Abstract
Purpose
The Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) study showed a 15.7-month survival benefit with the addition of pertuzumab to docetaxel and trastuzumab (THP) as first-line treatment for patients with human epidermal growth factor receptor 2 (HER2) –overexpressing metastatic breast cancer. We performed a cost-effectiveness analysis to assess the value of adding pertuzumab.
Patient and Methods
We developed a decision-analytic Markov model to evaluate the cost effectiveness of docetaxel plus trastuzumab (TH) with or without pertuzumab in US patients with metastatic breast cancer. The model followed patients weekly over their remaining lifetimes. Health states included stable disease, progressing disease, hospice, and death. Transition probabilities were based on the CLEOPATRA study. Costs reflected the 2014 Medicare rates. Health state utilities were the same as those used in other recent cost-effectiveness studies of trastuzumab and pertuzumab. Outcomes included health benefits expressed as discounted quality-adjusted life-years (QALYs), costs in US dollars, and cost effectiveness expressed as an incremental cost-effectiveness ratio. One- and multiway deterministic and probabilistic sensitivity analyses explored the effects of specific assumptions.
Results
Modeled median survival was 39.4 months for TH and 56.9 months for THP. The addition of pertuzumab resulted in an additional 1.81 life-years gained, or 0.62 QALYs, at a cost of $472,668 per QALY gained. Deterministic sensitivity analysis showed that THP is unlikely to be cost effective even under the most favorable assumptions, and probabilistic sensitivity analysis predicted 0% chance of cost effectiveness at a willingness to pay of $100,000 per QALY gained.
Conclusion
THP in patients with metastatic HER2-positive breast cancer is unlikely to be cost effective in the United States.
INTRODUCTION
Overexpression of the human epidermal growth factor receptor 2 (HER2/neu) occurs in 20% to 25% of patients with breast cancer.1,2 HER2 dimerization inhibitors are humanized monoclonal antibodies targeted at the HER2 receptor. Trastuzumab is the first approved therapy in this class and has been shown to improve outcomes in patients with HER2-positive metastatic breast cancer.3-5 Trastuzumab suppresses oncologic signaling by blocking HER2 homodimerization.6 Pertuzumab is less specific in that it also blocks heterodimerization with HER1, HER3, and HER4.7 The combination of trastuzumab and pertuzumab (HP) has been shown to be more effective than trastuzumab alone in both metastatic8,9 and nonmetastatic10,11 HER2-overexpressing breast cancers.
The NeoSphere and TRYPHAENA trials evaluated various combinations of docetaxel, trastuzumab, and pertuzumab for the neoadjuvant treatment of patients with operable, locally advanced, or inflammatory breast cancers > 2 cm. Patients on the NeoSphere trial were randomly assigned to one of four neoadjuvant schemas: docetaxel plus trastuzumab (TH); docetaxel, trastuzumab, and pertuzumab (THP); HP; or docetaxel plus pertuzumab (TP). Pathologic complete response rates were 29% for TH, 46% for THP, 17% for HP, and 24% for TP.11 Patients in the TRYPHAENA trial were randomly assigned to one of three arms: fluorouracil, epirubicin, and cyclophosphamide (FEC) followed by THP; concurrent FEC and HP followed by THP; or docetaxel, carboplatin, and trastuzumab (TCH) with pertuzumab. All patients received an additional year of trastuzumab after surgery. Rates of cardiotoxicity were acceptably low, with comparable rates between the two anthracycline-containing arms (5.6% and 5.3%) and the third arm (3.9%).10 Final results from the Adjuvant Pertuzumab and Herceptin in Initial Therapy of Breast Cancer (APHINITY) trial (ClinicalTrials.gov No. NCT01358877) will allow for characterization of pertuzumab in the adjuvant setting.
Pertuzumab is highly effective in the metastatic setting.8,9 The National Comprehensive Cancer Network recommends THP as preferred first-line agents for HER2-positive metastatic breast cancer based on the interim results from the phase III Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) study.8 The trial showed improved progression-free survival and a trend toward improved overall survival for patients treated with THP versus TH.8 After additional follow-up, the benefit in overall survival has reached statistical significance (hazard ratio, 0.68; P < .001), with median survival of 56.5 months for THP versus 40.8 months for TH.9 Both regimens were well-tolerated with similar safety profiles between the arms.8,9
These exceptional results come at a price. Our work shows that an insurer could expect to pay $2,942 per week for the THP regimen (Table 1, Appendix Table A1, online only) at Medicare rates. Private contractors and smaller entities would pay more. The cost effectiveness of THP has been evaluated in Canada for locally advanced, inflammatory, or early HER2-positive breast cancer18 on the basis of dual analyses of NeoSphere11 and TRYPHAENA10 clinical trials. In this setting, pertuzumab is likely to be cost effective at a cost between $25,388 and $46,196 per quality-adjusted life-year (QALY) gained.18 To our knowledge, no such study has been done in the United States, and no such study has been published for THP in the metastatic setting. Our analysis represents the first US-based cost-effectiveness study of pertuzumab in the treatment of HER2-overexpressing metastatic breast cancer.
Table 1.
