Abstract
Purpose
We performed a multi-institutional study to identify prognostic factors and determine outcomes for patients with ALK-rearranged non–small-cell lung cancer (NSCLC) and brain metastasis.
Patients and Methods
A total of 90 patients with brain metastases from ALK-rearranged NSCLC were identified from six institutions; 84 of 90 patients received radiotherapy to the brain (stereotactic radiosurgery [SRS] or whole-brain radiotherapy [WBRT]), and 86 of 90 received tyrosine kinase inhibitor (TKI) therapy. Estimates for overall (OS) and intracranial progression-free survival were determined and clinical prognostic factors were identified by Cox proportional hazards modeling.
Results
Median OS after development of brain metastases was 49.5 months (95% CI, 29.0 months to not reached), and median intracranial progression-free survival was 11.9 months (95% CI, 10.1 to 18.2 months). Forty-five percent of patients with follow-up had progressive brain metastases at death, and repeated interventions for brain metastases were common. Absence of extracranial metastases, Karnofsky performance score ≥ 90, and no history of TKIs before development of brain metastases were associated with improved survival (P = .003, < .001, and < .001, respectively), whereas a single brain metastasis or initial treatment with SRS versus WBRT were not (P = .633 and .666, respectively). Prognostic factors significant by multivariable analysis were used to describe four patient groups with 2-year OS estimates of 33%, 59%, 76%, and 100%, respectively (P < .001).
Conclusion
Patients with brain metastases from ALK-rearranged NSCLC treated with radiotherapy (SRS and/or WBRT) and TKIs have prolonged survival, suggesting that interventions to control intracranial disease are critical. The refinement of prognosis for this molecular subtype of NSCLC identifies a population of patients likely to benefit from first-line SRS, close CNS observation, and treatment of emergent CNS disease.
INTRODUCTION
CNS metastases are common in patients with non–small-cell lung cancer (NSCLC), developing in approximately 30% of patients with advanced-stage adenocarcinomas.1 It has been recognized that patients with brain metastases comprise a heterogeneous population.2-5 With regard to brain metastases from NSCLC, the disease-specific graded prognostic assessment (GPA) has identified patient groups for which median survival ranges from 3 to 14.8 months.6,7
Approximately 5% of NSCLCs have ALK rearrangement,8 and these patients achieve prolonged progression-free survival (PFS) when treated with crizotinib, an ALK-targeted tyrosine kinase inhibitor (TKI).9-12 However, brain metastases and disease progression in the brain are common in this subpopulation13-15 and may result from poor CNS penetration by crizotinib.16 Control of metastatic disease to the brain is now emerging as a crucial issue in the treatment of these patients, and it has been suggested that local control of disease at sites of oligoprogression may improve outcomes.14,17
Local treatment strategies for brain metastases include whole-brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), or surgical resection, either alone, in combination, or as multiple sequential treatments. Because the optimal treatment approach for brain metastases varies according to the specific patient subset and expected prognosis, we sought to describe outcomes for patients with NSCLC and ALK rearrangement and to identify prognostic factors that can be used to appropriately tailor treatment strategies. Our analysis of a large cohort of patients with brain metastases from ALK-rearranged NSCLC provides significant new insights into outcomes for this patient population. These findings establish a role for the use of molecularly defined prognostic indices to tailor treatment for patients with CNS metastases from NSCLC.
PATIENTS AND METHODS
Patient information was collected under individual institutional review board–approved protocols. Criteria for inclusion in this retrospective study were a diagnosis of ALK-rearranged NSCLC, brain metastasis, and evaluation for radiotherapy (RT). ALK translocation was determined by fluorescent in situ hybridization. A total of 90 patients with brain metastases diagnosed between 2007 and 2014 were identified from six institutions: University of Colorado (n = 33), Yale University (n = 17), Memorial Sloan Kettering Cancer Center (n = 14), Ohio State University (n = 11), Dana-Farber Cancer Institute (n = 9), and Vanderbilt University (n = 6).
