Abstract
Mercury (Hg) concentrations in aquatic environments have increased globally, exposing consumers of aquatic organisms to high Hg levels. For both aquatic and terrestrial consumers, exposure to Hg depends on their food sources as well as environmental factors influencing Hg bioavailability. The majority of the research on the transfer of methylmercury (MeHg), a toxic and bioaccumulating form of Hg, between aquatic and terrestrial food webs has focused on terrestrial piscivores. However, a gap exists in our understanding of the factors regulating MeHg bioaccumulation by non-piscivorous terrestrial predators, specifically consumers of adult aquatic insects. Because dissolved organic carbon (DOC) binds tightly to MeHg, affecting its transport and availability in aquatic food webs, we hypothesized that DOC affects MeHg transfer from stream food webs to terrestrial predators feeding on emerging adult insects. We tested this hypothesis by collecting data over two years from 10 low-order streams spanning a broad DOC gradient in the Lake Sunapee watershed in New Hampshire. We found that streamwater MeHg concentration increased linearly with DOC concentration. However, streams with the highest DOC concentrations had emerging stream prey and spiders with lower MeHg concentrations than streams with intermediate DOC concentrations; a pattern that is similar to fish and larval aquatic insects. Furthermore, high MeHg concentrations found in spiders show that MeHg transfer in adult aquatic insects is an overlooked but potentially significant pathway of MeHg bioaccumulation in terrestrial food webs. Our results suggest that although MeHg in water increases with DOC, MeHg concentrations in stream and terrestrial consumers did not consistently increase with increases in streamwater MeHg concentrations. In fact, there was a change from a positive to a negative relationship between aqueous exposure and bioaccumulation at streamwater MeHg concentrations associated with DOC above around 5 mg/L. Thus, our study highlights the importance of stream DOC for MeHg dynamics beyond stream boundaries, and shows that factors modulating MeHg bioavailability in aquatic systems can affect the transfer of MeHg to terrestrial predators via aquatic subsidies.
Keywords: aquatic insects, aquatic-terrestrial linkages, bioaccumulation, dissolved organic carbon, emergence, food web, mercury, non-linearity, resource subsidy, spiders, streams, threshold
INTRODUCTION
In addition to providing energy and nutrients, materials moving across ecosystem boundaries can be environmental stressors for the recipient food web (Rasmussen and Vander Zanden 2004, Paetzold et al. 2011, Kraus et al. 2014a). Aquatic contaminants such as polychlorinated biphenyls (PCBs) and heavy metals are examples of such potential environmental stressors, and their movement and propagation through aquatic to terrestrial food webs via consumption of aquatic prey by terrestrial predators has been well documented (Tremblay et al. 1998, Christensen et al. 2005, Walters et al. 2008, Raikow et al. 2011). However, little is known about the mechanisms controlling the movement of these contaminants. The ubiquity of aquatic contaminants and their potential to have negative impacts on wildlife (Scheuhammer et al. 2007, Brar et al. 2010, USEPA 2010), as well as the importance of aquatic prey for terrestrial consumers (Willson and Halupka 1995, Baxter et al. 2005), highlight the need for understanding what factors affect the movement of aquatic contaminants into terrestrial food webs.
Mercury (Hg) concentrations in aquatic environments have increased globally, exposing terrestrial consumers and ecosystems to elevated Hg levels (AMAP/UNEP 2013, Rowse et al. 2014). Hg is a toxic and naturally occurring element, but human activities such as the burning of coal for power production and artisanal small-scale gold mining have increased its presence in the environment by two-to-three fold since the Industrial Revolution (AMAP/UNEP 2013). The anthropogenic, less-toxic inorganic forms of Hg are transformed by microbes –mainly methanogens, and sulfate- and iron-reducing bacteria – into the more toxic methylmercury (hereafter: MeHg), which is prone to bioaccumulation, primarily in anaerobic aquatic environments (Fitzgerald and Clarkson 1991, Gilmour et al. 1992, Fleming et al. 2006, Hamelin et al. 2011, Kidd et al. 2012). Because aquatic food webs are disproportionally burdened by MeHg bioaccumulation (Driscoll et al. 2007, Chasar et al. 2009), studying the movement of Hg from those habitats onto land via linked aquatic and terrestrial (hereafter aquatic-terrestrial) processes is particularly relevant.
Emerging aquatic insects are potentially important vectors for MeHg transport from aquatic ecosystems to the diverse array of terrestrial consumers that feed on them (Power et al. 2004, Ballinger and Lake 2006, Speir et al. 2014). Researchers have reported high MeHg concentrations in non-piscivorous (e.g., insectivorous) terrestrial consumers that may be eating large amounts of adult aquatic insects (Cristol et al. 2008, Wada et al. 2010). These high MeHg concentrations may have negative reproductive, developmental, and behavioral effects on wildlife (Wolfe et al. 1998, Hopkins et al. 2013, Varian-Ramos et al. 2014). However, due to the link with human health, the majority of the research associated with MeHg moving from aquatic to terrestrial food webs has focused on piscivorous predators, especially fish that may be consumed by humans (Chan et al. 2003, Scheuhammer et al. 2007, Schoch et al. 2014), leaving a gap in understanding about MeHg bioaccumulation by terrestrial non-piscivorous predators. Furthermore, studies have reported high MeHg concentrations in endangered insectivorous wildlife such as little brown bats (Wada et al. 2010, Nam et al. 2012), and higher MeHg concentrations in birds consuming arthropods than birds consuming fish (Evers et al. 2005, Cristol et al. 2008).
Although the principal route of exposure to MeHg is through diet (Hall et al. 1997), factors influencing Hg bioavailability to Hg methylation and bioaccumulation lower in the food web could determine MeHg concentrations in terrestrial consumers. Dissolved organic carbon (DOC), a dominant ligand of freshwater Hg (Ravichandran 2004), modulates the amount of mercury available for methylation and MeHg bioavailability in freshwaters (Schuster et al. 2008, Brigham et al. 2009, Tsui et al. 2009). However, the role of DOC in the accumulation of Hg in food webs is complex (Pickhardt and Fisher 2007, Chasar et al. 2009, Ward et al. 2010, Chen et al. 2012, Lavoie et al. 2013) and less well understood. DOC modulates the transport of Hg in freshwater; studies report an increase in freshwater dissolved Hg and MeHg with increases in DOC concentration (Grigal 2002, Brigham et al. 2009). However, a linear positive relationship between freshwater DOC and dissolved Hg concentration may not translate to a linear increase in MeHg bioavailability to biota. Some studies have reported an initial increase in MeHg concentration in algae, aquatic invertebrates and fish with increasing DOC up to a threshold DOC concentration, after which DOC reduces MeHg uptake (Driscoll et al. 1994, Gorski et al. 2008, Tsui and Finlay 2011, Chiasson-Gould et al. 2014, French et al. 2014). Furthermore, recent studies by Chiasson-Gould et al. (2014) and French et al. (2014) showed that this attenuation, or a threshold-type effect, in MeHg uptake at high DOC concentrations is associated with decreasing Hg methylation and reduced MeHg bioavailability through the formation of strong DOC-Hg complexes. Therefore, we hypothesized that the effects of DOC on emerging aquatic insect MeHg concentrations would be reflected in MeHg concentrations of their terrestrial predators (Cremona et al. 2008, Kraus et al. 2014a).
There are few published studies on MeHg concentration in emerging insects, and the existing studies come mainly from lentic environments (Tremblay et al. 1998, Cremona et al. 2008, Wurtsbaugh et al. 2011), suggesting that more research, especially on MeHg bioaccumulation by terrestrial organisms that consume stream insects, is necessary. The physical and chemical characteristics of both lakes and streams, as well as a consumer’s trophic position, determine MeHg bioaccumulation in biota (Chasar et al. 2009, Ward et al. 2010). However, streams often have stronger linkages to land (Richardson et al. 2010), and the greater reliance of some stream biota on riparian organic matter can significantly reduce their body MeHg concentrations (Jardine et al. 2012), and thus the export of MeHg to riparian predators. The aim of this research was to investigate the transfer of MeHg from stream food webs to terrestrial predators feeding on emerging aquatic insects. Because DOC forms strong bonds with Hg species (Cai et al. 1999), potentially transporting both inorganic Hg (Hg available for methylation) and MeHg from methylating sites, we hypothesized that streamwater inorganic Hg and MeHg concentration would increase as DOC increases. We also predicted that DOC concentration would modulate MeHg bioaccumulation in both emerging stream insects and their riparian predators, with MeHg concentration in emerging stream insects and spiders increasing with streamwater MeHg concentration up to a DOC concentration above which the relationship between streamwater MeHg and MeHg bioaccumulation would decrease.
METHODS
To investigate the effects of DOC and streamwater Hg and MeHg on the concentration of MeHg in emerging aquatic insects and terrestrial predators, we studied 10 streams in the Lake Sunapee watershed (Sullivan County, New Hampshire, USA, Appendix S1). This region has little industrial or commercial development, and only low-density residential development. Streams in this watershed were ideal because the region receives atmospheric Hg deposition (NADP 2015b, a), and the streams exhibit mean DOC concentrations between 2.09 and 12.52 mg C/L.