Model Parameters and Assumptions
| Variable | Base Case and Modeled Distribution (95% CI) |
Reference and Note | |
|---|---|---|---|
| Control (TH) | CLEOPATRA (THP) | ||
| Transition probabilities: β distributed | |||
| Progression from stable state | 0.010250 | 0.006982 (0.005958 to 0.008209) | Swain et al9; uncertainty in both groups was attributed to a single arm |
| Death from stable state | 0.001762 | 0.001198 (0.000986 to 0.001479) | |
| Mortality after progression | 0.002610 | 0.001775 (0.001462 to 0.002193) | |
| Hospice after progression | 0.004131 | 0.002811 (0.002315 to 0.003471) | Dartmouth Institute for Health Policy Clinical Practice12; 61% of patients chose hospice at the end of life |
| Mortality in hospice | 0.109101 | Christakis and Escarce13 and Younis et al14; median survival for patients with breast cancer during hospice care was 6 weeks | |
| Serious adverse events† | 0.003582 | 0.003055 | Swain et al15; serious adverse events were reported in 29% and 36% of patients given TH and THP |
| Serious adverse event that is unmanageable | 0.182504† | 0.168162† | Swain et al15; unmanageable toxicities seen in 5% and 6% of patients given TH and THP |
| Background mortality | Age specific | Centers for Disease Control and Prevention16 | |
| Utilities: β distributed | |||
| Stable state | 0.65 (0.50 to 0.80) | Hedden et al17 and Attard et al18 | |
| Progressing state | 0.29 (0.16 to 0.41) | Same utilities used in recent cost-effectiveness analyses of trastuzumab and pertuzumab | |
| Hospice state | 0.48 | Casarett et al19 | |
| Toll for major toxicity | −0.28 | Launois et al20 | |
| Cost per cycle: γ distributed | |||
| Loading dose and first cycle of therapy, one-time cost | $5,628 ($5,083 to $6,195) | $14,344 ($12,956 to $15,791) | Primary costing per MPFS and ASP (Appendix Table A1, online only) |
| Stable state | $1,467 ($1,325 to $1,615) | $2,942 ($2,657 to $3,239) | Primary costing per MPFS and ASP (Appendix Table A1, online only) |
| Progressing state | $1,913 ($67 to $6,022) | Mariotto et al21; on the basis of annualized data in last year of life; good agreement with results reported by Chastek et al22 | |
| Hospice state | $628 ($534 to $728) | Chastek et al22 $2,464 for last month of life on hospice; good agreement with Mariotto et al21 and Zhang et al23 | |
| Toll for major toxicity, one-time cost | $2,126 ($109 to $6,368) | Hansen et al24; on the basis of incremental monthly cost for grade 3 or 4 adverse events with second-line capecitabine | |
| Toll for death outside hospice, one-time cost | $3,284 ($2,773 to $3,831) | Zhang et al23 | |
Abbreviations: ASP, average sales price, as reported to Medicare by the manufacturer; CLEOPATRA, Clinical Evaluation of Pertuzumab and Trastuzumab trial; H, trastuzumab; MPFS, Medicare physician fee schedule; P, pertuzumab; T, docetaxel.
Distributions show 95% confidence intervals.
Cycle-specific probabilities of toxicity are lower for patients receiving THP because they spend more cycles in the stable state. Cumulative probabilities are well matched to the target.
PATIENTS AND METHODS
Documentation of our methods adhere to the recommendations of the Society for Medical Decision Making good research practices for model transparency and validation.25,26
Patients and Intervention
Our treatment schema was modeled after the CLEOPATRA trial of patients with HER2-positive metastatic or recurrent breast cancer. Eligible patients had an Eastern Cooperative Oncology Group performance status of 0 or 1 and had received no more than one hormonal treatment for metastatic disease. Patients were randomly assigned to receive THP versus TH.9 Pertuzumab was administered at a fixed loading dose of 840 mg, followed by 420 mg every 3 weeks. Trastuzumab was initiated with a loading dose of 8 mg/kg, followed by a maintenance dose of 6 mg/kg every 3 weeks. Docetaxel was prescribed at 75 mg/m2 over 60 minutes every 3 weeks for at least six cycles. The dose was decreased by 25% for toxicity or increased to 100 mg/m2 if it was well tolerated. Patients continued with their respective regimens until progression or unmanageable toxicity.
Decision-Analytic Markov Model
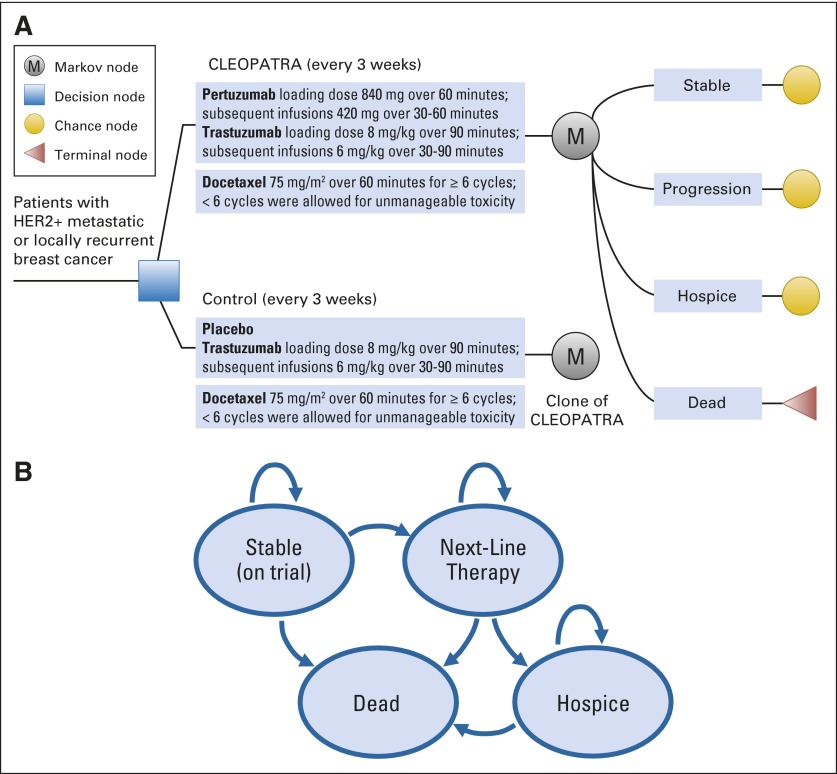
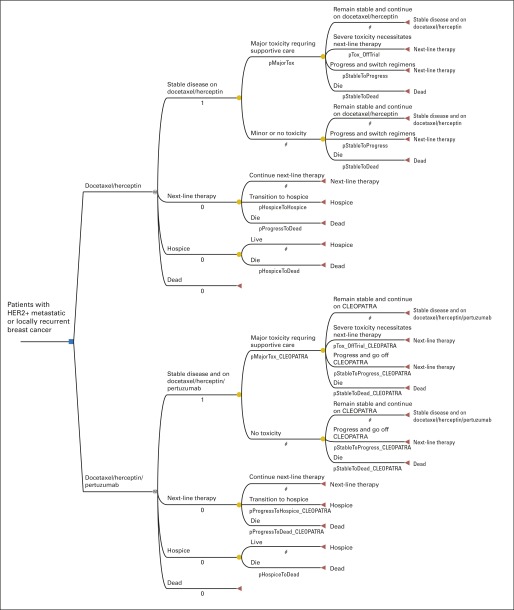
We developed a decision-analytic Markov model using TreeAge Pro 2014 software (TreeAge, Williamstown, MA). We used the model to perform a cost-effectiveness analysis of THP versus TH from the societal perspective. The health states were stable disease, progressing disease, hospice, and dead (Fig 1, Appendix Fig A1, online only). The model followed patients weekly over their remaining lifetimes. Toxicity rates were tracked and validated against those followed in the trial. Patients in the stable disease state were treated with TH or THP until progression, unmanageable toxicity, or death. We explicitly modeled serious adverse events, including the 5.3% of patients given TH and 6.1% given THP, whose toxicities could not be managed and, therefore, had to stop trial participation. Patients could experience multiple adverse events. All patients received at least six cycles of docetaxel, except for those with unmanageable toxicities. Adverse events in the progressing disease state were not explicitly modeled but were inherently accounted for in assigned utilities and costs. After progression, patients were treated with the next-line regimen until hospice or death. Patients in hospice had marginally lower costs and higher utilities during the last several weeks of life than those who died before entering hospice.19,22
Fig 1.