Age, sex, Karnofsky performance score (KPS), smoking history, stage at diagnosis, time interval to developing brain metastasis, number of metastatic brain lesions, and presence of extracranial metastasis (ECM) at the time of brain metastasis diagnosis were recorded. Systemic disease status at the time of brain metastasis was also classified as stable, oligoprogressive (≤ four sites of worsening extracranial disease), or progressive. Treatment dates, follow-up, CNS disease control, and characteristics describing RT, chemotherapy, TKI treatment, or neurosurgical intervention were also recorded.
Statistical analysis was performed using STATA software (version 13.1; STATA, College Station, TX). Kaplan-Meier analysis was used to estimate overall survival (OS) and intracranial PFS stratified by patient or treatment characteristics, and the log-rank test was used to assess for differences. OS was calculated from the date of brain metastasis diagnosis to the time of death. Intracranial progression was calculated from the date of brain metastasis diagnosis to first progression in the brain. Multivariable analysis was performed using the Cox proportional hazards regression model. A two-sided P value ≤ .05 was considered statistically significant. Independent predictors of OS were identified by multivariable analysis, and Kaplan-Meier analysis was used to estimate median survival and 2-year survival for patients with zero, one, two, or three factors.
RESULTS
Patient characteristics are summarized in Table 1. Median follow-up was 38.1 months (range, 0.95 to 185.5 months) when calculated from the date of lung cancer diagnosis and 16.0 months (range, 0.16 to 82.2 months) from the date of first brain metastasis. Patients from this cohort were relatively young (median age, 52 years; range, 23 to 80 years), with a large proportion of nonsmokers (67%). Most patients in this study presented with stage IV disease, and 30% of patients had brain metastases at the time of diagnosis. In the remaining 70% of patients, brain metastases developed at a median of 27 months from initial diagnosis of lung cancer (range, 2 to 174 months). Brain metastases were detected by magnetic resonance imaging in essentially all patients (98%), and nearly half of the patients had ≥ four brain metastases at the time of presentation. ECM was found in 69% of patients at the time of brain metastasis diagnosis, whereas 30% of patients had only brain metastases. At the time of brain metastasis development, patient KPS was 90 to 100 (50% of patients), 70 to 80 (30% of patients), and < 70 (10% of patients; KPS was not available for nine patients). The excellent KPS for this cohort suggests that brain metastases were likely minimally symptomatic, responsive to steroid therapy, or asymptomatic for most of these patients.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. (%) |
|---|---|
| Median follow-up, months | |
| From date of lung cancer diagnosis | 38.1 |
| From date of brain metastasis diagnosis | 16.0 |
| Age, years | |
| < 60 | 72 (80) |
| ≥ 60 | 18 (20) |
| Sex | |
| Male | 48 (53) |
| Female | 42 (47) |
| Smoking history, pack-years | |
| Never-smoker | 60 (67) |
| ≤ 10 | 18 (20) |
| < 10 | 12 (13) |
| Stage at diagnosis | |
| I | 3 (3) |
| II | 3 (3) |
| IIIA | 11 (12) |
| IIIB | 6 (7) |
| IV | 67 (74) |
| Brain metastasis at time of diagnosis | |
| No | 63 (70) |
| Yes | 27 (30) |
| First brain imaging | |
| CT | 2 (2) |
| MRI | 73 (81) |
| Both | 15 (17) |
| No. of brain metastases at first imaging | |
| 1 | 23 (26) |
| 2 | 15 (17) |
| 3 | 10 (11) |
| ≥ 4 | 42 (47) |
| Extracranial metastasis present | |
| Yes | 62 (69) |
| No | 27 (30) |
| Unknown | 1 (1) |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
A total of 84 of 90 patients received RT for treatment of brain metastases, and six patients received TKI therapy alone. Surgical resection preceded RT in eight patients. RT was delivered with either localized SRS (Gamma Knife or linear accelerator based) or WBRT. SRS was delivered to 64 patients, to a median of two lesions per session (range, one to 18 lesions) and a median prescribed dose of 20 Gy (range, 15 to 27.5 Gy). Forty-five patients received WBRT, to a median dose of 30 Gy (range, 20 to 50.4 Gy). Twenty-two craniotomies were performed in 17 patients, with gross tumor resected in 15, necrosis resected in five, biopsy performed in one, and thermocoagulation performed in one. Eighty-four patients received crizotinib, and 41 received a second-generation ALK inhibitor (ceritinib, n = 21; AP-26113, n = 16; alectinib, n = 2; and X-396, n = 2). Patients categorized as having received prior TKI therapy received crizotinib for at least 4 months. Of the 44 patients who received an ALK-targeted TKI before developing brain metastases, 38 received crizotinib, and six received crizotinib followed by a second-generation ALK inhibitor before developing metastatic disease in the brain.
