Abstract
Purpose
Evidence-based treatments for metastatic, human epidermal growth factor receptor 2 (HER2)–positive breast cancer in the CNS are limited. Neratinib is an irreversible inhibitor of erbB1, HER2, and erbB4, with promising activity in HER2-positive breast cancer; however, its activity in the CNS is unknown. We evaluated the efficacy of treatment with neratinib in patients with HER2-positive breast cancer brain metastases in a multicenter, phase II open-label trial.
Patients and Methods
Eligible patients were those with HER2-positive brain metastases (≥ 1 cm in longest dimension) who experienced progression in the CNS after one or more line of CNS-directed therapy, such as whole-brain radiotherapy, stereotactic radiosurgery, and/or surgical resection. Patients received neratinib 240 mg orally once per day, and tumors were assessed every two cycles. The primary endpoint was composite CNS objective response rate (ORR), requiring all of the following: ≥50% reduction in volumetric sum of target CNS lesions and no progression of non-target lesions, new lesions, escalating corticosteroids, progressive neurologic signs/symptoms, or non-CNS progression—the threshold for success was five of 40 responders.
Results
Forty patients were enrolled between February 2012 and June 2013; 78% of patients had previous whole-brain radiotherapy. Three women achieved a partial response (CNS objective response rate, 8%; 95% CI, 2% to 22%). The median number of cycles received was two (range, one to seven cycles), with a median progression-free survival of 1.9 months. Five women received six or more cycles. The most common grade ≥ 3 event was diarrhea (occurring in 21% of patients taking prespecified loperamide prophylaxis and 28% of those without prophylaxis). Patients in the study experienced a decreased quality of life over time.
Conclusion
Although neratinib had low activity and did not meet our threshold for success, 12.5% of patients received six or more cycles. Studies combining neratinib with chemotherapy in patients with CNS disease are ongoing.
INTRODUCTION
Patients with metastatic, human epidermal growth factor receptor 2 (HER2)–positive breast cancers often face significant challenges once their disease progresses through standard trastuzumab-based regimens, and approximately one half of patients will ultimately develop parenchymal brain metastases.1-7 Upfront therapy for newly diagnosed brain metastases typically includes whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), or both, and a small proportion of patients undergo surgical resection for limited disease. Historically, survival after diagnosis of CNS disease was poor, and many patients died of progressive disease8; however, recent studies have suggested that median survival for patients with CNS disease may be improving, particularly for those with HER2-positive disease, likely as a result of advances in systemic therapy.9-13 Unfortunately, standard therapies after CNS progression remain undefined. Few prospective clinical trials have been conducted in the modern era, and many trials evaluating novel agents specifically exclude patients with active CNS disease.
Neratinib is a potent, oral, irreversible-binding inhibitor of the erbB family of receptor tyrosine kinases that inhibits signal transduction through erbB1, HER2, and erbB4.14-17 Although CNS penetration by neratinib is not well described, preclinical models suggest limited penetration,18 with concentrations similar to those observed in the brains of healthy animals who received lapatinib,19 an agent known to produce responses in the brain.20,21 Thus, low CNS concentrations do not necessarily preclude potential CNS activity, particularly in the setting of a disrupted blood-tumor barrier. Given the promising activity for neratinib monotherapy16 and the potential for CNS penetration as a small molecule, we conducted a two-stage, phase II, single-arm study to evaluate the efficacy of treatment with neratinib in women with HER2-positive brain metastases.
PATIENTS AND METHODS
Study Cohort
Adult patients with HER2-positive,22 invasive breast cancer and measurable CNS disease (one or more parenchymal brain lesions measuring ≥ 10 mm in the longest dimension) whose cancers progressed in the CNS after any previous CNS-directed therapy (WBRT, SRS, surgery, or any combination) were eligible. Other key inclusion criteria included Eastern Cooperative Oncology Group performance status of 0 to 2 and a cardiac ejection fraction of ≥ 50%. There was no limit on the number of previous therapy lines; previous lapatinib, but not previous neratinib, was allowed. Patients were excluded if they had escalating corticosteroids during the week before baseline imaging, more than two seizures over the 4 weeks before study entry, significant malabsorption syndrome, inability to tolerate oral medications, or any pre-existing, chronic, grade ≥ 2 diarrhea.