(A) Abbreviated decision tree and Markov model used to compare two strategies for treating metastatic human epidermal growth factor receptor 2–positive (HER2+) breast cancer explored in the Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) trial. (B) Influence diagram shows a network of four health states linked by transitional variables. M, Markov node.
We assessed cost effectiveness by calculating the incremental cost-effectiveness ratio. In our main analysis, we assumed a willingness-to-pay (WTP) threshold of $100,000 per QALY gained, but we also explored the implications of thresholds up to $500,000 per QALY gained. One- and multi-way deterministic sensitivity analyses were conducted to probe the effects of uncertainty in our assumptions about treatment efficacy, utilities, and cost.27 Probabilistic sensitivity analysis with 10,000 Monte Carlo simulations was performed to probe the stochastic effects of transition probabilities and parameter uncertainty in utilities and cost.27,28
Transition Probabilities
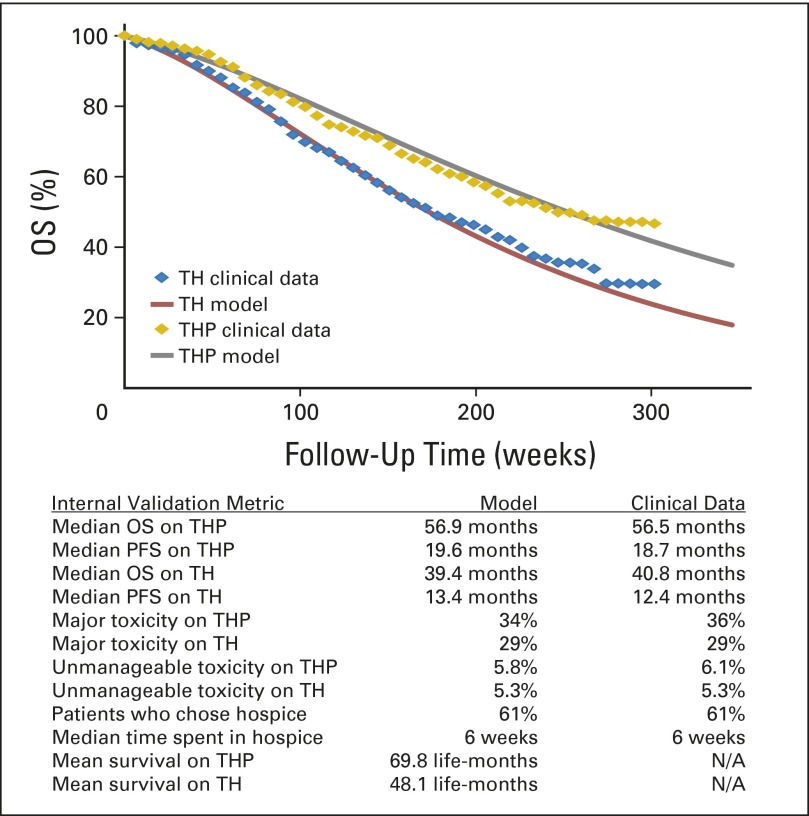
We inferred the cycle-specific transition probabilities from the CLEOPATRA trial.9 First, graphical data were extracted from the published Kaplan-Meier curves by using a validated graphical digitizer (WebPlotDigitizer version 3.4; Ankit Rohatgi, Austin, TX). Next, we calibrated our cycle-specific transition probabilities to the CLEOPATRA data. We used an iterative, optimizing algorithm to minimize the difference between a target (actual data) and a model derived from our Markov states by using a nonlinear least-squares objective function. The solution was constrained by the hazard ratios reported by Swain et al9 and real world truisms (eg, overall survival is greater than progression-free survival). Finally, we used the same method to model the 29% of patients given TH and 36% given THP who had serious adverse events, and the 5.3% given TH and 6.1% given THP whose toxicities could not be managed and, therefore, had to stop trial participation (Fig 2).
Fig 2.
Internal validation of our model shows agreement with target data across overall survival (OS), progression-free survival (PFS), toxicity rates, and choice for hospice. Clinical data refer to the published results of the Clinical Evaluation of Pertuzumab and Trastuzumab (CLEOPATRA) trial.9 Hazard ratios for OS and PFS were used as constraints in our optimization algorithm and, therefore, were exactly the same as those reported by Swain et al.9 H, trastuzumab; P, pertuzumab; T, docetaxel.