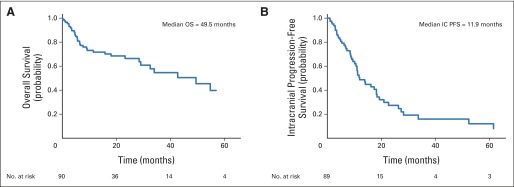
Median OS after diagnosis of brain metastasis was 49.5 months (range, 0.2 to 82.2 months; 95% CI, 29.0 months to not reached). Kaplan-Meier estimates of 1- and 2-year OS were 72% and 66%, respectively (Fig 1A). At the time of analysis, 51 patients had experienced progression in the CNS. Median intracranial PFS was 11.9 months (range, 1 to 81.4 months; 95% CI, 10.1 to 18.2 months; Fig 1B).
Fig 1.
Kaplan-Meier estimate of (A) overall (OS) and (B) intracranial progression-free survival (IC PFS) from date of diagnosis of brain metastasis.
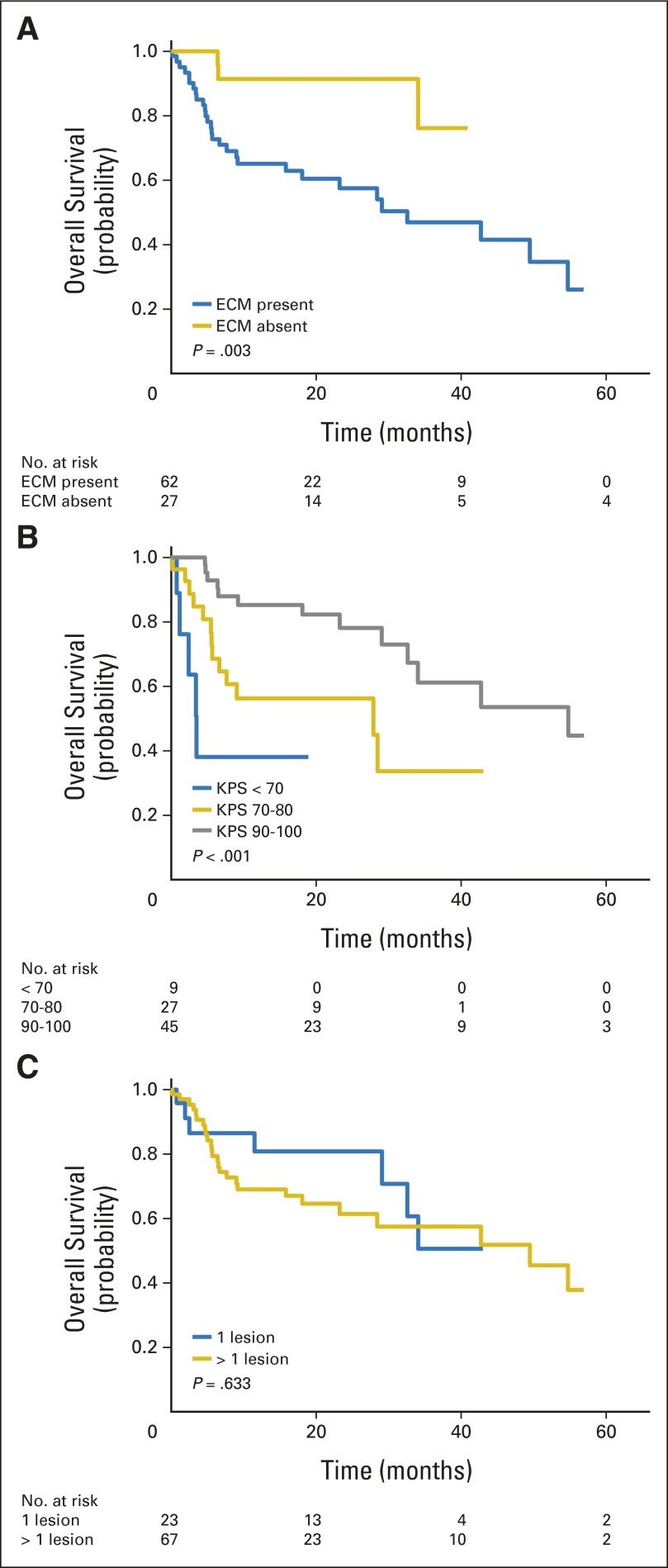
OS was significantly longer for patients with metastatic disease limited to the brain (median survival, not reached) compared with patients with ECM and brain metastases (median survival, 32.6 months; P = .003; Fig 2A). There was no survival difference for patients with stable or no evidence of systemic disease versus progressive systemic disease (P = .644). OS did vary significantly by KPS, with median survival of 54.8 months for KPS of 90 to 100, 27.8 months for KPS of 70 to 80, and 3.5 months for KPS < 70 (P < .001; Fig 2B). There was no survival difference for patients presenting with a single brain metastasis versus < one metastasis (63.3 v 49.5 months; P = .633; Fig 2C).
Fig 2.
Kaplan-Meier estimate of overall survival from date of diagnosis of brain metastasis, stratified by (A) presence or absence of extracranial metastasis (ECM) at time of brain metastases diagnosis, (B) Karnofsky performance score (KPS) at time of brain metastasis diagnosis, and (C) number of brain metastases at time of diagnosis.