The study was conducted through the Translational Breast Cancer Research Consortium, and all women signed an informed consent approved by the institutional review boards of each participating institution. Participating centers included the Dana-Farber/Harvard Cancer Center (Dana-Farber Cancer Institute, Beth Israel Deaconess Medical Center, and Massachusetts General Hospital), Baylor College of Medicine and its affiliate Ben Taub Hospital, Johns Hopkins University, University of California, San Francisco, University of Michigan, Duke University, and the University of Pittsburgh.
Treatment Plan
This was a two-stage, phase II, open-label, single-arm study. Oral neratinib 240 mg was administered once per day without breaks, and cycle duration was 28 days. All patients were evaluated with a neurologic exam on the first day of each cycle. Evaluations included neurologic symptoms (ie, headache, dizziness, etc), cranial nerve and motor strength assessments, presence of aphasia and dysphasia, ataxia, somnolence sensation, and global assessment of worsening, stability, or improvement. Patients were reimaged every two cycles with magnetic resonance imaging of the brain and computed tomography scans of the chest-abdomen-pelvis.
Patients were to remain on study treatment until tumor progression, unacceptable toxicity and/or adverse event, severe intercurrent illness, request to come off study, study closure, or any changes in the condition of the participant that rendered the participant unacceptable for further treatment. Treatment holds and/or dose reductions were required for patients who experienced prolonged grade ≥ 2 diarrhea, grade ≥ 2 pneumonitis, grade ≥ 3 nausea and vomiting or rash, or any grade 4 toxicity. An amendment mandating loperamide 2 mg once per day during cycle 1 was approved on May 14, 2012, at the Dana-Farber Cancer Institute (and subsequently approved at all sites). In total, 28 of 40 patients received prophylactic loperamide.
In an optional extension cohort, patients who experienced non-CNS progression by Response Evaluation Criteria in Solid Tumors (RECIST) 1.123,24 but who had stable disease (SD), a partial response (PR), or a complete response (CR) in the CNS had the option to continue therapy with neratinib (at the current dose) along with standard dose trastuzumab (administration weekly or every three weeks).
Two additional cohorts are still enrolling patients in this protocol, including a cohort of patients deemed eligible for surgical resection (cohort 2) and a cohort of women with progressive CNS disease who receive concurrent neratinib and capecitabine (cohort 3). Results of cohort 1 are presented here.
Correlative Studies
Correlative studies for women in our study included a research blood draw at baseline to be processed using the CellSearch Circulating Tumor Cells (CTC) method (Janssen Diagnostics, Raritan, NJ).25,26 We also examined longitudinal neurocognitive function and quality of life (QOL) for women at baseline compared with end of treatment. If progression occurred before cycle 2 or 3 testing, the most recent testing served as end of treatment. Neurocognitive testing included the Hopkins Verbal Learning Test-Revised (HVLT-R; learning and memory), the Trail Making Test Parts A (for processing speed) and B (for executive function), and Controlled Oral Word Association (for executive function). To assess QOL, patients completed the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30)/BN20.27-29
Statistical Analyses
The primary end point assessed was objective response rate (ORR; to include CR and PR) in the CNS, according to composite criteria.21 Measurement of CNS lesions was performed centrally by the Harvard Tumor Imaging Metrics Core. Non-CNS evaluation was completed by local investigators, per RECIST 1.1.23,24 An objective CNS PR was defined as ≥ 50% reduction in the sum volume of CNS target lesions, without new lesions, systemic disease progression, clearly worsening neurologic status (either by neurologic examination or by neurologic symptoms), or increase in corticosteroid dose. A CR was defined as disappearance of all target lesions in addition to the PR criteria. Progressive disease (PD) was defined as ≥ 40% increase in the sum of target lesions, any new lesion ≥ 6 mm, systemic progression, clearly worsening neurologic status, or significant increase in corticosteroid dose. If a tumor progressed in a non-CNS site first or a patient died or withdrew from the study for any reason after receiving at least one dose of the drug and before a response was determined, she was considered a nonresponder. In the few cases (n = 3) for which CNS progression was suspected locally but not confirmed centrally, patients were allowed to continue on the study until the next imaging assessment. If progression was then noted, the time of progression was documented as the first suspected progression.