We assumed that 61% of patients chose hospice in the last weeks of life, based on data from the Dartmouth Institute for Health Policy and Clinical Practice.12 The time spent in hospice matched published Medicare claims data and a retrospective review from the Center for Hospice and Palliative Care of Buffalo.13,14 Age-specific mortality for other causes were based on life tables from the Centers for Disease Control.16 Deterministic sensitivity analysis addressed subgroups of patients who may derive greater benefit from THP, including patients with visceral metastases, patients older than age 65 years, and black patients.9 For probabilistic sensitivity analysis, uncertainty in transition probabilities was modeled by using β distributions based on the hazard ratio CIs reported in the trial.9
Costs and Utilities
Costs of targeted therapies and chemotherapies were 106% of the manufacturer's average sales price, consistent with Medicare pricing policy (Table 1).29 Cost of administration was derived from the national payment amount listed in the Medicare physician fee schedule for 2014 (Appendix Table A1).30 Dosing calculations assumed a 70-kg patient with a 1.8-m2 body surface area. Costs for second- and third-line regimens were based on published data from the SEER and linked Medicare data, and the OptumInsight claims database.21,22
Health state utilities were the same as those used in previously published cost-effectiveness analyses of trastuzumab and pertuzumab,17,18 which were based on primary utility data derived by using a standard gamble and visual analog scale.20,31,32 We did not explicitly model utilities for the 1% of patients receiving THP or the 2% of patients receiving TH with symptomatic left ventricular systolic dysfunction.15 Minor toxicities were considered to be inherent to the metastatic cancer state and, therefore, were not explicitly modeled. Major toxicities were modeled with a one-time disutility.
Patients who spent their last months of life in hospice had higher utilities than those who were aggressively managed based on a published assessment of primary patient data using a conjoint analysis.19 Uncertainty was modeled by β distribution, which is bounded by 0 and 1.27
We did not explicitly account for costs associated with grade 1 or 2 adverse events, nor did we account for the cost of measuring left ventricular ejection fraction every 9 weeks during treatment and at regular intervals after progression. We discounted all costs and benefits incurred in the future at a 3% annual rate to adjust for inflation.26 Costs from past sources were adjusted to 2014 US dollars according to the Consumer Price Index health care services group.33 Uncertainty was minimal for patients in the stable disease state because the exact treatment regimen was known and costs were derived directly from Medicare. Published data showed substantially greater uncertainty for patients in the progressing-disease state receiving second- and third-line therapies.21,22,24 Uncertainty in cost was modeled by γ distribution, which is bounded by 0 and infinity.27,34
Patient Population Cost Impacts
We estimated the societal cost of treating all patients for whom THP is recommended in the metastatic setting. Costs were calculated, as previously mentioned, recognizing that many patients would be covered by insurers reimbursing at higher fee-for-service rates than Medicare. We calculated both direct and indirect costs; the former were costs directly related to THP, and the latter were all costs associated with the longer time spent caring for patients with metastatic cancer.
Estimates were derived from data provided by the SEER program35 and epidemiologic studies of HER2-positive prevalence1,2 and metastasis.36 We assumed that all eligible patients would consent to treatment and be medically fit to receive THP.
RESULTS
Main Analysis
After all patients were followed through their remaining lifetimes, 61% died in hospice and 39% outside of hospice (Appendix Fig A2, online only). Modeled outcomes were consistent with empirical study target data in terms of overall survival, progression-free survival, major toxicities, and time spent in each health state (Fig 2). The addition of pertuzumab to TH resulted in an additional 1.81 life-years and 0.62 QALYs. Gains were achieved at an incremental cost of $294,747. Taken together, the addition of pertuzumab to TH cost $472,668 per QALY gained.
Sensitivity Analysis
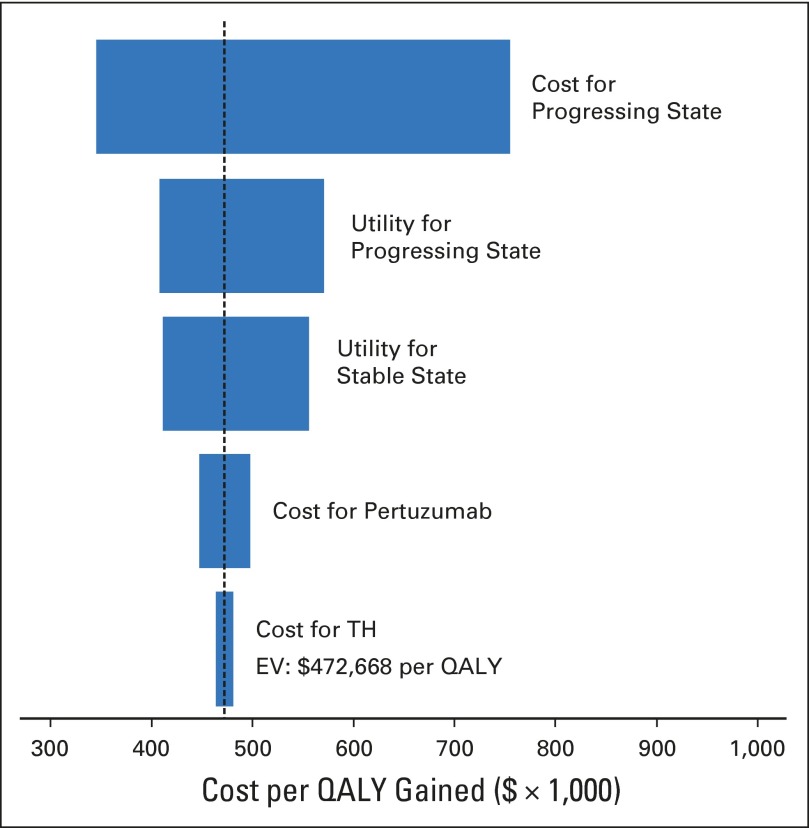
The cost remained higher than $100,000 per QALY gained in our subgroup analysis, which included patients with visceral metastases, age 65 years or older, and black patients (Table 2). Costs and utilities for patients with progressing disease contributed substantial uncertainty to the model (Fig 3). When more optimistic utilities of 0.80 and 0.51 are used for the stable and progressive metastatic states, the cost of THP decreased to $327,899 per QALY gained. When perfect utilities were assigned to both stable and progressing disease states, the cost of THP decreased to $206,335 per QALY gained (Table 2). When the regimen prices for TH and THP were reduced by 50% and 90%, cost decreased to $302,259 and $165,931 per QALY gained, respectively (Table 2).