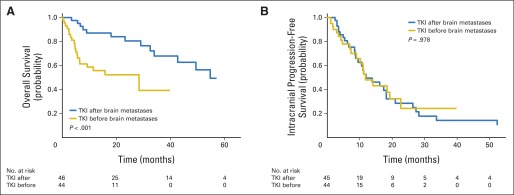
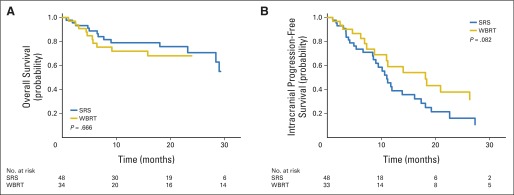
ALK-targeted TKIs were initiated before the development of brain metastases in 49% of patients, and OS was significantly inferior compared with those patients who began TKI therapy after diagnosis of brain metastases (median survival, 28.4 v 54.8 months, respectively; P < .001; Fig 3A). In contrast, intracranial progression of brain metastases was equivalent for both patient groups (median intracranial PFS, 11.7 v 11.9 months, respectively; P = .978; Fig 3B), demonstrating no difference in durability of CNS metastasis control. Initial RT was delivered with single-fraction SRS for 48 patients and with WBRT for 34 patients (seven patients received no RT), and survival did not differ between these two groups (P = .666). A nonsignificant trend toward improved intracranial PFS for those patients who received WBRT first was observed (P = .082; Appendix Fig A1, online only).
Fig 3.
Kaplan-Meier estimate of (A) overall (B) and intracranial progression-free survival from date of diagnosis of brain metastasis, stratified by treatment with tyrosine kinase inhibitor (TKI) before development of brain metastasis or initiation of TKI after diagnosis of brain metastasis.
On multivariable analysis, no prior treatment with ALK-targeted TKIs, absence of ECM, and KPS ≥ 90 were found to be independent predictors of OS (Table 2). Notably, age, smoking history, number of metastatic lesions in the brain (one v multiple), and initial type of RT (SRS v WBRT) were not observed to significantly influence prognosis.