DOC
To explore the degree to which the concentration of DOC was associated with MeHg in emerging aquatic and riparian consumers, we collected one DOC sample per stream on 10 total sampling dates between May and July of 2011 and 2012. To ensure comparability of results, all streams were sampled the same day, in each sampling date. Stream water samples were collected in flowing water using a 60 mL syringe and filtered in the field using pre-combusted Whatman GF/F filters rinsed three times with stream water prior to collection. Samples were kept on ice and then frozen until they were analyzed at the Analytical Lab, Cary Institute of Ecosystem Studies, NY. Samples were analyzed on a Shimadzu® 5050 TC Analyzer as described by Findlay et al. (2010). For each stream we calculated a mean DOC concentration (mg C/L) for each year.
Stream temperature, pH and nutrient
We measured temperature, pH and nutrients in each study stream to account for potential effects of these stream variables on the MeHg concentration in stream water (Ullrich et al. 2001), as well as in emerging aquatic insects and riparian consumers. Temperature and pH were measured in situ using a pH/Conductivity/TDS Hanna meter (model number HI98129) at the same time samples were collected for DOC analysis. Due to logistical constraints temperature and pH were not sampled for two streams in 2011 (Appendix S2). We collected one sample for total phosphorus (TP) and total nitrogen (TN) in April, June and July 2012. Samples were kept on ice and then frozen until analysis. For TN, we used a basic persulfate digestion and measured nitrate using the second-derivative method (Crumpton et al. 1992). For TP, we used the persulfate digestion and the molybdate colorimetric method (Method 4500-P, American Public Health Association 1980).
Streamwater Hg and MeHg
To determine the Hg available for uptake by bacteria and algae and subsequent incorporation by stream consumers, we measured MeHg and total Hg (sum of MeHg and inorganic Hg) concentration in streamwater collected in June 2011, and April and June 2012, following methods in Chen et al. (2012). We collected water in certified clean polyethylene terephthalate bottles (500 mL) using the USEPA “clean hands/dirty hands” method (EPA Method 1669) by submergence into free-flowing surface water, following three rinses with stream water. We took duplicate samples and stored them double-bagged in 1% trace metal grade nitric acid-rinsed plastic bags on ice in the dark until filtering. The samples were filtered within 6 hrs of collection through pre-combusted (4 hrs at 550 °C) quartz fiber filters (particle retention of 0.3 μm). Blanks were 125 mL of distilled de-ionized (DI) water filtered before each duplicate set. Equipment was rinsed with DI water and 1% Optima grade HCl acid between samples from different streams. The filtrate was stored in certified clean 250 mL amber glass bottles in the dark at 4 °C before analyzing the actual form of the Hg molecules or ions present in the samples (i.e., Hg speciation analysis).
Terrestrial predator
We collected adult females of Tetragnatha elongata (Araneae: Tetragnathidae), a long-jawed orb weaver spider, from their webs using acid cleaned, non-metallic sampling gear. T. elongata’s low mobility and high dependence on aquatic insects (Gillespie 1987, Aiken and Coyle 2000) made this species ideal for our study because we could be confident that most of the Hg in the spiders came from consuming emerging stream insects rather than terrestrial insects. We collected spiders in June 2011, and June and July 2012. Spiders were found on their webs overhanging the rocks and riparian vegetation within 1 m from the stream at a maximum height of 2 m. Since T. elongata in the north temperate zone has one generation per year and overwinters in the antepenultimate instar (Aiken and Coyle 2000) performing collections in these months allowed us to specifically sample adult females of T. elongata. Thus we could control for spider gender and instar, which may confound Hg accumulation among individuals (Driscoll et al. 1994). Additionally, working only with females allowed us to avoid confounding potential diet differences between sexes (Sanzone et al. 2003). Females were freeze-dried in a trace metal-clean laboratory prior to Hg speciation analysis.
Quantifying spider diet
To characterize T. elongata’s diet we collected webs from all 10 streams during the last week of June 2011, the second and third weeks of May 2012, and the third weeks of June and July 2012. To identify the peak time of day for spider predation we performed collections at each stream as early as 0500 hrs and as late as 0100 hrs, and at multiple times in between, in June 2011 and May 2012. Once the time of peak predation was established, collections were made during the peak, which was between 2100 hrs and 0100 hrs, in June and July 2012. These months coincide with the bulk of aquatic insect emergence in these streams (Baer et al., unpublished data). Collections were performed from webs with active spiders to ensure that the insects reflected prey items rather than bycatch from uninhabited webs. We preserved the webs along with prey in 70% ethanol for identification in the lab. Similar to other web-building spiders, tetragnathids are non-selective predators (Lesar and Unzicker 1978, Culin and Yeargan 1982), making prey collection from webs a robust way to characterize their diet. Webs collected in May 2012 were occupied by juvenile spiders that had not developed the sexual characters that allow gender identification in the field. June and July collections were from webs occupied by adult females. Prey were identified down to family when possible using McCafferty (1983) and Merritt et al. (2008), otherwise taxa were identified to order. We also counted and measured individual prey insects to obtain number and biomass per web using published length-weight relationships (Sabo et al. 2002). We measured invertebrate body lengths (head to abdomen) under a dissecting scope with a 0.1 mm graduated handheld miniscale. Insects with an incomplete body (15% of total insects collected) were assigned the average body mass of the corresponding taxa in the same stream. To characterize the diet of spiders, and to estimate the dominant prey (by biomass) for Hg analysis in each stream, we took an average of the number and biomass of emerging aquatic insects in each web, and weighted this average by the number of webs collected each month, which allowed us to standardize diet for differences in collection effort across months in 2012.
Emerging stream insects
To measure the Hg concentration of the spider’s prey we collected emerging stream insects in 2012 with constructed metal-free emergence traps (Appendix S3). Spider webs collect heavy metals from the environment (Hose et al. 2002, Xiao-li et al. 2006), thus using emerging stream insects rather than those insects collected directly from spider webs allowed us to avoid insect contamination with web material. Additionally, collecting emerging insects rather than those in webs allowed us to obtain sufficient biomass (>20 mg) for Hg speciation analyses of most of T. elongata’s potential prey taxa. Although terrestrial prey can alter MeHg concentrations in riparian predators (Bartrons et al. 2015), our focal riparian predator relies heavily on stream insect subsidies for its diet, with >97% of the potential prey biomass collected from the webs being aquatic origin (see Results). Thus we only analyzed MeHg concentrations in emerging stream prey. Emergence trap collection canisters (1 L Nalgene bottles) were acid cleaned sequentially in Citranox soap and DI water (≤ 10 mL soap in 2 L water), 1 N nitric acid (280 mL trace-metal-grade acid to 4 L DI water), and 1.4 N hydrochloric acid (1 L trace-metal-grade acid to 4 L DI water). Emergence traps had a capture surface area of 0.50 m2. We deployed five traps per stream from May through July and collected insects from the traps twice a week. We removed the collecting canisters and placed them on ice until sorting. Insects were sorted and identified to family using acid-cleaned, non-metallic sampling gear in a laboratory set up for trace metal sample processing.
We selected a subset of the emerging insect taxa to analyze for Hg speciation based on their biomass dominance in T. elongata’s webs (Table 1). This was necessary due to the low biomass of emerging stream insects collected and the biomass required for accurate Hg speciation analyses (>20 mg). Thus we could not test the relationship between MeHg concentration in spiders and the MeHg concentrations of all the spiders’ insect prey. For Chironomidae, the only biomass-dominant group present across all the focal streams, we had sufficient biomass to pool individuals by month for May, June and July. For the rest of the prey taxa, we analyzed MeHg in aquatic prey that, combined, made up at least 70 % of the prey biomass in the spiders’ webs in 2012.
Table 1.
Main elements of T. elongata’s diet across the focal streams. Streams are presented from low to high DOC concentrations. Dominant prey taxa are those that made up >70% biomass in the web. Taxa in italics were not analyzed for MeHg concentration due to low (<20 mg) biomass.