Table 2.
Summary of One- and Multi-Way Deterministic and Probabilistic Sensitivity Analyses
| Assumption | Life-Years Gained | Incremental Cost | Incremental Benefit, QALY | ICER, per QALY | Probability of Cost-Effectiveness (%) |
|---|---|---|---|---|---|
| Base case | |||||
| WTP $100,000/QALY | 1.81 | $294,747 | 0.62 | $472,668 | 0 |
| WTP $200,000/QALY | 1.81 | $294,747 | 0.62 | $472,668 | 1 |
| WTP $500,000/QALY | 1.81 | $294,747 | 0.62 | $472,668 | 59 |
| Subgroup | |||||
| Visceral metastases* | 2.54 | $356,662 | 0.82 | $432,656 | 0 |
| Age ≥ 65 years† | 3.46 | $450,236 | 1.17 | $385,529 | 0 |
| Black patients‡ | 4.88 | $543,343 | 1.43 | $380,450 | 4 |
| Utilities | |||||
| Stable disease utility 1.0 | 1.81 | $294,747 | 0.84 | $350,137 | 0 |
| Progressing utility 1.0 | 1.81 | $294,747 | 1.21 | $243,539 | 3 |
| Stable and progressing utilities 1.0 | 1.81 | $294,747 | 1.43 | $206,335 | 4 |
| Cost | |||||
| Pertuzumab at 50% cost | 1.81 | $215,081 | 0.62 | $344,913 | 0 |
| Pertuzumab at 10% cost | 1.81 | $151,349 | 0.62 | $242,709 | 4 |
| Pertuzumab free | 1.81 | $135,416 | 0.62 | $217,158 | 12 |
| TH and THP at 50% cost | 1.81 | $188,483 | 0.62 | $302,259 | 4 |
| TH and THP at 10% cost | 1.81 | $103,472 | 0.62 | $165,931 | 47 |
| TH and THP free | 1.81 | $82,219 | 0.62 | $131,849 | 60 |
| All therapies and supportive care at 50% cost | 1.81 | $147,307 | 0.62 | $236,228 | 1 |
| All therapies and supportive care at 10% cost | 1.81 | $29,356 | 0.62 | $47,076 | 98 |
| All therapies and supportive care free | 1.81 | ($132) | 0.62 | Dominated | 100 |
Abbreviations: H, trastuzumab; HR, hazard ratio; ICER, incremental cost-effectiveness ratio; OS, overall survival; P, pertuzumab; PFS, progression-free survival; QALY, quality-adjusted life-years; T, docetaxel; WTP, willingness to pay.
OS HR, 0.59; PFS HR, 0.64.
OS HR, 0.53; PFS HR, 0.50.
OS HR, 0.41; PFS HR, 0.54.
Fig 3.
Tornado diagram shows one-way sensitivity analyses within the 95% CIs for each variable. Costs and utilities for the progressing disease state contribute substantial uncertainty to the model. EV, expected value; H, trastuzumab; QALY, quality-adjusted life-year; T, docetaxel.
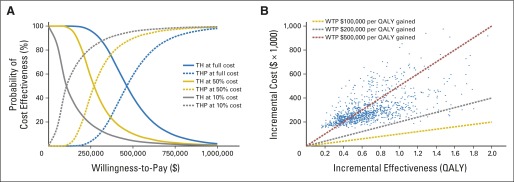
Probabilistic sensitivity analysis showed 0% chance of cost effectiveness at a WTP of $100,000 per QALY gained for both our base case and the more optimistic utilities of 0.80 and 0.51 for the stable and progressive metastatic states. The conclusion remained unchanged even with a WTP of up to $200,000 per QALY gained (Fig 4). When the cost of pertuzumab was reduced by 90%, THP still cost $242,709 per QALY gained (Table 2). When the cost of all first-line therapies, TH and THP, were reduced by 90%, THP cost $165,931 per QALY gained and was preferred in 47% of simulations (Table 2, Fig 4). When cost of all therapies, first and second line, and supportive care was reduced by 90%, the addition of pertuzumab to TH cost $47,076 per QALY gained and was preferred in 98% of simulations with a WTP of $100,000 per QALY gained (Table 2).
Fig 4.
Probabilistic sensitivity analysis. (A) Cost-effectiveness acceptability curve shows the effect of cost on probability of cost effectiveness. (B) Incremental cost-effectiveness scatterplot of 10,000 Monte Carlo simulations shows low probability of cost effectiveness, even at willingness to pay (WTP) well above commonly accepted thresholds. H, trastuzumab; P, pertuzumab; QALY, quality-adjusted life-year; T, docetaxel.
Patient Population Cost Impacts
We estimated an annual incidence of 17,450 patients with metastatic HER2 breast cancer eligible for THP. Direct costs associated with treating a single patient were $114,676 for TH and $327,072 for THP. Total costs, direct and indirect, were estimated to be $326,678 for TH and $621,425 for THP per patient. Incremental direct and total costs were $212,396 and $294,747 per patient. Direct costs associated with treating all incidences of eligible US patients were $2.00 billion for TH and $5.71 billion for THP. The incremental cost of adding pertuzumab was $3.71 billion. The incremental cost rose to $5.14 billion when the indirect costs were also considered.