Table 2.
Multivariable Analysis for OS
| Variable | HR | 95% CI | P |
|---|---|---|---|
| All Variables | |||
| Age at diagnosis of brain metastasis: < 50 years v | |||
| 50-60 years | 0.993 | 0.374 to 2.639 | .989 |
| < 60 years | 0.805 | 0.282 to 2.296 | .684 |
| Smoking history: never-smoker v | |||
| ≤ 10 pack-years | 0.411 | 0.119 to 1.415 | .159 |
| < 10 pack-years | 1.043 | 0.281 to 3.869 | .950 |
| KPS: < 70 v | |||
| 70 to 80 | 0.398 | 0.090 to 1.751 | .223 |
| 90 to 100 | 0.191 | 0.038 to 0.945 | .042 |
| Absence of extracranial metastasis | 0.241 | 0.067 to 0.866 | .029 |
| No. of metastases: one v < one | 0.810 | 0.301 to 2.181 | .676 |
| TKI initiated after brain metastasis diagnosis | 0.382 | 0.149 to 0.978 | .045 |
| Initial treatment: SRS v WBRT | 0.672 | 0.256 to 1.763 | .419 |
Abbreviations: HR, hazard ratio; KPS, Karnofsky performance score; OS, overall survival; TKI, tyrosine kinase inhibitor.
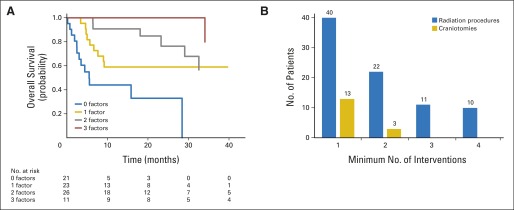
The number of RT procedures (SRS or WBRT) and neurosurgical procedures to the brain for this patient cohort are shown in Figure 4B. Overall, 119 SRS treatments in 64 patients, 48 courses of WBRT in 45 patients, and 21 neurosurgical procedures in 16 patients were documented. Repeat use of RT was frequent in this patient cohort, with 43 of 84 patients receiving a repeat RT procedure and 21 of 84 receiving ≥ three procedures. On average, for each year of life after diagnosis of brain metastasis, 1.4 brain treatments were performed in year 1, 0.6 in year 2, 0.7 in year 3, and 0.5 in year 4 for those patients still alive and with follow-up.
Fig 4.
(A) Kaplan-Meier estimates of overall survival for patients with brain metastases and ALK rearrangement and zero, one, two, or three positive prognostic risk factors. (B) Prevalence of brain interventions in this patient cohort. Number of patients receiving at least one, two, three, or four radiotherapy (blue) or craniotomy (gold) interventions is presented.
The status of brain disease at the time of death was examined for the 34 patients who died. Thirteen of 29 patients with follow-up had progressive brain metastases at the time of death, and 10 (33%) of 30 had symptomatic brain metastases, with seven of 10 requiring steroid therapy. Cause of death was unknown for most patients, but was determined to be the result of neurologic complications in five patients.
To assess the utility of the GPA to predict survival in patients with ALK-rearranged NSCLC and brain metastasis, patients were divided into NSCLC-specific GPA groups,7 and median survival was calculated for each of these groups (Appendix Table A1, online only). GPA was not prognostic for this patient population, and patients with ALK-rearranged disease significantly exceeded the GPA-predicted survival. Survival outcomes for patients with zero, one, two, or three of the independent factors identified by multivariable analysis are shown in Figure 4A. Patients with KPS < 90, ECM, and prior TKI therapy had 2-year survival of 33% (Appendix Table A2, online only). For patients with one, two, or three positive prognostic factors, 2-year survival was estimated to be 59%, 76% and 100%, respectively (P < .001).