| Stream | Year | # spider webs | # insects from webs | Mean ± 1 SE # insect/web | Mean ± 1 SE biomass/web | Dominant prey taxa by mass |
|---|---|---|---|---|---|---|
| Beck Brook | 2011 | 11 | 149 | 13.1 ± 9.9 | 3.0 ± 1.5 | Chironomidae (58%) |
| Tipulidae (28%) | ||||||
| 2012 | 32 | 92 | 2.7 ± 0.4 | 1.4 ± 0.4 | Tipulidae (42%) | |
| Chironomidae (34%) | ||||||
| Bartlett Brook | 2011 | 6 | 19 | 3.0 ± 0.7 | 2.4 ± 1.0 | Ephemerellidae (79%) |
| 2012 | 40 | 133 | 3.3 ± 0.5 | 1.1 ± 0.3 | Chironomidae (63%) | |
| Tipulidae (19%) | ||||||
| Blodgett South | 2011 | 7 | 21 | 3.0 ± 1.0 | 1.0 ± 0.6 | Leuctridae (52%) |
| Chironomidae (44%) | ||||||
| 2012 | 27 | 114 | 4.0 ± 0.9 | 1.5 ± 0.3 | Chironomidae (44%) | |
| Tipulidae (34%) | ||||||
| Otter Pond | 2011 | 3 | 11 | 3.0 ± 2.0 | 0.9 ± 0.4 | Chironomidae (100%) |
| 2012 | 29 | 222 | 7.6 ± 2.3 | 2.0 ± 0.6 | Chironomidae (65%) | |
| Hydropsychidae (29%) | ||||||
| Pike Brook | 2011 | 6 | 18 | 2.7 ± 0.6 | 2.8 ± 1.7 | Tipulidae (63%) |
| 2012 | 42 | 258 | 6.0 ± 1.5 | 3.2 ± 0.9 | Heptageniidae (46%) | |
| Chironomidae (36%) | ||||||
| Kidder Brook | 2011 | 13 | 200 | 15.1 ± 8.2 | 3.3 ± 1.4 | Chironomidae (70%) |
| 2012 | 42 | 193 | 4.5 ± 0.9 | 2.5 ± 1.1 | Ephemerellidae (41%) | |
| Chironomidae (25%) | ||||||
| Leuctridae (18%) | ||||||
| King Hill | 2011 | 8 | 10 | 1.25 ± 0.2 | 0.6 ± 0.17 | Leptohyphidae (35%) |
| Tipulidae (28%) | ||||||
| Chironomidae (19%) | ||||||
| 2012 | 35 | 132 | 3.5 ± 0.6 | 2.0 ± 0.5 | Heptageniidae (18%) | |
| Chironomidae (17%) | ||||||
| Leuctridae (15%) | ||||||
| Tipulidae (10%) | ||||||
| Perlodidae (9%) | ||||||
| Blodgett North | 2011 | 6 | 19 | 3.2 ± 1.4 | 0.9 ± 0.5 | Chironomidae (97%) |
| 2012 | 35 | 170 | 4.7 ± 1.2 | 1.8 ± 0.7 | Chironomidae (41%) | |
| Perlodidae (39%) | ||||||
| Red Water Creek | 2011 | 10 | 44 | 4.4 ± 0.8 | 5.0 ± 2.2 | Zygoptera (45%) |
| Ephemeroptera (27%) | ||||||
| 2012 | 30 | 102 | 3.3 ± 0.5 | 1.9 ± 0.5 | Chironomidae (32%) | |
| Leptophlebiidae (17%) | ||||||
| Heptageniidae (17%) | ||||||
| Tipulidae (16%) | ||||||
| Herrick Cove | 2011 | 13 | 57 | 4.3 ± 1.1 | 1.4 ± 0.4 | Chironomidae (96%) |
| 2012 | 35 | 102 | 2.8 ± 0.3 | 1.2 ± 0.2 | Chironomidae (60%) | |
| Hydropsychidae (18%) |
Numbers inside parenthesis represent contribution to spider diet. Collections were performed during the last week of June 2011, the second and third weeks of May 2012, and the third weeks of June and July 2012.
We freeze-dried the insects and pooled individuals collected during the three months of sampling to obtain sufficient biomass for Hg speciation analyses. If the minimum biomass for Hg speciation analyses was not obtained from the emergence traps for one of these prey taxa, we analyzed the next-most-abundant taxon by biomass in the spider’s webs until 80% of total prey biomass in webs was reached (Table 1, Appendix S4). However, this minimum percent biomass was not attained for five sites where we were unable to obtain 20 mg of sample for some of the taxa (Appendix S4). For those sites we analyzed taxa that comprised between 34% and 69% of the total biomass in the spider’s web (Table 1, Appendix S4).
Hg speciation analysis
All Hg speciation analyses were performed by the Dartmouth Trace Element Analysis Core Facility using isotope dilution gas chromatography-inductively coupled plasma mass spectrometry (ICP-MS). Filtrates from water samples were spiked with labeled Hg and MeHg to give a ~1:1 ratio of spike to natural 202Hg concentration of enriched inorganic 199Hg (Hgi) and enriched methyl 201Hg (MeHg), and then extracted in 10 ml of 4M HNO3 overnight at 60°C. Ionic Hg and MeHg were determined in the water sample filtrate following the ultra-low level methods described in Jackson et al. (2009). We calculated mean streamwater inorganic Hg and MeHg concentration for samples collected in each year. For determination of Hg speciation in biota, samples were freeze-dried, spiked with an appropriate amount of enriched inorganic 199Hg and enriched Me201Hg (Taylor et al. 2008) and then extracted in 2–3 ml of tetramethyl ammonium hydroxide, 25% w/v. MeHg and inorganic Hg were determined by species-specific isotope dilution purge and trap ICP-MS. Total Hg was calculated as the sum of MeHg and inorganic Hg (Point et al. 2007, Taylor et al. 2008). The method detection limit (MDL) for streamwater averaged 0.01 ng/L ± 0.01 (mean ± 1 SE) for MeHg and 0.1 ng/L ± 0.01 for inorganic Hg. Percent recovery for streamwater samples spiked in the range of sample concentrations averaged 107.5% ± 6.7 for MeHg and 96.2% ± 2.3 for inorganic Hg. The MDL for the spider samples averaged 0.4 ng ± 0.1 for both MeHg and inorganic Hg. Spiders were large enough (>20 mg) that we analyzed three individuals from each stream in each year for MeHg and inorganic Hg, then calculated the mean MeHg concentration in the spiders for each stream in each year. For the emerging aquatic insects, the MDL was 0.7 ng ± 0.4 for MeHg and 1.5 ng ± 0.7 for inorganic Hg. Quality control for biological samples was further evaluated by analyzing a standard reference material (SRM) for every batch of 20 samples. Recovery in SRMs averaged 101.6% ± 2.5 for MeHg (DORM 4, NRC, Ottawa, 354 ng/g MeHg). For THg, recovery was 100.3% ± 3.0 (DORM 4, NRC, Ottawa, 410 ng/g THg).
Data analysis
To test the hypothesis that streamwater inorganic Hg and MeHg concentrations increase with DOC, we used a general linear model relating average summer DOC concentration and average summer streamwater MeHg concentration, including an indicator variable for the year (2011 or 2012). Associations among streamwater MeHg, streamwater inorganic Hg, and stream parameters (temperature, pH, TN, and TP) were quantified using Spearman rank correlation coefficients. We used Spearman rank correlation instead of Pearson’s correlation due to the presence of outliers and non-linearities in the relationships.
Additionally, we tested our prediction that DOC concentration would modulate MeHg bioaccumulation in chironomid and non-chironomid prey and spiders using general linear models. Specifically, we tested whether DOC concentration and streamwater MeHg concentration interacted to determine the MeHg concentrations of these focal organisms. Explanatory variables were centered to minimize multicollinearity when interactions were included in the model (Chatterjee and Hadi 2012). We examined the degree of multicollinearity using the variance inflation factor (VIF). Although DOC concentration and streamwater MeHg concentrations were linearly related, all VIFs were lower than 3.3, indicating that effects of multicollinearity were minimal (Chatterjee and Hadi 2012). Because spiders were collected in 2011 and 2012, we included year of collection in that model. We analyzed chironomids separately from other stream prey to obtain information on the temporal variability in MeHg concentration in emerging stream insects, as well as the variability in prey MeHg concentration within a family. Thus, we included month of collection (May, June or July) as an indicator variable in that model. We removed one outlier (563.6 ng/g) from the chironomid data set after several outlier analyses (Mahalanobis distances, Jackknife distances, and Cook’s D influence). Following a marginally significant outcome for the spiders, we performed a posthoc power analysis in JMP using an α of 0.05.
Data were checked to confirm that they met the assumptions for each statistical test. We used a Breusch-Pagan test (Breusch and Pagan 1979) to confirm that the residual variance did not depend on month or year (P > 0.05), and a Durbin-Watson test to confirm that there was no temporal autocorrelation (P > 0.05). The Breusch-Pagan test was performed using R statistical software (R development core team 2013), while all other statistical analyses were performed using JMP statistical software (SAS institute Inc. 2013).
RESULTS
Mercury concentrations in streamwater samples were dominated by inorganic Hg (79.3% ± 2.5; mean ± 1 SE). Streamwater inorganic Hg and MeHg were positively correlated (ρ = 0.64, P = 0.002). Streamwater MeHg concentrations across the studied streams increased with DOC concentration, with streamwater MeHg concentrations being marginally higher in 2011 compared to 2012 (Table 2a; Fig. 1). Stream temperature ranged from 12.5 to 21.2°C (median 15.2°C, interquartile range 14.1-17.5°C), all streams had circumneutral pH (median 6.7, interquartile range 6.3-6.9), TN ranged from 222.7 to 1125.9 μg/L (median 319.0 μg/L, interquartile range 276.4-487.6 μg/L), and total phosphorus ranged from 6.4 to 40.5 μg/L (median 13.6 μg/L, interquartile range 10.5-20.6 μg/L). TP was associated with streamwater MeHg (ρ = 0.62, p = 0.05), but the rest of the variables were not related to streamwater MeHg concentration (|ρ| < 0.39, P > 0.23; Appendices S2 and S5).
Table 2.