DISCUSSION
The addition of pertuzumab to a standard regimen of TH for treatment of metastatic HER2-overexpressing breast cancer is unlikely to provide reasonable value for money spent in the United States compared with other interventions generally deemed cost effective. We also find that widespread use of this new regimen in the population with metastatic disease could contribute an additional $5.14 billion to health care spending. For perspective, the total cost of cancer-related care is projected to be between $173 billion and $207 billion by 2020 in 2010 US dollars.21
Our results agree with those from an analysis by the manufacturer (Genentech/Roche) for the United Kingdom's National Institute for Health and Care Excellence, as reported by Fleeman et al.37 This analysis was conducted for a markedly different health care system with markedly different costs. Even so, the manufacturers reported a 0% chance of cost effectiveness at a WTP of £30,000, or approximately $46,000 per QALY.37 Likewise, the National Centre for Pharmacoeconomics in Ireland estimated a 2.5% chance of cost effectiveness at a threshold of €45,000 per QALY and recommended against reimbursement.38
Sensitivity analysis shows that our findings against cost effectiveness are multifactorial. In the simplest terms, our model highlights the reality that better progression-free survival in a noncurable setting means more time spent accruing costs for expensive therapies. Each cycle of THP given every 3 weeks costs $8,642 plus ancillary care, modeled as $2,942 per week. These costs are already well above $100,000 per year, even before one considers less-than-perfect health state utilities. This seeming paradox explains why THP cannot be cost effective even with the most favorable assumptions (Table 2).
Our model has limitations. Quality-of-life estimates come with uncertainty and must strike a balance between practicality and realism. We did not explicitly model additional costs or disutilities for minor adverse events incurred while in the stable disease state. We did not model cost for measuring left ventricular ejection fraction every 9 weeks during the trial and at regular intervals after progression. We did not consider multiple permutations of second- and third-line therapies. Costs in the progressing disease state were based on a broad population with metastatic breast cancer, whereas patients in the CLEOPATRA trial received costly targeted therapies, including lapatinib, trastuzumab, and ado-trastuzumab emtansine.9 Effectively, this means that patients in our model realized an overall survival benefit from therapies without incurring the cost.
Despite these limitations, we believe our conclusions are justified. Our model was informed by high-quality data. Weekly follow-up allowed for high temporal resolution and all patients had lived out their remaining lives by the time the model terminated. Costing was sound, and our methods are transparent in accordance with the Society for Medical Decision Making task force guidelines.25 The utilities we used are the same as those used in recent cost-effectiveness analyses of trastuzumab and pertuzumab,17,18 and quality-of-life data gathered as a secondary end point from the trial seem to support our assumptions of equivocal quality-of-life between the arms and worsening quality of life after progression.39 The costing limitations identified above most likely resulted in underestimate of the cost per QALY gained; that is, the model was generous in its assumptions. Sensitivity analysis confirmed that THP is unlikely to be cost effective even under the most favorable set of assumptions.
WTP thresholds used in most US analyses range from $50,000 per QALY to three times the US per-capita gross domestic product, or about $160,000 per QALY.40 Our base case scenario yielded a cost of $472,668 per QALY gained, which is well above any commonly used threshold and well above the de facto threshold of cost effectiveness for interventions already in practice. Expensive targeted therapies are far more likely to be cost effective in the nonmetastatic setting. Liberato et al41 report a cost of $18,970 per QALY gained for patients treated with trastuzumab for early HER2-overexpresssing breast cancer. Kurian et al42 report $39,982 per QALY gained for the same intervention in the same population. Attard et al18 report costs of $25,388 and $46,196 per QALY gained for use of THP in Canada based on their dual analysis of the NeoSphere and TRYPHAENA trials. In the metastatic setting, the cost of even a single targeted agent is increasingly more per QALY gained. Elkin et al43 report a cost of $125,000 per QALY gained for HER2 testing and treatment with trastuzumab. It is notable that these findings were based on cost assumptions that were substantially similar to ours.43
The choice to adopt a highly effective but low-value strategy is not unprecedented. The switch from film to digital screening mammography cost $331,000 per QALY gained.44 An analysis of ondansetron showed a cost of $407,667 per QALY gained shortly after it was approved for cisplatin-induced nausea and vomiting.45 Conversely, some low-value interventions are being recognized as such and are being prescribed less often. Use of intensity-modulated radiotherapy in locally advanced pancreatic cancer is one such example, with a cost of over $1 million per QALY gained.46
This analysis highlights the economic challenges of extending life for patients with noncurable disease. It also typifies the broader observation that half of our health care dollars are spent on 5% of the population.47 The results of this study contribute to a broader discussion of value in health care. Here, we have a therapy that is highly effective but not cost effective. Cost-effectiveness studies should not be viewed as definitive recommendations but rather serve as one piece of a broader discussion in how we allocate resources to treat cancer.
Supplementary Material
Appendix
Patient Population Cost Impacts
In 2014, an estimated 232,670 new cases and 40,000 deaths resulted from breast cancer.35 Estimates of recurrence and metastasis have been as high as 30%.36 Human epidermal growth factor receptor 2 overexpression is seen in 20% to 25% of cases.1,2 We conservatively assumed the same ratio for the metastatic subgroup. Given these figures, we estimate 17,450 cases of human epidermal growth factor receptor 2 metastatic cancer per year.
Table A1.