DISCUSSION
NSCLC brain metastasis is often considered a final stage of advanced disease and an ominous sign of disease progression and death. Clinical factors including age, performance status, and extent of disease are frequently used to predict survival in the setting of brain metastasis and are essential for patient counseling and for guiding treatment recommendations.2-7 The recent revolution in characterizing predictive biomarkers for targeted therapy also provides an opportunity for integrating molecular information into prognostic models and better informing both physicians and patients about anticipated outcomes. In this study, we investigated outcomes for patients with ALK-rearranged NSCLC and brain metastases. We found that patients with NSCLC with ALK rearrangement and brain metastases had a median OS < 4 years and intracranial PFS after treatment was nearly 1 year. These favorable survival outcomes are noted despite 74% of patients presenting with multiple brain metastases, nearly half with ≥ four metastases. We also found that three significant clinical factors (KPS, presence of ECM, and prior TKI treatment) could be used to further refine estimates of prognosis. Our multi-institutional study thus provides the first large data set to our knowledge of long-term outcomes for ALK-rearranged NSCLC with brain metastases.
In this study, patients were predominantly treated with RT, using either SRS or WBRT. An analysis of first-line RT delivery (SRS v WBRT) showed no evidence of a survival difference, although a trend toward increased brain failure for those patients who received first-line SRS was observed. These results are similar to data from the Japanese Radiation Oncology Study Group 99-1 and European Organisation for Research and Treatment of Cancer 22952-26001 randomized trials, which showed equivalent survival but increased brain failure in patients who received SRS without WBRT.18,19 This latter study of patients with one to three brain metastases convincingly demonstrated that the tradeoff for increased brain control with WBRT is significant decreases in physical and cognitive functioning and health-related quality of life20 and was in agreement with a smaller randomized study by Chang et al,21 which prospectively demonstrated cognitive benefits for those patients who did not receive immediate WBRT. These characterizations of delayed sequelae from WBRT are particularly important for the cohort of patients described herein and are further supported by a recent meta-analysis that showed a survival advantage for SRS alone versus SRS plus WBRT for patients age < 50 years.22 Patients with ALK-rearranged NSCLC and brain metastases have a relatively long survival, and thus, SRS and deferred WBRT should be strongly considered for these patients.
Recursive partitioning analysis2,3 and improved GPA4-7 are established prognostic indices for patients with brain metastases. For NSCLC, the most favorable GPA (age ≤ 50 years, KPS ≥ 90, absent ECM, and single brain metastasis) has an estimated survival of 14.8 months.6,7 In comparison, we found that median survival in patients with tumors harboring an ALK rearrangement was 49.5 months, demonstrating that in the context of modern TKI treatment and RT, ALK rearrangement alone is a strong predictor for improved survival in patients with NSCLC brain metastases. Multivariable analysis of patient and treatment characteristics revealed that high KPS, absence of ECM, and no prior treatment with a TKI were all associated with improved survival. In patients who were TKI naive at the time of CNS disease presentation, we found that median survival was nearly doubled compared with patients in whom brain metastases were detected during treatment with an ALK inhibitor (median survival, 28.4 v 54.8 months, respectively), raising the possibility that control of CNS disease with RT may be an important determinant of OS.
Extended survival for patients with a single brain metastasis has been observed in the Radiation Therapy Oncology Group 9508 and Patchell randomized trials, the GPA, and a recent meta-analysis,22-24 suggesting that a single brain metastasis is a marker for low disease burden, and thus, intensification of local therapy affects survival. In our study, a single metastasis was not prognostic. This difference indicates that the number of brain metastases is not a reliable surrogate for disease burden in the setting of ALK rearrangement, TKI therapy, and the ability to deliver focal radiation with SRS. This finding also implies that patients with control of their systemic disease burden might also derive a survival benefit from intensification of therapy at multiple intracranial sites. This hypothesis could not be explored in our patient cohort, because SRS was used as salvage therapy in 15 of 34 patients who received WBRT. However, the concept of local intensification of therapy to sites of progression has been examined in patients with oligoprogressive extracranial disease using SBRT or surgery, and an apparent survival benefit has been suggested.14,17,25
Brain metastases have metachronous presentation because of either divergent growth rates or intermittent metastatic events to the brain. Thus, in the setting of extended survival, the brain remains at high risk for recurrence with either focal (SRS or surgery) or regional therapy (WBRT). In our patient cohort, intracranial progression frequently required management with additional RT, and although the median time to intracranial progression was nearly 1 year, more than half of the patients experienced progression in the brain after initial management and required additional treatment with RT. One fourth of the patients were re-treated with RT at least three times. Considering that most patients in this cohort were alive at last follow-up, these data reveal and also significantly underestimate the prevalence of repeat RT in this population. The observed frequency and likely necessity for repeat RT procedures in patients with brain metastases from ALK-rearranged NSCLC provide a second rationale for deferring WBRT when feasible.