Secondary objectives assessed included time to progression, overall survival (OS), response by bidirectional CNS response criteria (PR, ≥ 50% decrease; PD, ≥ 25% increase in the sum of the longest diameters),30 response according to Response Assessment in Neuro-Oncology Brain Metastases Working Group criteria31 (PR, ≥ 30% decrease in the sum of the longest diameters of CNS disease for ≥ 4 weeks with no new lesions, stable and/or improved clinical condition, and stable and/or decreased corticosteroid dose), safety and tolerability, and associations of baseline CTC count and OS. In exploratory analyses, we examined changes in neurocognitive function and QOL at end of treatment versus baseline.
The study had a two-stage design on the basis of a binomial distribution with constant probability of response, with an accrual goal of 40 patients. In the first stage, if one or more patients of 18 had a response, another 22 patients would be enrolled. All patients who received one dose of the study drug were included in analyses. If five or more patients of 40 achieved a CNS response, the drug would be deemed worthy of future study as a single agent. This two-stage design had 92% power to detect a true response of ≥ 20% (alternative hypothesis) against the true response rate of 6% (null hypothesis) while maintaining a one-sided type I error rate of 9%.
CTC Analysis
We examined associations of CTC count at baseline and OS for women in the study. We tested whether the percent of patients living more than 6 months after study entry in the group with less than five CTC per 7.5 mL of whole blood was significantly greater than the percent of patients living more than 6 months in the group with five or more CTC per 7.5 mL (one-sided Fisher’s exact test) on the basis of a previous work demonstrating the importance of this threshold.32
Neurocognitive Function and QOL
We compared baseline values to end-of-treatment values for HVLT-R Total Recall, HVLT-R Delayed Recall, HVLT-R Delayed Recognition, Trail Making Test Parts A and B, and Controlled Oral Word Association in two ways: by using standardized scores adjusted for age and education,33-35 and using the Reliable Change Index.36 The standardized scores were compared using the Wilcoxon signed rank test for paired data.
To assess QOL, we focused on seven EORTC QLQ-C30 QOL measures: global health status, physical functioning, cognitive functioning, diarrhea, fatigue, nausea and vomiting, and emotional functioning. These measures were constructed from the individual item values using the formulae in the EORTC QLQ-C30 version 3.0 manual. We compared baseline values to end-of-treatment values by using the Wilcoxon signed rank test for paired data.
RESULTS
Patient Characteristics
A total of 40 women enrolled in the study between February 2012 and June 2013 at seven Translational Breast Cancer Research Consortium centers. Baseline characteristics are shown in Table 1.
Table 1.
Patient Characteristics for the Cohort
| Characteristic | Value |
|---|---|
| Median age, years (range) | 51 (35-65) |
| Female | 40 (100) |
| ECOG PS | |
| 0 | 15 (38) |
| 1 | 13 (33) |
| 2 | 12 (30) |
| Race | |
| White | 34 (85) |
| Black/other | 6 (15) |
| Non-CNS sites of disease | |
| Lung | 15 (38) |
| Liver | 16 (40) |
| Bone | 26 (65) |
| Chest wall | 2 (5) |
| ER-positive disease | 18 (45) |
| Previous CNS therapy* | |
| WBRT alone | 15 (38) |
| SRS alone | 9 (23) |
| WBRT and SRS | 16 (40) |
| Previous surgical resection of CNS disease | 12 (30) |
| Previous systemic therapy for metastatic disease | |
| No previous chemotherapy | 2 (5) |
| ≥ 2 previous lines | 33 (83) |
| Trastuzumab | 36 (90) |
| Lapatinib | 34 (85) |
NOTE. N = 40. All data are given as No. (%) unless otherwise specified.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; PS, performance status; SRS, stereotactic brain surgery; WBRT, whole brain radiotherapy.
Patients may have received more than one previous CNS therapy. Totals do not sum to 100.