General linear model results, for streams in the Lake Sunapee Watershed, NH. Explanatory variables were centered for the analyses that included interaction terms.
| Model | Estimate | Parameter Estimates Std. Error | P |
|---|---|---|---|
| a. Streamwater [MeHg] | R2=0.66, F2,17=19.72, p<0.0001 | ||
| Intercept | -0.07 | 0.07 | 0.35 |
| [DOC] | 0.06 | 0.01 | <0.0001 |
| Year | 0.06 | 0.03 | 0.09 |
| b. Chironomid prey [MeHg] | R2=0.61, F5,23=9.91, p<0.0001 | ||
| Intercept | 317.38 | 18.22 | <0.0001 |
| Streamwater [MeHg] | 671.75 | 113.82 | <0.0001 |
| [DOC] | -18.18 | 7.02 | 0.02 |
| Streamwater [MeHg]*[DOC] | -133.42 | 42.07 | 0.004 |
| Month [May] | -8.82 | 18.48 | 0.64 |
| Month [June] | 10.26 | 18.48 | 0.58 |
| c. Non-chironomid prey [MeHg] | R2=0.76, F3,5=9.52, p=0.02 | ||
| Intercept | 183.03 | 17.52 | <0.0001 |
| Streamwater [MeHg] | -161.27 | 106.42 | 0.19 |
| [DOC] | 22.14 | 6.77 | 0.02 |
| Streamwater [MeHg]*[DOC] | -99.57 | 39.47 | 0.05 |
| d. Spider [MeHg] | R2=0.33, F4,14=3.26, p=0.04 | ||
| Intercept | 1180.02 | 150.63 | <0.0001 |
| Streamwater [MeHg] | 1514.89 | 833.74 | 0.09 |
| [DOC] | -38.94 | 60.55 | 0.53 |
| Streamwater [MeHg]*[DOC] | -321.67 | 174.83 | 0.09 |
| Year [2012] | -268.19 | 123.84 | 0.05 |
Figure 1.
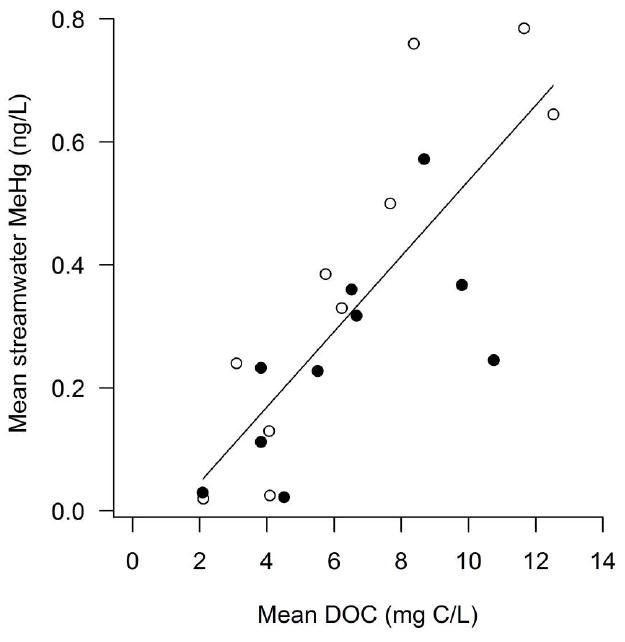
Relationship between streamwater MeHg concentration and DOC concentration in the Lake Sunapee, NH, watershed. Open symbols show data collected in 2011 and closed symbols show data from 2012.
Aquatic insects were the dominant potential prey of T. elongata with a weighted mean percent ± 1 SE of 96.7% ± 0.3 individuals and 97.9% ± 1.1 of the insect biomass in their webs. However, we found considerable variation in the taxonomic composition of the aquatic insects in T. elongata’s diet among the study sites, with Chironomidae being the only taxon that was common in webs at all streams (Table 1).
Most of the total Hg in the emerging insects and the riparian spiders was in the form of MeHg, with 90.8% ± 1.0 MeHg in chironomids, 70.5% ± 4.0 in non-chironomid emerging stream insects, and 78.5% ± 1.4 MeHg in spiders. Non-chironomid and chironomid prey had MeHg concentrations that were much higher than the ambient water (ranging from 180 to 4500 and 480 to 4100 times higher, respectively). Spiders also had MeHg concentrations that were much higher than the stream water, with MeHg concentrations ranging from 690 to 11000 times higher than water in 2011, and from 1400 to 32000 times higher in 2012.
Streamwater MeHg concentration and DOC concentration interacted to explain MeHg concentration in emerging stream insects and riparian spiders, although the effect size and statistical significance varied among responses (Table 2b-d). In both cases, the association between streamwater MeHg concentration and both chironomid and spider MeHg switched from positive at low DOC concentrations to negative as DOC increased (Fig. 3a, c). The interaction was statistically significant for emerging stream insects and marginally significant for spiders, whose MeHg concentrations were lower in 2011 than in 2012 (Table 2b-d; Fig. 2a-f). This marginally significant outcome was associated with low statistical power (0.40). Temperature, pH, and nutrients were not associated with MeHg concentrations in stream emerging insects or spiders (|ρ| < 0.52, P > 0.15; Appendices S2 and S5).
Figure 3.
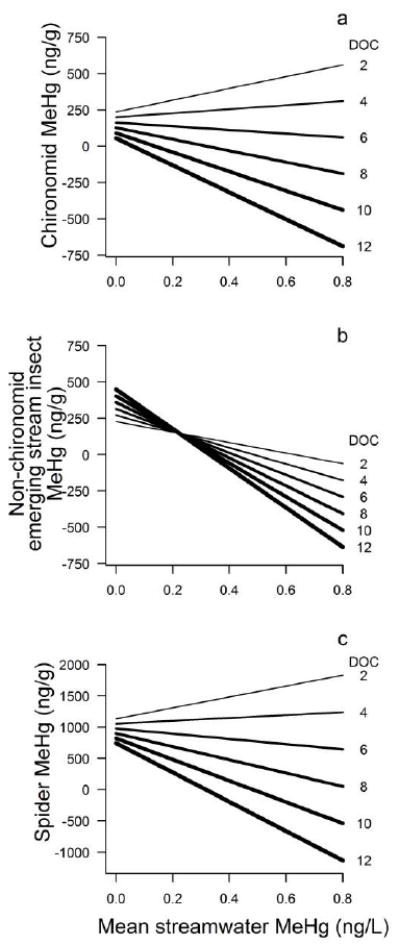
Response surfaces for the interactive effects of DOC concentration and streamwater MeHg concentration on the body MeHg concentration of the focal organisms associated with stream in the Lake Sunapee watershed, NH. Each line represents the relationship between body MeHg concentration and streamwater MeHg concentration at a particular DOC concentration, in mg C/L, based on the statistical models reported in Table 2.
Figure 2.
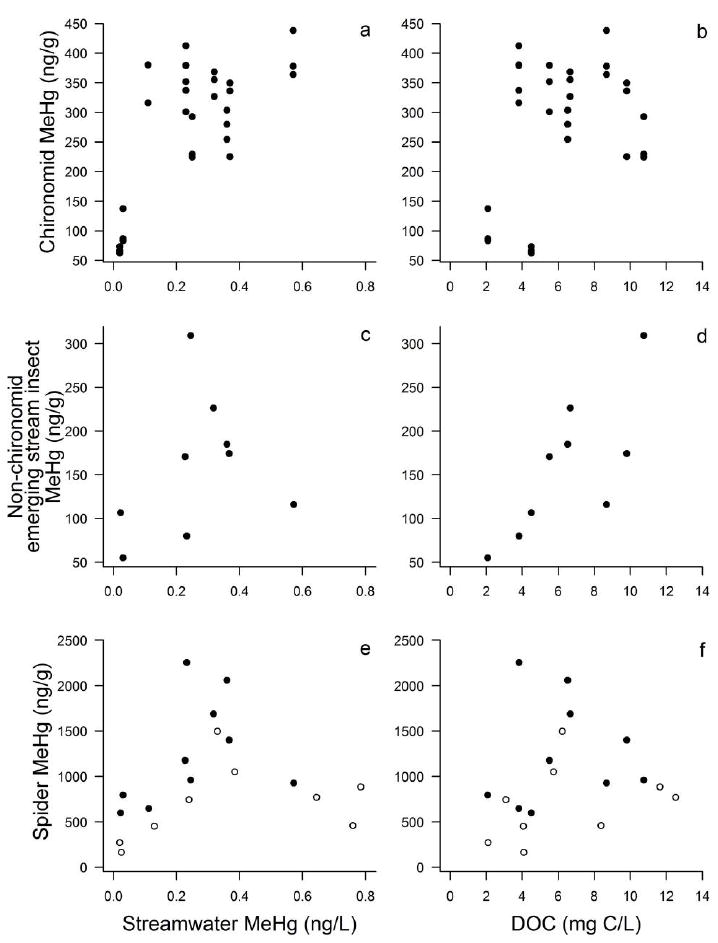
Relationship between mean streamwater MeHg concentration and mean DOC concentration, and mean MeHg concentration in emerging stream insects and riparian spiders for streams in the Lake Sunapee Watershed, NH. Open symbols show data collected in 2011 and closed symbols show data from 2012.
To facilitate interpretation of the interactions between aqueous MeHg, DOC, and MeHg bioaccumulation, we used the parameter estimates from the regression models to generate response surfaces for the relationship between streamwater MeHg concentration and biota MeHg concentration as a function of DOC (Fig. 3). The response surface showed similar responses for chironomids and spiders. In streams with DOC concentrations below 5.03 and 4.65 mg C/L (respectively), there was a positive relationship between streamwater and either chironomid or spider MeHg concentrations (Fig. 3a,c). In contrast, there was a negative relationship between streamwater and either chironomid or spider MeHg concentrations in streams with more than 5.03 and 4.65 mg C/L (respectively). For the non-chironomid emerging stream insects, the response surface showed a negative relationship between the insect and streamwater MeHg concentrations regardless of the DOC concentration in the stream, although the slope became increasingly negative as DOC concentration increased (Fig. 3b).