This study also shows that nearly 40% of patients were found to have progressive brain metastases at the time of death, a majority of which were symptomatic, indicating that strategies to manage CNS progression in these patients are of considerable importance. Together these findings reinforce the need for routine brain surveillance with magnetic resonance imaging and evaluation for repeat CNS treatment.26 They also illustrate the need for ALK-targeted TKIs with improved CNS penetrance and trials that allow for comparison of intracranial progression rates with those of crizotinib. Even a modest increase in CNS availability is likely to have a significant impact on this patient population, and evaluations of second-generation ALK inhibitors for efficacy in the CNS are currently under way.27,28
This study is limited by its retrospective nature. Bias may also have been introduced by the patient cohort, which was obtained from National Cancer Institute–designated cancer centers that have access to investigational second-generation ALK TKIs through clinical trials and may attract patients with better performance status. However, it is encouraging that outcomes from our patient cohort are comparable to those from the PROFILE 1005 and 1007 studies, which demonstrated an intracranial time to progression with crizotinib of 13.2 months,29 similar to our finding of 11.9 months. Together these data provide a useful benchmark for evaluating and guiding the design of clinical trials to test the efficacy of second-generation ALK-targeted TKIs on brain metastases. Another limitation of this study is that we were unable to capture quality-of-life or neurocognitive follow-up data. Thus, because of the limitations of these data, a prospective study would be required to confirm that SRS is a superior first-line treatment for patients with ALK rearrangement and brain metastasis.
In summary, our hypothesis that the prognosis for patients with NSCLC with brain metastases could be further refined according to molecular subtype was confirmed by this multi-institutional analysis of patients with ALK-rearranged NSCLC. We found that patients with this genetic marker had a prolonged survival when treated with systemic TKIs and brain RT and that repeat interventions to achieve intracranial disease control were an important component of treatment. In addition, clinical factors that classify patients into distinct prognostic groups were identified for this patient population (KPS, absence of ECM, and no history of ALK-directed TKI therapy before developing brain metastases) and will be useful for stratifying patients into risk groups in the setting of a clinical trial or for guiding decision making between the use of SRS or WBRT. On the basis of the data from this study, we make a strong recommendation to treat patients with either KPS ≥ 90, no ECM, or no prior TKI therapy with SRS. As advances in the treatment of this disease are made with both local and systemic therapies, favorable SRS side effect profiles or other factors such as patient eligibility to receive effective systemic therapy may also warrant consideration of SRS over WBRT for treatment of brain metastases.
Appendix
Table A1.
Survival Compared With GPA Estimates
| GPA Group | Median Survival (months) |
|
|---|---|---|
| GPA | Patients With ALK Rearrangement (95% CI) | |
| 1 | 3.0 | 7.7 (4.4 to NR) |
| 2 | 5.5 | 54.8 (15.8 to NR) |
| 3 | 9.4 | 32.6 (23.2 to NR) |
| 4 | 14.8 | 34.0 (6.4 to NR) |
Abbreviations: GPA, graded prognostic assessment; NR, not reached.
Table A2.
Survival Groups
| No. of Prognostic Factors* | 2-Year OS (%) | Median Survival (months) | P |
|---|---|---|---|
| 0 | 33 | 5.5 | < .001 |
| 1 | 59 | NR | < .001 |
| 2 | 76 | 42.7 | < .001 |
| 3 | 100 | NR | < .001 |
Abbreviations: NR, not reached; OS, overall survival.