Efficacy
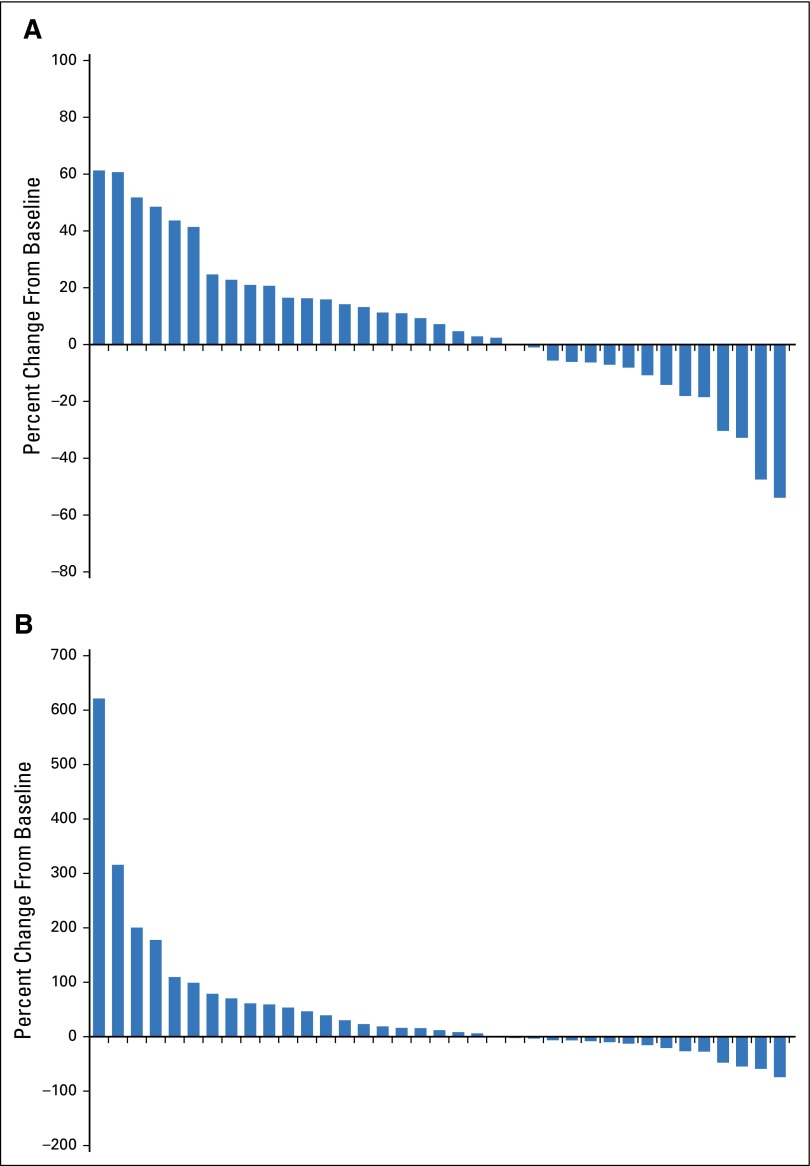
As of January, 1, 2015, no patients remained on the study treatment; 34 patients had died, two were lost to follow-up, and four remained alive. The median number of cycles received before any extension therapy was two (range, one to seven cycles). Five women received six or more cycles of therapy. One patient experienced a PR in the first stage of the study (n = 18), and the second stage enrolled to completion (n = 22). Three patients had an ORR (8%; 95% CI, 2% to 22%) and no patients experienced a CR (Fig 1; Table 2). In the three responders, duration of response was six cycles in one patient and four cycles in two patients; all three responders had received previous treatment with lapatinib. Of those without previous WBRT (n = 9), one patient experienced a PR, four had SD, and three had PD as the best CNS response; one patient was not evaluable. Of the six women who had not received previous treatment with lapatinib, five came off treatment as a result of progression by four cycles (range, one to four cycles) and one came off treatment as a result of toxicity during cycle 1.
Fig 1.
Waterfall plot for best (A) volumetric response and (B) bidirectional response in each patient who had baseline imaging and at least one staging exam (n = 37).
Table 2.
Best CNS Response According to Composite Criteria
| Best Response | Cohort 1 (N = 40) |
|---|---|
| CR | 0 (0) |
| PR | 3* (8; 95% CI, 2% to 22%) |
| SD six or more cycles | 4 (10) |
| SD less than six cycles | 12 (30) |
| PD | |
| PD in CNS only | 10 (25) |
| Symptomatic deterioration/clinical progression (CNS or non-CNS) | 7 (18) |
| PD in CNS and non-CNS | 2 (5) |
| Off treatment before restaging for toxicity | 2 (5) |
NOTE. Composite CNS objective response rate required all of the following: ≥ 50% reduction in volumetric sum of target CNS lesions, no progression of nontarget lesions, no new lesions, no escalating steroids, no progressive neurologic signs and symptoms, and no non-CNS progression. Data are given as No. (%) unless otherwise specified.