Micro-Costing for One-Time and Cycle-Specific Costs Associated With CLEOPATRA Trial Arms
| CPT Code | Description | Fee Category | Unit Cost (MPFS/ASP) | Multiplier | Subtotal | QTY | One-Time Cost | Cycle-Specific Cost (per week) |
|---|---|---|---|---|---|---|---|---|
| First-line therapy | ||||||||
| J9306 | Pertuzumab injection 1 mg, loading* | Drug | $10.22 | 840.00 | $8,583.12 | 1.00 | $8,583.12 | |
| J9306 | Pertuzumab injection 1 mg, subsequent* | Drug | $10.22 | 420.00 | $4,291.56 | 0.33 | $1,430.52 | |
| 96413 | Pertuzumab 1 unit for 60 minutes, loading* | Administration | $133.26 | 1.00 | $133.26 | 1.00 | $133.26 | |
| 96413 | Pertuzumab 1 unit for 60 minutes, subsequent* | Administration | $133.26 | 1.00 | $133.26 | 0.33 | $44.42 | |
| J9355 | Trastuzumab Injection 10 mg, loading† | Drug | $82.49 | 56.00 | $4,619.44 | 1.00 | $4,619.44 | |
| J9355 | Trastuzumab Injection 10 mg, subsequent† | Drug | $82.49 | 42.00 | $3,464.58 | 0.33 | $1,154.86 | |
| 96413 | Trastuzumab 1 unit for 90 minutes, loading† | Administration | $133.26 | 1.00 | $133.26 | 1.00 | $133.26 | |
| 96417 | Trastuzumab 1 unit for 60 minutes, subsequent† | Administration | $61.97 | 1.00 | $61.97 | 0.33 | $20.66 | |
| J9171 | Docetaxel injection 1 mg, initial cycle‡ | Drug | $4.66 | 135.00 | $629.10 | 1.00 | $629.10 | |
| J9171 | Docetaxel injection 1 mg, subsequent‡ | Drug | $4.66 | 135.00 | $629.10 | 0.33 | $209.70 | |
| 96417 | Docetaxel 1 unit for 60 minutes, initial cycle‡ | Administration | $61.97 | 1.00 | $61.97 | 1.00 | $61.97 | |
| 96417 | Docetaxel 1 unit for 60 minutes, subsequent‡ | Administration | $61.97 | 1.00 | $61.97 | 0.33 | $20.66 | |
| Supportive | ||||||||
| J7030 | Normal saline, 1-L unit price | Drug | $1.35 | 1.00 | $1.35 | 0.33 | $1.35 | $0.45 |
| 96360 | Hydration for first hour | Administration | $56.96 | 1.00 | $56.96 | 0.33 | $56.96 | $18.99 |
| 96361 | Hydration after first hour | Administration | $15.05 | 3.00 | $45.15 | 0.33 | $45.15 | $15.05 |
| J2405 | Ondansetron 8 mg IV, 1-mg unit price | Drug | $0.08 | 8.00 | $0.64 | 0.33 | $0.64 | $0.21 |
| 96374 | Ondansetron IV push | Administration | $56.24 | 1.00 | $56.24 | 0.33 | $56.24 | $18.75 |
| J1100 | Decadron 10 mg, 1-mg unit price | Drug | $0.14 | 10.00 | $1.42 | 0.33 | $1.42 | $0.47 |
| 96375 | Decadron IV push | Administration | $22.21 | 1.00 | $22.21 | 0.33 | $22.21 | $7.40 |
Abbreviations: ASP, average sales price, as reported to Medicare by the manufacturer; CLEOPATRA, Clinical Evaluation of Pertuzumab and Trastuzumab trial; CPT, current procedural terminology; IV, intravenous; MPFS, Medicare physician fee schedule; QTY, quantity.
Pertuzumab dosing every 3 weeks: 840-mg loading dose for 60 minutes and 420 mg for 30 to 60 minutes for subsequent infusions.
Trastuzumab dosing every 3 weeks: 8-mg/kg loading dose for 90 minutes and 6 mg/kg for 30 to 90 minutes for subsequent infusions.
Docetaxel dosing every 3 weeks: 75 mg/m2 for 60 minutes for ≥ six cycles. Dose could be decreased by 25% because of toxicity or increased to 100 mg/m2 in patients who could tolerate this dose; < six cycles were allowed for unmanageable toxicity.
Fig A1.
Detailed view of the decision tree and Markov model. CLEOPATRA, Clinical Evaluation of Pertuzumab and Trastuzumab; HER2, human epidermal growth factor receptor 2.
Fig A2.
Probability of existing in one of four health states: stable disease, during the trial; progressing disease, next-line therapy; hospice; and dead.
Footnotes
See accompanying editorial on page 889
Processed as a Rapid Communication manuscript.
Supported in part by the Henry S. Kaplan Research Fund, Department of Radiation Oncology, Stanford University (to B.Y.D.), and by Career Development Award No. K01AG037593-01A1, National Institute on Aging of the National Institutes of Health (to J.D.G.-F.).
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Ben Y. Durkee, Yushen Qian, Erqi L. Pollom, Jeremy D. Goldhaber-Fiebert, Kathleen C. Horst
Collection and assembly of data: Ben Y. Durkee, Yushen Qian, Erqi L. Pollom, Sara A. Dudley, Jeremy D. Goldhaber-Fiebert
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cost-Effectiveness of Pertuzumab in Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Ben Y. Durkee
No relationship to disclose
Yushen Qian
No relationship to disclose
Erqi L. Pollom
No relationship to disclose
Martin T. King
No relationship to disclose
Sara A. Dudley
No relationship to disclose
Jenny L. Shaffer
No relationship to disclose
Daniel T. Chang
Stock or Other Ownership: ViewRay
Iris C. Gibbs
No relationship to disclose
Jeremy D. Goldhaber-Fiebert
No relationship to disclose
Kathleen C. Horst
No relationship to disclose
REFERENCES
- 1.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 2.Yaziji H, Goldstein L, Barry T, et al. HER-2 testing in breast cancer using parallel tissue- based methods. JAMA. 2004;291:1972–1977. doi: 10.1001/jama.291.16.1972. [DOI] [PubMed] [Google Scholar]
- 3.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of Chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: The M77001 Study Group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 6.Tzahar E, Waterman H, Chen X, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbeck N, Beckmann MW, Rody A, et al. HER2 Dimerization inhibitor pertuzumab: Mode of action and clinical data in breast cancer. Breast Care (Basel) 2013;8:49–55. doi: 10.1159/000346837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 11.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 12.Dartmouth Institute for Health Policy Clinical Practice, Center for Health Policy Research. Percent of Cancer Patients Enrolled in Hospice, by Interval Before Death, The Dartmouth Atlas of Health Care, 2010. http://www.dartmouthatlas.org.