DISCUSSION
To date, most of our knowledge about the factors affecting the movement of Hg through food webs comes from aquatic systems (Driscoll et al. 2007, Chasar et al. 2009). This is likely because MeHg production generally occurs under reducing conditions, such as in wetlands and the anoxic sediment bed of streams and lakes. Furthermore, human health concerns over the neurotoxic effects of eating contaminated fish has intensified research on MeHg bioaccumulation on aquatic food webs (Fitzgerald and Clarkson 1991, Hamelin et al. 2011, Driscoll et al. 2013). However, the strong connection between aquatic and certain terrestrial food webs caused by emerging aquatic insects (Baxter et al. 2005) suggests that factors affecting Hg methylation and bioaccumulation in aquatic systems may impact a wider array of terrestrial consumers than previously thought. Recent research has focused on the movement of MeHg from freshwater to terrestrial food webs via aquatic insects (Wyman et al. 2011, Kraus et al. 2014b). However, as in aquatic food webs, the factors governing the movement of Hg between aquatic and terrestrial food webs are complex and not clearly understood. Our study shows that higher DOC concentrations may alter the bioavailability of MeHg to aquatic and terrestrial consumers. Taken together, this work extends our understanding of the ‘dark side’ of resource subsidies (sensu Walters et al. 2008), by revealing that streamwater chemistry (DOC) may alter the quality of subsidies to terrestrial consumers.
Our data suggest that DOC plays a dual role in MeHg dynamics. On the one hand, DOC concentration may affect MeHg bioaccumulation in emerging stream insects and their spider predators by increasing MeHg transport to streams from methylating sites. In contrast, DOC concentration may reduce the availability of MeHg for bioaccumulation. Streamwater MeHg concentration increased with DOC concentration, reinforcing the idea that DOC concentration is an important factor explaining streamwater MeHg concentrations (Brigham et al. 2009, Tsui et al. 2009). However, the increase in streamwater MeHg concentration did not translate into higher body MeHg concentrations in emerging stream insects and riparian spiders. Although streamwater MeHg concentrations increased with DOC (Fig. 1), our results suggest the MeHg bound to the DOC at higher DOC concentrations may be less readily available for uptake by the microorganisms that are the food source and pathway for MeHg uptake for many stream insects. This threshold effect of DOC on MeHg is supported by the decreasing slope with increasing DOC in all three panels of Fig. 3. Thus as DOC concentration increases there is an attenuation of MeHg bioaccumulation in chironomids and spiders. The negative relationship between streamwater MeHg concentrations and MeHg concentration in non-chironomid emerging stream insects along the entire DOC concentration range, depicted in the response surface, was unexpected, and may be related to the small sample size (n=3) for this group from streams below around 5 mg C/L DOC. Although the interaction between streamwater MeHg concentration and DOC concentration had low power and was marginally significant for the terrestrial consumer (P=0.09), the high reliance of T. elongata on emerging stream insects (here and by others, Gillespie 1987, Aiken and Coyle 2000), along with previous evidence of DOC attenuation of MeHg bioaccumulation, support our results.
The dual role of DOC in both transport of MeHg from methylating sites, and uptake attenuation has been hypothesized for years (Driscoll et al. 1994, Driscoll et al. 1995, Ravichandran 2004, Gorski et al. 2008, Tsui et al. 2009, Gerbig et al. 2011). Driscoll et al. (1994, 1995) suggested that MeHg concentrations in yellow perch from Adirondack lakes increased as DOC concentration increased up to 8 mg C/L, after which MeHg in perch was lower. Similarly, recent studies in Arctic lakes have quantitatively shown a threshold-type relationship between DOC and MeHg uptake by amphipods (French et al. 2014). Although the absolute concentration of DOC at which MeHg becomes attenuated has been highlighted, the absolute concentration may be context dependent (i.e., variable due to differences in consumer trophic level or system-specific DOC quality). Nonetheless, the attenuation of MeHg bioaccumulation and a threshold-type effect of DOC are common and have application to understanding MeHg flow through food webs. The present study extends the observation of the threshold effects of DOC to emerging aquatic insects and their riparian predators along these streams. To our knowledge, this is the first study showing that attenuation of MeHg bioaccumulation at high DOC concentrations extends from aquatic to terrestrial food webs via predation on aquatic insects.
Obtaining data on MeHg concentrations in multiple food web compartments is typically limited by the costs of sample analyses and the need for a minimum biomass of prey, which is difficult to obtain for small organisms such as plankton and emerging aquatic invertebrates. Although stream characteristics such as cold temperatures, low pH, and low nutrient concentrations have been associated with high MeHg bioaccumulation in other systems (Ullrich et al. 2001, Chen and Folt 2005, Jardine et al. 2013, Lavoie et al. 2013), these characteristics did not explain the variability in MeHg concentrations in our focal organisms across the studied streams, perhaps in part because of the limited range in these variables across streams. Other biotic factors not investigated in this study have also been shown to impact MeHg bioaccumulation including microbial species composition and sediment or periphyton methylation potential in lakes and streams (Macalady et al. 2000, Hamelin et al. 2011, Buckman et al. 2015). These factors were beyond the scope of this study. However, the wide range of DOC concentrations among the focal streams in the Lake Sunapee system likely enabled us to detect effects of DOC on MeHg bioaccumulation.
Although it was beyond the scope of this study to speciate DOC, we hypothesize that heterogeneity in the functional groups of DOC might explain its attenuating effect on MeHg bioaccumulation observed at high DOC concentrations. Landscape variables, such as the presence of wetlands and differences in soil types, may be influencing the variation in DOC concentrations, and these factors may also affect the chemical structure of the DOC in the study streams (Nguyen and Hur 2011). Increases in DOC concentrations have been associated with increases in less bioavailable humic acids and aromatic DOC (Wu et al. 2007, Tsui and Finlay 2011, French et al. 2014). Thus, high DOC streams may have high MeHg concentrations but low bioavailability of it because the DOC-MeHg complexes are too large for microbial uptake or have strong binding sites that restrict ligand exchange (Kerner et al. 2003, Gorski et al. 2006). Another potential mechanism decreasing MeHg bioavailability is a decrease in Hg bioavailability to methylating bacteria (Chiasson-Gould et al. 2014). However, the positive, linear relationship between streamwater MeHg concentration and DOC suggests that the rate of methylation is not a limiting factor in our high DOC streams.
Differences in dietary sources, physiologies of the prey, and assimilation efficiency of taxa by predators may be among the factors influencing how DOC concentration affects organismal MeHg concentration within and across ecosystems. These factors can alter the effects of water chemistry on MeHg bioaccumulation (Jardine et al. 2013). For example, researchers found a decrease in MeHg bioaccumulation above a DOC concentration of 5 mg C/L in phytoplankton, seston, and hydropsychid larvae (Gorski et al. 2008, Tsui and Finlay 2011), while this concentration was reported at 8 mg C/L for amphipods and fish (Driscoll et al. 1994, French et al. 2014). Thus, the effect of DOC on MeHg concentration is inherently variable, and this variability is compounded with each additional trophic level, potentially explaining the variability in spider MeHg concentration. Because percent MeHg typically increases with trophic level (Mason et al. 2000, Driscoll et al. 2007, Chen et al. 2009), and spider percent MeHg was less than that of chironomids but greater than that of non-chironomid emerging stream insects, non-chironomids are likely a more important source of assimilated food, and therefore MeHg, than chironomids. This suggests that the relative abundance of diet items found in webs may not be an accurate reflection of what was actually consumed and assimilated. Further research is needed to understand the factors affecting the role that DOC plays in MeHg’s movement through different parts of the food web and in different food webs.
Furthermore, temporal changes in water chemistry and environmental factors affect MeHg availability and bioaccumulation in food webs (Chen et al. 2012, Eklof et al. 2015). In this study, there was little temporal variation of MeHg in chironomids during summer, however there may be large interannual variation. Although streamwater MeHg and DOC concentrations were higher in 2011, spiders collected in 2011 had lower MeHg concentrations than those collected in 2012. These interannual differences in spider MeHg are likely the result of spiders collected in 2012 having fed as juveniles on stream insects emerging in 2011; hence, MeHg bioaccumulated by the juveniles may have impacted MeHg loads in adult spiders. Further research is warranted to understand how temporal variation of DOC and streamwater MeHg concentrations affect MeHg bioaccumulation in stream-terrestrial food webs. Thus there is inherent variability in bioaccumulation of MeHg in terrestrial consumers due to myriad factors. The interaction between MeHg and DOC concentration is decipherable nonetheless, and merits further study.
Spiders connect aquatic and terrestrial food webs by preying on emerging aquatic insects and serving as prey for terrestrial wildlife such as birds and lizards (Ballinger and Lake 2006, Richardson et al. 2010). Furthermore, some studies report higher Hg concentrations in terrestrial invertivores than omnivores or piscivores, especially birds living near water (Cristol et al. 2008, Keller et al. 2014). Gann et al. (2015) found that Tetragnatha sp. near Caddo Lake and its associated wetlands exceeded the minimum tissue MeHg concentrations (19.4 -256 ng/g) that would cause physiological impairment in arachnivorous birds (USEPA 1995) – and the mean MeHg concentration found in Tetragnatha spiders in this study was 3.9 times higher than the highest MeHg concentration reported by Gann et al. (2015). Moreover, MeHg concentrations in spiders in our study sites were also higher than in fish collected from the same streams (Broadley et al. unpublished manuscript), emphasizing the importance of MeHg bioaccumulation across aquatic-terrestrial boundaries in non-piscivorous terrestrial consumers. Because spiders are easy to collect, have enough biomass for accurate Hg speciation analyses, and reflect the effects of DOC on streamwater MeHg concentrations, we propose that they are good indicator species for monitoring Hg risk.