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
One responder received treatment for six cycles and was not included in the SD for six or more cycles category; two responders received four cycles of study treatment.
Reasons for discontinuation of study treatment included radiographic CNS progression (n = 23), clinical CNS progression (n = 6), non-CNS progression (n = 2), both CNS and non-CNS progression (n = 3), and toxicity (n = 6). Two patients who experienced non-CNS progression received trastuzumab-neratinib on the extension cohort after one and two cycles of neratinib, respectively. One of these patients received two extension cycles and the other received 15; neither experienced a CNS response during extension therapy.
Regarding secondary clinical outcomes, four women (10%) experienced a CNS response by bidirectional criteria. Seven patients had discordant results for their best volumetric and bidirectional response (five patients had PD by volume but SD by bidirectional criteria; one patient had SD by volume but PR by bidirectional criteria; and one patient had SD by volume but PD by bidirectional criteria). All three partial responders by volume also had responses by bidirectional criteria. For responses by Response Assessment in Neuro-Oncology Brain Metastases Working Group criteria,31 two (5%) women had confirmed PRs and two (5%) had unconfirmed PRs (response of ≥ 4 weeks could not be confirmed).
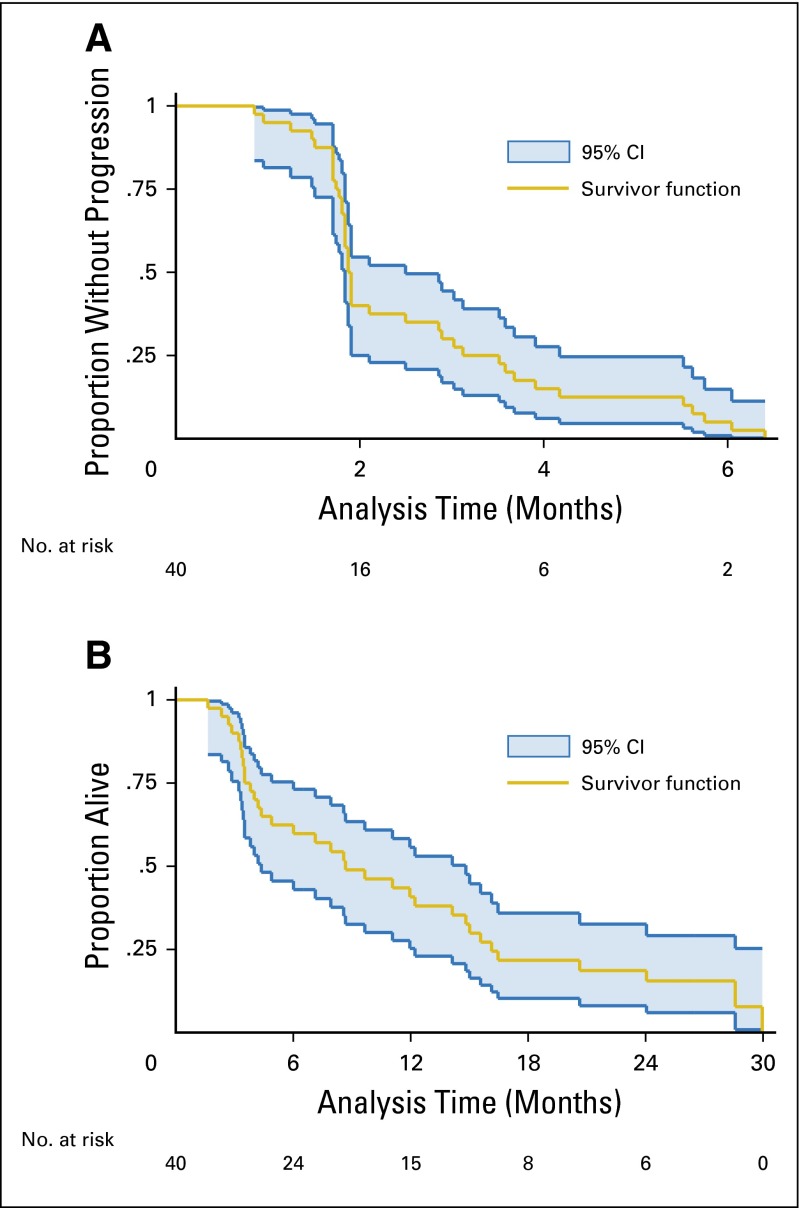
The median time to progression for women in the study was 1.9 months (all progressed, no censoring), and the median OS was 8.7 months, with 30% of women still alive at 12 months after study registration (Figs 2A and 2B).