- 13.Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med. 1996;335:172–178. doi: 10.1056/NEJM199607183350306. [DOI] [PubMed] [Google Scholar]
- 14.Younis T, Milch R, Abul-Khoudoud N, et al. Length of survival of patients with cancer in hospice: A retrospective analysis of patients treated at a major cancer center versus other practice settings. J Palliative Med. 2007;10:381–389. doi: 10.1089/jpm.2006.0071. [DOI] [PubMed] [Google Scholar]
- 15.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arias E. United States life tables, 2010. Natl Vital Stat Rep. 2014;63:1–63. [PubMed] [Google Scholar]
- 17.Hedden L, O'Reilly S, Lohrisch C, et al. Assessing the real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu positive breast cancer. Oncologist. 2012;17:164–171. doi: 10.1634/theoncologist.2011-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attard CL, Pepper AN, Brown ST, et al. Cost-effectiveness analysis of neoadjuvant pertuzumab and trastuzumab therapy for locally advanced, inflammatory, or early HER2-positive breast cancer in Canada. J Med Econ. 2015;18:173–188. doi: 10.3111/13696998.2014.979938. [DOI] [PubMed] [Google Scholar]
- 19.Casarett D, Fishman J, O'Dwyer PJ, et al. How should we design supportive cancer care? The patient's perspective. J Clin Oncol. 2008;26:1296–1301. doi: 10.1200/JCO.2007.12.8371. [DOI] [PubMed] [Google Scholar]
- 20.Launois R, Reboul-Marty J, Henry B, et al. A cost-utility analysis of second-line chemotherapy in metastatic breast cancer: Docetaxel versus paclitaxel versus vinorelbine. Pharmacoeconomics. 1996;10:504–521. doi: 10.2165/00019053-199610050-00008. [DOI] [PubMed] [Google Scholar]
- 21.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chastek B, Harley C, Kallich J, et al. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8:75s–80s. doi: 10.1200/JOP.2011.000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med. 2009;169:480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen RN, Ramsey SD, Lalla D, et al. Identification and cost of adverse events in metastatic breast cancer in taxane and capecitabine based regimens. Springerplus. 2014;3:259. doi: 10.1186/2193-1801-3-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32:733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 26.Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 27.Briggs AH, Weinstein MC, Fenwick EAL, et al. Model parameter estimation and uncertainty analysis: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32:722–732. doi: 10.1177/0272989X12458348. [DOI] [PubMed] [Google Scholar]
- 28.Doubilet P, Begg CB, Weinstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation: A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Medicare and Medicaid Services. Medicare Part B Drug Average Sale Price. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html.
- 30.Centers for Medicare and Medicaid Services. Medicare Physician Fee Schedule 2014. https://www.cms.gov/apps/physician-fee-schedule/
- 31.Hutton J, Brown R, Borowitz M, et al. A new decision model for cost-utility comparisons of chemotherapy in recurrent metastatic breast cancer. Pharmacoeconomics. 1996;9(suppl 2):8–22. doi: 10.2165/00019053-199600092-00004. [DOI] [PubMed] [Google Scholar]
- 32.Hillner BE, Smith TJ, Desch CE. Efficacy and cost-effectiveness of autologous bone marrow transplantation in metastatic breast cancer: Estimates using decision analysis while awaiting clinical trial results. JAMA. 1992;267:2055–2061. [PubMed] [Google Scholar]
- 33.US Department of Labor. Inflation & Prices Online Calculator: Bureau of Labor Statistics, 2015. http://www.bls.gov/data/#calculators.
- 34.Briggs AH, Gray AM. Handling uncertainty in economic evaluations of healthcare interventions. BMJ. 1999;319:635–638. doi: 10.1136/bmj.319.7210.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Research Data 1973-2011, 2014. www.seer.cancer.gov.
- 36.O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10:20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 37.Fleeman N, Bagust A, Beale S, et al. Pertuzumab in combination with trastuzumab and docetaxel for the treatment of HER2-positive metastatic or locally recurrent unresectable breast cancer. Pharmacoeconomics. 2015;33:13–23. doi: 10.1007/s40273-014-0206-2. [DOI] [PubMed] [Google Scholar]
- 38.National Centre for Pharmacoeconomics. National Centre for Pharmacoeconomics. Rialto Gate, St. James's Hospital; Cost effectiveness of pertuzumab (perjeta) in combination with trastuzumab and docetaxel in adults with HER2-positive metastatic or locally recurrent unresectable breast cancer who have not received previous anti-HER2 Therapy or chemotherapy. Dublin 8, Ireland, 2013. [Google Scholar]
- 39.Cortés J, Baselga J, Im YH, et al. Health-related quality-of-life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer. Ann Oncol. 2013;24:2630–2635. doi: 10.1093/annonc/mdt274. [DOI] [PubMed] [Google Scholar]
- 40.Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 41.Liberato NL, Marchetti M, Barosi G. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2–positive breast cancer. J Clin Oncol. 2007;25:625–633. doi: 10.1200/JCO.2006.06.4220. [DOI] [PubMed] [Google Scholar]
- 42.Kurian AW, Thompson RN, Gaw AF, et al. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol. 2007;25:634–641. doi: 10.1200/JCO.2006.06.3081. [DOI] [PubMed] [Google Scholar]
- 43.Elkin EB, Weinstein MC, Winer EP, et al. HER-2 testing and trastuzumab therapy for metastatic breast cancer: A cost-effectiveness analysis. J Clin Oncol. 2004;22:854–863. doi: 10.1200/JCO.2004.04.158. [DOI] [PubMed] [Google Scholar]
- 44.Tosteson ANA, Stout NK, Fryback DG, et al. Cost-effectiveness of digital mammography breast cancer screening. Ann Intern Med. 2008;148:1–10. doi: 10.7326/0003-4819-148-1-200801010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zbrozek AS, Cantor SB, Cardenas MP, et al. Pharmacoeconomic analysis of ondansetron versus metoclopramide for cisplatin-induced nausea and vomiting. Am J Hosp Pharm. 1994;51:1555–1563. [PubMed] [Google Scholar]
- 46.Murphy JD, Chang DT, Abelson J, et al. Cost-effectiveness of modern radiotherapy techniques in locally advanced pancreatic cancer. Cancer. 2012;118:1119–1129. doi: 10.1002/cncr.26365. [DOI] [PubMed] [Google Scholar]
- 47.Kaiser Family Foundation. Contribution to total health expenditures by individuals: Analysis of medical expenditure panel survey. Agency for Healthcare Research and Quality: US Department of Health and Human Services; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.