Because MeHg is transmitted through food webs (Hall et al. 1997), increased MeHg in prey is an important risk factor for MeHg exposure in both aquatic and terrestrial consumers. Our study shows that higher DOC concentrations may alter the relationship between MeHg in streamwater and its bioavailability to aquatic and terrestrial consumers. Thus our results highlight the complexity of the relationship between DOC, streamwater MeHg and MeHg concentration in biota, and suggest caution when using DOC as an indicator of biotic sensitivity to Hg hotspots under the assumption of a linear relationship between DOC and MeHg bioaccumulation in biota (Driscoll et al. 2007, Evers et al. 2007). Moreover, DOC concentrations and chemical composition of freshwaters are changing globally (Findlay 2005, Evans et al. 2006, Dawson et al. 2009, Ritson et al. 2014); how these changes interact with MeHg risk to aquatic and terrestrial organisms warrants further study.
Supplementary Material
Appendix S1. Map of focal stream sites.
Appendix S2. Watershed characteristics and streamwater chemistry of the focal streams.
Appendix S3. Image of emergence traps used to collect emerging stream insects.
Appendix S4. MeHg concentrations in emerging stream prey.
Appendix S5. Correlation coefficients between stream variables and streamwater MeHg concentration, and the MeHg concentration in focal biota.
Acknowledgments
We gratefully acknowledge the undergraduate students that made this research possible: Amy Van Scoyoc helped make the emergence traps; Ellen Gawarkiewicz, Katie Irwin, Chelsea Slogic, Caroline Watzon, and Molly Zegans helped with field collections; Ian Moore and Phurchhoki Sherpa helped with sample processing in the laboratory; and Paul Boynton and Jenisha Shrestha helped with making emergence traps, field collections, and sample processing. In addition, Kate Buckman, Jessica Warkentien, and Eli Wyman helped with field collections. We also thank Mark Townley for help with spider taxonomy, natural history, and advice. Zak J. Gezon provided feedback on various stages of this manuscript and made the final figures. We acknowledge the Dartmouth Trace Element Analysis Core Facility and the contributions of Brian Jackson, Vivien Taylor, and Arthur Baker for Hg speciation analysis of streamwater and biotic samples. Lastly, David Fischer and Amanda Lindsey conducted the DOC analyses, managed data, and created Appendix S1. The Lake Sunapee Protective Association provided lodging and logistical support during the field portion of the study. Thanks also to the anonymous reviewers who offered valuable feedback and greatly improved this manuscript. This publication was made possible by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National institutes of Health, grant number P20GM103506; the Dartmouth Superfund Research Program funded by NIH Grant Number P42 ES007373 from the National Institute of Environmental Health Sciences; National Science Foundation grant numbers EF-0842267, EF-0842112, and EF-0842125; the Dartmouth College Cramer Fund, and the Arts and Sciences Graduate Alumni Research Award. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or the National Institute of Environmental Health Sciences.
LITERATURE CITED
- Aiken M, Coyle FA. Habitat distribution, life history and behavior of Tetragnatha spider species in the Great Smoky Mountains National Park. Journal of Arachnology. 2000;28:97–106. [Google Scholar]
- AMAP/UNEP. Technical background report for the global mercury assessment 2013. Arctic Monitoring and Assessment Programme, Oslo, Norway/UNEP Chemical Branch; Geneva, Switzerland: 2013. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater. American Public Health Association; Washington, D.C., USA: 1980. [Google Scholar]
- Ballinger A, Lake PS. Energy and nutrient fluxes from rivers and streams into terrestrial food webs. Marine and Freshwater Research. 2006;57:15–28. [Google Scholar]
- Bartrons M, Gratton C, Spiesman BJ, Vander Zanden MJ. Taking the trophic bypass: aquatic-terrestrial linkage reduces methylmercury in a terrestrial food web. Ecological Applications. 2015;25:151–159. doi: 10.1890/14-0038.1. [DOI] [PubMed] [Google Scholar]
- Baxter CV, Fausch KD, Saunders WC. Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biology. 2005;50:201–220. [Google Scholar]
- Brar NK, Waggoner C, Reyes JA, Fairey R, Kelley KM. Evidence for thyroid endocrine disruption in wild fish in San Francisco Bay, California, USA. Relationships to contaminant exposures. Aquatic Toxicology. 2010;96:203–215. doi: 10.1016/j.aquatox.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Breusch TS, Pagan AR. A simple test for heteroscedasticity and random coefficient variation. Econometrica. 1979;47:1287–1294. [Google Scholar]
- Brigham ME, Wentz DA, Aiken GR, Krabbenhoft DP. Mercury Cycling in Stream Ecosystems. 1. Water Column Chemistry and Transport. Environmental Science & Technology. 2009;43:2720–2725. doi: 10.1021/es802694n. [DOI] [PubMed] [Google Scholar]
- Buckman KL, Marvin-DiPasquale M, Taylor VF, Chalmers A, Broadley HJ, Agee J, Jackson BP, Chen CY. Influence of a chlor-alkali superfund site on mercury bioaccumulation in periphyton and low-trophic level fauna. Environmental Toxicology and Chemistry. 2015;34:1649–1658. doi: 10.1002/etc.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Jaffe R, Jones RD. Interactions between dissolved organic carbon and mercury species in surface waters of the Florida Everglades. Applied Geochemistry. 1999;14:395–407. [Google Scholar]
- Chan HM, Scheuhammer AM, Ferran A, Loupelle C, Holloway J, Weech S. Impacts of mercury on freshwater fish-eating wildlife and humans. Human and Ecological Risk Assessment. 2003;9:867–883. [Google Scholar]
- Chasar LC, Scudder BC, Stewart AR, Bell AH, Aiken GR. Mercury cycling in stream ecosystems. 3. Trophic dynamics and methylmercury bioaccumulation. Environmental Science & Technology. 2009;43:2733–2739. doi: 10.1021/es8027567. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Hadi AS. Regression analysis by example. Fifth. John Wiley & Sons, Inc; Hoboken, New Jersey: 2012. [Google Scholar]
- Chen CY, Dionne M, Mayes BM, Ward DM, Sturup S, Jackson BP. Mercury Bioavailability and Bioaccumulation in Estuarine Food Webs in the Gulf of Maine. Environmental Science & Technology. 2009;43:1804–1810. doi: 10.1021/es8017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Folt CL. High plankton densities reduce mercury biomagnification. Environmental Science & Technology. 2005;39:115–121. [PubMed] [Google Scholar]
- Chen CY, Kamman N, Williams J, Bugge D, Taylor V, Jackson B, Miller E. Spatial and temporal variation in mercury bioaccumulation by zooplankton in Lake Champlain (North America) Environmental Pollution. 2012;161:343–349. doi: 10.1016/j.envpol.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson-Gould SA, Blais JM, Poulain AJ. Dissolved organic matter kinetically controls mercury bioavailability to bacteria. Environmental Science & Technology. 2014;48:3153–3161. doi: 10.1021/es4038484. [DOI] [PubMed] [Google Scholar]
- Christensen JR, Macduffee M, Macdonald RW, Whiticar M, Ross PS. Persistent organic pollutants in British Columbia grizzly bears: Consequence of divergent diets. Environmental Science & Technology. 2005;39:6952–6960. doi: 10.1021/es050749f. [DOI] [PubMed] [Google Scholar]
- Cremona F, Planas D, Lucotte M. Assessing the importance of macroinvertebrate trophic dead ends in the lower transfer of methylmercury in littoral food webs. Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:2043–2052. [Google Scholar]
- Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, White AE. The movement of aquatic mercury through terrestrial food webs. Science. 2008;320:335–335. doi: 10.1126/science.1154082. [DOI] [PubMed] [Google Scholar]
- Crumpton WG, Isenhart TM, Mitchell PD. Nitrate and organic N analyses with 2nd-derivative spectroscopy. Limnology and Oceanography. 1992;37:907–913. [Google Scholar]
- Culin JD, Yeargan KV. Feeding behavior and prey of Neoscona arabesca [Araneae: Araneidae] and Tetragnatha laboriosa [Araneae: Tetragnathidae] in soybean fields. Entomophaga. 1982;27:417–423. [Google Scholar]
- Dawson JJC, Malcolm IA, Middlemas SJ, Tetzlaff D, Soulsby C. Is the composition of dissolved organic carbon changing in upland acidic streams? Environmental Science & Technology. 2009;43:7748–7753. doi: 10.1021/es901649b. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Blette V, Yan C, Schofield CL, Munson R, Holsapple J. The role of dissolved organic-carbon in the chemistry and bioavailability of mercury in remote Adirondack lakes. Water Air and Soil Pollution. 1995;80:499–508. [Google Scholar]
- Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK. Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. Bioscience. 2007;57:17–28. [Google Scholar]
- Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N. Mercury as a global pollutant: Sources, pathways, and effects. Environmental Science & Technology. 2013;47:4967–4983. doi: 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll CT, Yan C, Schofield CL, Munson R, Holsapple J. The mercury cycle and fish in the Adirondack lakes. Environmental Science & Technology. 1994;28:A136–A143. doi: 10.1021/es00052a721. [DOI] [PubMed] [Google Scholar]
- Eklof K, Kraus A, Futter M, Schelker J, Meili M, Boyer EW, Bishop K. Parsimonious Model for Simulating Total Mercury and Methylmercury in Boreal Streams Based on Riparian Flow Paths and Seasonality. Environmental science & technology. 2015;49:7851–7859. doi: 10.1021/acs.est.5b00852. [DOI] [PubMed] [Google Scholar]
- Evans CD, Chapman PJ, Clark JM, Monteith DT, Cresser MS. Alternative explanations for rising dissolved organic carbon export from organic soils. Global Change Biology. 2006;12:2044–2053. [Google Scholar]
- Evers DC, Burgess NM, Champoux L, Hoskins B, Major A, Goodale WM, Taylor RJ, Poppenga R, Daigle T. Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology. 2005;14:193–221. doi: 10.1007/s10646-004-6269-7. [DOI] [PubMed] [Google Scholar]
- Evers DC, Han Y-J, Driscoll CT, Kamman NC, Goodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T. Biological mercury hotspots in the northeastern United States and southeastern Canada. Bioscience. 2007;57:29–43. [Google Scholar]
- Findlay S, McDowell WH, Fischer D, Pace ML, Caraco N, Kaushal SS, Weathers KC. Total carbon analysis may overestimate organic carbon content of fresh waters in the presence of high dissolved inorganic carbon. Limnology and Oceanography-Methods. 2010;8:196–201. [Google Scholar]
- Findlay SEG. Increased carbon transport in the Hudson River: unexpected consequence of nitrogen deposition? Frontiers in Ecology and the Environment. 2005;3:133–137. [Google Scholar]
- Fitzgerald WF, Clarkson TW. Mercury and monomethylmercury: present and future concerns. Environmental Health Perspectives. 1991;96:159–166. doi: 10.1289/ehp.9196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming EJ, Mack EE, Green PG, Nelson DC. Mercury methylation from unexpected sources: Molybdate-inhibited freshwater sediments and an iron-reducing bacterium. Applied and Environmental Microbiology. 2006;72:457–464. doi: 10.1128/AEM.72.1.457-464.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French TD, Houben AJ, Desforges JPW, Kimpe LE, Kokelj SV, Poulain AJ, Smol JP, Wang XW, Blais JM. Dissolved organic carbon thresholds affect mercury bioaccumulation in Arctic Lakes. Environmental Science & Technology. 2014;48:3162–3168. doi: 10.1021/es403849d. [DOI] [PubMed] [Google Scholar]
- Gann GL, Powell CH, Chumchal MM, Drenner RW. Hg-contaminated terrestrial spiders pose a potential risk to songbirds at Caddo Lake (Texas/Louisiana, USA) Environmental Toxicology and Chemistry. 2015;34:303–306. doi: 10.1002/etc.2796. [DOI] [PubMed] [Google Scholar]
- Gerbig CA, Ryan JN, Aiken GR. The Effects of dissolved organic matter on mercury biogeochemistry. In: Liu G, C Y, O’Driscoll N, editors. Environmental chemistry and toxicology of mercury. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2011. [Google Scholar]
- Gillespie RG. The Mechanism of habitat selection in the Long-jawed orb-weaving spider Tetragnatha elongata (Araneae, Tetragnathidae) Journal of Arachnology. 1987;15:81–90. [Google Scholar]
- Gilmour CC, Henry EA, Mitchell R. Sulfate stimulation of mercury methylation in freshwater sediments. Environmental Science & Technology. 1992;26:2281–2287. [Google Scholar]
- Gorski PR, Armstrong DE, Hurley JP, Krabbenhoft DP. Influence of natural dissolved organic carbon on the bioavailability of mercury to a freshwater alga. Environmental Pollution. 2008;154:116–123. doi: 10.1016/j.envpol.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Gorski PR, Armstrong DE, Hurley JP, Shafer MM. Speciation of aqueous methylmercury influences uptake by a freshwater alga (Selenastrum capricornutum) Environmental Toxicology and Chemistry. 2006;25:534–540. doi: 10.1897/04-530r.1. [DOI] [PubMed] [Google Scholar]
- Grigal DF. Inputs and outputs of mercury from terrestrial watersheds: a review. Environmetal Reviews. 2002;10:1–39. [Google Scholar]
- Hall BD, Bodaly RA, Fudge RJP, Rudd JWM, Rosenberg DM. Food as the dominant pathway of methylmercury uptake by fish. Water Air and Soil Pollution. 1997;100:13–24. [Google Scholar]
- Hamelin S, Amyot M, Barkay T, Wang Y, Planas D. Methanogens: Principal methylators of mercury in lake periphyton. Environmental Science & Technology. 2011;45:7693–7700. doi: 10.1021/es2010072. [DOI] [PubMed] [Google Scholar]
- Hopkins BC, Willson JD, Hopkins WA. Mercury exposure is associated with negative effects on turtle reproduction. Environmental Science & Technology. 2013;47:2416–2422. doi: 10.1021/es304261s. [DOI] [PubMed] [Google Scholar]
- Hose GC, James JM, Gray MR. Spider webs as environmental indicators. Environmental Pollution. 2002;120:725–733. doi: 10.1016/s0269-7491(02)00171-9. [DOI] [PubMed] [Google Scholar]
- Jackson B, Taylor V, Baker RA, Miller E. Low-level mercury speciation in freshwaters by isotope dilution GC-ICP-MS. Environmental Science & Technology. 2009;43:2463–2469. doi: 10.1021/es802656p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine TD, Kidd KA, O’Driscoll N. Food web analysis reveals effects of pH on mercury bioaccumulation at multiple trophic levels in streams. Aquatic Toxicology. 2013;132:46–52. doi: 10.1016/j.aquatox.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Jardine TD, Kidd KA, Rasmussen JB. Aquatic and terrestrial organic matter in the diet of stream consumers: implications for mercury bioaccumulation. Ecological Applications. 2012;22:843–855. doi: 10.1890/11-0874.1. [DOI] [PubMed] [Google Scholar]
- Keller RH, Xie LT, Buchwalter DB, Franzreb KE, Simons TR. Mercury bioaccumulation in Southern Appalachian birds, assessed through feather concentrations. Ecotoxicology. 2014;23:304–316. doi: 10.1007/s10646-013-1174-6. [DOI] [PubMed] [Google Scholar]
- Kerner M, Hohenberg H, Ertl S, Reckermann M, Spitzy A. Self-organization of dissolved organic matter to micelle-like microparticles in river water. Nature. 2003;422:150–154. doi: 10.1038/nature01469. [DOI] [PubMed] [Google Scholar]
- Kidd KA, Clayden M, Jardine T. Bioaccumulation and biomagnification of mercury in food webs. In: Liu G, Cai Y, O’Driscoll N, editors. Environmental chemistry and toxicology of mercury. John Wiley & Sons, Inc; New Jersey: 2012. pp. 455–500. [Google Scholar]
- Kraus JM, Schmidt TS, Walters DM, Wanty RB, Zuellig RE, Wolf RE. Cross-ecosystem impacts of stream pollution reduce resource and contaminant flux to riparian food web. Ecological Applications. 2014a;24:235–243. doi: 10.1890/13-0252.1. [DOI] [PubMed] [Google Scholar]
- Kraus JM, Walters DM, Wesner J, Stricker CA, Schmidt TS, Zuellig R. Metamorphosis alters contaminants and chemical tracers in insects: implications for food webs. Environmental Science and Technology. 2014b;48:10957–10965. doi: 10.1021/es502970b. [DOI] [PubMed] [Google Scholar]
- Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM. Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Environmental Science & Technology. 2013;47:13385–13394. doi: 10.1021/es403103t. [DOI] [PubMed] [Google Scholar]
- Lesar CD, Unzicker JD. Life history, habits, and prey preferences of Tetragnatha laboriosa (Araneae: Tetragnathidae) Environmental Entomology. 1978;7:879–884. [Google Scholar]
- Macalady JL, Mack EE, Nelson DC, Scow KM. Sediment microbial community structure and mercury methylation in mercury-polluted Clear Lake, California. Applied and Environmental Microbiology. 2000;66:1479–1488. doi: 10.1128/aem.66.4.1479-1488.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Laporte JM, Andres S. Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Archives of Environmental Contamination and Toxicology. 2000;38:283–297. doi: 10.1007/s002449910038. [DOI] [PubMed] [Google Scholar]
- McCafferty WP. Aquatic entomology: the fishermen’s and ecologists illustrated guide to insects and their relatives. 83. Jones & Bartlett Publishers; Sudbury, MA: 1983. [Google Scholar]
- Merritt RW, Cummins KW, Berg MB. An introduction to the aquatic insects of North America. 4. Kendall/Hunt; Iowa: 2008. [Google Scholar]
- NADP. Total mercury wet deposition, 2011. University of Illinois; Champaign, IL 61820: 2015a. [Google Scholar]
- NADP. Total mercury wet deposition, 2012. University of Illinois; Champaign, IL 61820: 2015b. [Google Scholar]
- Nam D-H, Yates D, Ardapple P, Evers DC, Schmerfeld J, Basu N. Elevated mercury exposure and neurochemical alterations in little brown bats (Myotis lucifugus) from a site with historical mercury contamination. Ecotoxicology. 2012;21:1094–1101. doi: 10.1007/s10646-012-0864-9. [DOI] [PubMed] [Google Scholar]