Prognostic factors included Karnofsky performance score 90 to 100, no extracranial metastasis, and tyrosine kinase inhibitor initiated after brain metastasis.
Fig A1.
Kaplan-Meier estimate of (A) overall and (B) intracranial progression-free survival from the date of diagnosis of brain metastasis, stratified by initial type of radiotherapy as treatment for brain metastasis. SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Footnotes
See accompanying editorial on page 107
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Kimberly L. Johung, Joseph N. Contessa
Collection and assembly of data: All authors
Data analysis and interpretation: Kimberly L. Johung, Norman Yeh, Neil B. Desai, Terence M. Williams, Tim Lautenschlaeger, Nils D. Arvold, Albert Attia, Christine M. Lovly, Sarah Goldberg, Kathryn Beal, James B. Yu, Brian D. Kavanagh, Veronica L. Chiang, D. Ross Camidge, Joseph N. Contessa
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non–Small-Cell Lung Cancer and Brain Metastasis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Kimberly L. Johung
No relationship to disclose
Norman Yeh
Stock or Other Ownership: Merck
Neil B. Desai
No relationship to disclose
Terence M. Williams
Consulting or Advisory Role: Covidien
Tim Lautenschlaeger
No relationship to disclose
Nils D. Arvold
No relationship to disclose
Matthew S. Ning
No relationship to disclose
Albert Attia
No relationship to disclose
Christine M. Lovly
Honoraria: Harrison and Star
Consulting or Advisory Role: Pfizer, Novartis, Sequenom, Genoptix
Speakers' Bureau: Abbott Molecular, Qiagen
Research Funding: AstraZeneca, Novartis
Sarah Goldberg
Consulting or Advisory Role: Boehringer Ingelheim, Clovis
Research Funding: AstraZeneca, Merck (Inst), Genentech (Inst), Immunogen (Inst), Kadmon (Inst)
Kathryn Beal
No relationship to disclose
James B. Yu
Research Funding: 21st Century Oncology
Brian D. Kavanagh
No relationship to disclose
Veronica L. Chiang
No relationship to disclose
D. Ross Camidge
Honoraria: Pfizer, Novartis, ARIAD Pharmaceuticals, Roche/Genentech
Joseph N. Contessa
No relationship to disclose
REFERENCES
- 1.Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: Frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 2.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 3.Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 4.Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 5.Sperduto CM, Watanabe Y, Mullan J, et al. A validation study of a new prognostic index for patients with brain metastases: The graded prognostic assessment. J Neurosurg. 2008;109(suppl):87–89. doi: 10.3171/JNS/2008/109/12/S14. [DOI] [PubMed] [Google Scholar]
- 6.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 9.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non- small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 12.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 13.Chun SG, Choe KS, Iyengar P, et al. Isolated central nervous system progression on Crizotinib: An Achilles heel of non-small cell lung cancer with EML4-ALK translocation? Cancer Biol Ther. 2012;13:1376–1383. doi: 10.4161/cbt.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johung KL, Yao X, Li F, et al. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin Cancer Res. 2013;19:5523–5532. doi: 10.1158/1078-0432.CCR-13-0836. [DOI] [PubMed] [Google Scholar]
- 16.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 17.Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892–898. doi: 10.1016/j.ijrobp.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 19.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality-of-life results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 21.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 22.Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: Individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91:710–717. doi: 10.1016/j.ijrobp.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 24.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 25.Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25:415–422. doi: 10.1093/annonc/mdt572. [DOI] [PubMed] [Google Scholar]
- 26.Lester SC, Taksler GB, Kuremsky JG, et al. Clinical and economic outcomes of patients with brain metastases based on symptoms: An argument for routine brain screening of those treated with upfront radiosurgery. Cancer. 2014;120:433–441. doi: 10.1002/cncr.28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 28.Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10:232–236. doi: 10.1097/JTO.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non–small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]