Meer J. (2016) A paradox in individual-based models of populations. Conserv Physiol 4: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk WCEP, Overgaard J, Ern R, Bayley M, Wang T, Boardman L, Terblanche JS (2016) Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comp Biochem Physiol A Mol Integr Physiol 192: 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Overgaard J (2007) Ecology. The heartbreak of adapting to global warming. Science 315: 49–50. [DOI] [PubMed] [Google Scholar]
- Wang T, Lefevre S, Iversen NK, Findorf I, Buchanan R, McKenzie DJ (2014) Anaemia only causes a small reduction in the upper critical temperature of sea bass: is oxygen delivery the limiting factor for tolerance of acute warming in fishes. J Exp Biol 217: 4275–4278. [DOI] [PubMed] [Google Scholar]
- Ward TD, Algera DA, Gallagher AJ, Hawkins E, Horodysky A, Jørgensen C, Killen SS, McKenzie DJ, Metcalfe JD, Peck MA et al. (2016) Understanding the individual to implement the ecosystem approach to fisheries management. Conserv Physiol 4: cow005; doi:10.1093/conphys/cow005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware DM. (1982) Power and evolutionary fitness of teleosts. Can J Fish Aquat Sci 39: 3–13. [Google Scholar]
- Whitlock RE, Hazen EL, Walli A, Farwell C, Bograd SJ, Foley DG, Castleton M, Block BA (2015) Direct quantification of energy intake in an apex marine predator suggests physiology is a key driver of migrations. Sci Adv 1: e1400270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BK. (2011) Adaptive management of natural resources—framework and issues. J Environ Manag 92: 1346–1353. [DOI] [PubMed] [Google Scholar]
- Wilson RW. (2014) Chapter 3.6 Fish In: Laffoley D, Baxter J, Thevenon F, Oliver J, eds, The Significance and Management of Natural Carbon Stores in the Open Ocean. Full report. IUCN, Gland, Switzerland, pp 81–94. ISBN: 978-208317-1692-3. [Google Scholar]
- Wilson RW, Millero FJ, Taylor JR, Walsh PJ, Christensen V, Jennings S, Grosell M (2009) Contribution of fish to the marine inorganic carbon cycle. Science 323: 359–362. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Hinch SG, Eliason EJ, Farrell AP, Cooke SJ (2013) Calibrating acoustic acceleration transmitters for estimating energy use by wild adult Pacific salmon. Comp Biochem Physiol A Mol Integr Physiol 164: 491–498. [DOI] [PubMed] [Google Scholar]
- Wright S, Metcalfe JD, Hetherignton S, Wilson RP (2014) Estimating activity-specific energy expenditure in a teleost fish, using accelerometer loggers. Mar Ecol Prog Ser 496: 19–32. [Google Scholar]
- Zambonino-Infante JL, Claireaux G, Ernande B, Jolivet A, Quazuguel P, Sévère A, Huelvan C, Mazurais D (2013) Hypoxia tolerance of common sole juveniles depends on dietary regime and temperature at the larval stage: evidence for environmental conditioning. Proc Biol Sci 280: 20123022. [DOI] [PMC free article] [PubMed] [Google Scholar]