- Nguyen HVM, Hur J. Tracing the sources of refractory dissolved organic matter in a large artificial lake using multiple analytical tools. Chemosphere. 2011;85:782–789. doi: 10.1016/j.chemosphere.2011.06.068. [DOI] [PubMed] [Google Scholar]
- Paetzold A, Smith M, Warren PH, Maltby L. Environmental impact propagated by cross-system subsidy: Chronic stream pollution controls riparian spider populations. Ecology. 2011;92:1711–1716. doi: 10.1890/10-2184.1. [DOI] [PubMed] [Google Scholar]
- Pickhardt PC, Fisher NS. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environmental Science & Technology. 2007;41:125–131. doi: 10.1021/es060966w. [DOI] [PubMed] [Google Scholar]
- Point D, Davis WC, Garcia Alonso JI, Monperrus M, Christopher SJ, Donard OFX, Becker PR, Wise SA. Simultaneous determination of inorganic mercury, methylmercury, and total mercury concentrations in cryogenic fresh-frozen and freeze-dried biological reference materials. Analytical and Bioanalytical Chemistry. 2007;389:787–798. doi: 10.1007/s00216-007-1516-4. [DOI] [PubMed] [Google Scholar]
- Power ME, Rainey WE, Parker MS, Sabo JL, Smyth A, Khandwala S, Finlay JC, McNeely FC, Marsee K, Anderson C. River to watershed subsidies in an old-growth conifer forest. In: Polis GA, Power ME, Huxel GR, editors. Food webs at the landscape level. University of Chicago Press; Chicago, IL: 2004. pp. 217–240. [Google Scholar]
- R development core team. R: A language and environment for statistical computing. R foundation for statistical computing; Vienna, Austria: 2013. http://www.R-project.org. [Google Scholar]
- Raikow DF, Walters DM, Fritz KM, Mills MA. The distance that contaminated aquatic subsidies extend into lake riparian zones. Ecological Applications. 2011;21:983–990. doi: 10.1890/09-1504.1. [DOI] [PubMed] [Google Scholar]
- Rasmussen JB, Vander Zanden MJ. The variation of lake food webs across the landscape and its effect on contaminant dynamics. In: Polis GA, Power MA, Huxel GR, editors. Food Webs at the landscape level. The University of Chicago Press; Chicago, USA: 2004. pp. 169–182. [Google Scholar]
- Ravichandran M. Interactions between mercury and dissolved organic matter - a review. Chemosphere. 2004;55:319–331. doi: 10.1016/j.chemosphere.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Richardson JS, Zhang YX, Marczak LB. Resource subsidies across the land-freshwater interface and responses in recipient communities. River Research and Applications. 2010;26:55–66. [Google Scholar]
- Ritson JP, Graham NJD, Templeton MR, Clark JM, Gough R, Freeman C. The impact of climate change on the treatability of dissolved organic matter (DOM) in upland water supplies: A UK perspective. Science of the Total Environment. 2014;473:714–730. doi: 10.1016/j.scitotenv.2013.12.095. [DOI] [PubMed] [Google Scholar]
- Rowse LM, Rodewald AD, Sullivan SMP. Pathways and consequences of contaminant flux to Acadian flycatchers (Empidonax virescens) in urbanizing landscapes of Ohio, USA. Science of the Total Environment. 2014;485:461–467. doi: 10.1016/j.scitotenv.2014.03.095. [DOI] [PubMed] [Google Scholar]
- Sanzone DM, Meyer JL, Marti E, Gardiner EP, Tank JL, Grimm NB. Carbon and nitrogen transfer from a desert stream to riparian predators. Oecologia. 2003;134:238–250. doi: 10.1007/s00442-002-1113-3. [DOI] [PubMed] [Google Scholar]
- SAS institute Inc. JMP® version 11. Cary, NC, USA: 2013. [Google Scholar]
- Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO: A Journal of the Human Environment. 2007;36:12–19. doi: 10.1579/0044-7447(2007)36[12:eoemot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schoch N, Glennon MJ, Evers DC, Duron M, Jackson AK, Driscoll CT, Ozard JW, Sauer AK. The impact of mercury exposure on the common loon (Gavia immer) population in the Adirondack Park, New York, USA. Waterbirds. 2014;37:133–146. [Google Scholar]
- Schuster PF, Shanley JB, Marvin-Dipasquale M, Reddy MM, Aiken GR, Roth DA, Taylor HE, Krabbenhoft DP, DeWild JF. Mercury and organic carbon dynamics during runoff episodes from a northeastern USA watershed. Water Air and Soil Pollution. 2008;187:89–108. [Google Scholar]
- Speir SL, Chumchal MM, Drenner RW, Cocke WG, Lewis ME, Whitt HJ. Methyl mercury and stable isotopes of nitrogen reveal that a terrestrial spider has a diet of emergent aquatic insects. Environmental Toxicology and Chemistry. 2014;33:2506–2509. doi: 10.1002/etc.2700. [DOI] [PubMed] [Google Scholar]
- Taylor VF, Jackson BP, Chen CY. Mercury speciation and total trace element determination of low-biomass biological samples. Analytical and Bioanalytical Chemistry. 2008;392:1283–1290. doi: 10.1007/s00216-008-2403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay A, Cloutier L, Lucotte M. Total mercury and methylmercury fluxes via emerging insects in recently flooded hydroelectric reservoirs and a natural lake. Science of the Total Environment. 1998;219:209–221. [Google Scholar]
- Tsui MTK, Finlay JC. Influence of dissolved organic carbon on methylmercury bioavailability across Minnesota stream ecosystems. Environmental Science & Technology. 2011;45:5981–5987. doi: 10.1021/es200332f. [DOI] [PubMed] [Google Scholar]
- Tsui MTK, Finlay JC, Nater EA. Mercury bioaccumulation in a stream network. Environmental Science & Technology. 2009;43:7016–7022. doi: 10.1021/es901525w. [DOI] [PubMed] [Google Scholar]
- Ullrich SM, Tanton TW, Abdrashitova SA. Mercury in the aquatic environment: A review of factors affecting methylation. Critical Reviews in Environmental Science and Technology. 2001;31:241–293. [Google Scholar]
- USEPA. Great Lakes Water Quality Initiative: Criteria documents for the protection of wildlife:DDT, Mercury, 2,3,7,8-TCDD, PCBs. Washington, DC: 1995. [Google Scholar]
- USEPA. Technical fact sheet: EPA-820-F-11-009. 2010. National listing of fish advisories. September 2011. [Google Scholar]
- Varian-Ramos CW, Swaddle JP, Cristol DA. Mercury reduces avian reproductive success and imposes selection: an experimental study with adult- or lifetime-exposure in zebra finch. PloS one. 2014;9:e95674–e95674. doi: 10.1371/journal.pone.0095674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Yates DE, Evers DC, Taylor RJ, Hopkins WA. Tissue mercury concentrations and adrenocortical responses of female big brown bats (Eptesicus fuscus) near a contaminated river. Ecotoxicology. 2010;19:1277–1284. doi: 10.1007/s10646-010-0513-0. [DOI] [PubMed] [Google Scholar]
- Walters DM, Fritz KM, Otter RR. The dark side of subsidies: adult stream insects export organic contaminants to riparian predators. Ecological Applications. 2008;18:1835–1841. doi: 10.1890/08-0354.1. [DOI] [PubMed] [Google Scholar]
- Ward DM, Nislow KH, Folt CL. Year in Ecology and Conservation Biology 2010. Blackwell Publishing; Oxford: 2010. Bioaccumulation syndrome: identifying factors that make some stream food webs prone to elevated mercury bioaccumulation; pp. 62–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson MF, Halupka KC. Anadromous fish as keystone species in vertebrate communities. Conservation Biology. 1995;9:489–497. [Google Scholar]
- Wolfe MF, Schwarzbach S, Sulaiman RA. Effects of mercury on wildlife: A comprehensive review. Environmental Toxicology and Chemistry. 1998;17:146–160. [Google Scholar]
- Wu FC, Kothawala DN, Evans RD, Dillon PJ, Cai YR. Relationships between DOC concentration, molecular size and fluorescence properties of DOM in a stream. Applied Geochemistry. 2007;22:1659–1667. [Google Scholar]
- Wurtsbaugh WA, Gardberg J, Izdepski C. Biostrome communities and mercury and selenium bioaccumulation in the Great Salt Lake (Utah, USA) Science of the Total Environment. 2011;409:4425–4434. doi: 10.1016/j.scitotenv.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Wyman KE, Rodenhouse NL, Bank MS. Mercury bioaccumulation, speciation, and influence on web structure in orb-weaving spiders from a forested watershed. Environmental Toxicology and Chemistry. 2011;30:1873–1878. doi: 10.1002/etc.572. [DOI] [PubMed] [Google Scholar]
- Xiao-li S, Yu P, Hose GC, Iian C, Feng-xiang L. Spider webs as indicators of heavy metal pollution in air. Bulletin of Environmental Contamination and Toxicology. 2006;76:271–277. doi: 10.1007/s00128-006-0917-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Map of focal stream sites.
Appendix S2. Watershed characteristics and streamwater chemistry of the focal streams.
Appendix S3. Image of emergence traps used to collect emerging stream insects.
Appendix S4. MeHg concentrations in emerging stream prey.
Appendix S5. Correlation coefficients between stream variables and streamwater MeHg concentration, and the MeHg concentration in focal biota.