Fig 2.
(A) Time to progression and (B) overall survival for women treated with neratinib (n = 40).
Toxicity
Adverse events for women in the study are shown in Table 3. The most common grade 3 event was diarrhea (23%), but the event rate decreased for patients who received prophylactic loperamide. Of 28 patients who received prophylaxis, 11% had grade 2 diarrhea and 21% had grade 3 diarrhea. Of those who did not receive prophylaxis (n = 12), 25% had grade 2 diarrhea, 25% had grade 3 diarrhea, and 4% (n = 1) had grade 4 diarrhea. Six patients required dose reductions (for diarrhea in three patients, for nausea and diarrhea in two patients, and for liver function in one patient). No additional grade ≥ 2 toxicities were reported for the two patients who received extension therapy with trastuzumab.
Table 3.
Grade ≥ 2 Adverse Events (No., %) for Women in the Study
| Toxicity | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Diarrhea*† | 6 (15) | 9 (23) | 1 (3) |
| Nausea and vomiting | 3 (8) | 2 (5) | |
| Constipation | 2 (5) | ||
| Anorexia | 3 (8) | ||
| Dehydration† | 1 (3) | ||
| Dyspepsia | 2 (5) | ||
| Mucositis | 1 (3) | 1 (3) | |
| Fatigue | 6 (15) | ||
| Decreased LVEF | 1 (3) | ||
| Hypotension† | 1 (3) | ||
| Sinus tachycardia† | 1 (3) | ||
| Peripheral sensory neuropathy | 1 (3) | ||
| Liver function tests | 1 (3) | 1 (3) | |
| Headache | 1 (3) |
NOTE. N = 40. Toxicities are graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Each grade is counted once as the worst grade experienced by the patient for each event. Included toxicities are those deemed by the investigator to be possibly, probably, or definitely related to treatment.
Abbreviation: LVEF, left ventricular ejection fraction.
Of 28 patients who received prophylaxis, three had grade 2 diarrhea (11%) and six had grade 3 diarrhea (21%). Among the 12 patients who did not receive prophylaxis, three had grade 2 diarrhea (25%), three had grade 3 diarrhea (25%), and one had grade 4 diarrhea (4%).
All three events occurred in the same patient and were associated with grade 3 diarrhea.
CTCs
Thirty-one patients had usable CTC samples drawn at baseline and were included in this analysis, nine of whom had a CTC of five or more. Baseline CTCs ranged from zero to 379 (median of 0; zero CTC [55%]; one to 10 [26%]; 11 to 20 [3%]; 21 to 50 [10%]; and > 50 [6%]). Of patients with a CTC five or more, five (56%) lived longer than 6 months (95% CI, 21% to 86%) compared with 13 of those with a CTC less than five (59%; 95% CI, 36% to 79%; Fisher’s exact P = 1.00).
Neurocognitive Function and QOL
Results for patients who completed baseline neurocognitive testing are shown in Appendix Table A1 (online only). Median test performance was generally in the average range at baseline, though large variability was observed. Median difference scores over time declined with a measure of memory (HVLT-R Delayed Recall) on five of six tests, reaching significance (P < .05) on the basis of the Wilcoxon signed rank (Table 4). According to the Reliable Change Index criteria, the majority of patients remained stable whereas a subgroup (0% to 36% on one or more tests) exhibited objective decline, with the most frequently observed decline occurring on a measure of learning and memory (HVLT-R). Global QOL, fatigue, and diarrhea all worsened with time (Appendix Table A2, online only).
Table 4.
Results for Changes in Neurocognitive Function From Baseline to Off Treatment for Women With Paired Results Available
| Test | No. of Patients With Data Available | Median Difference | Wilcoxon SRT P | Improved (%)* | Stable (%)* | Worsened (%)* |
|---|---|---|---|---|---|---|
| HVLT-R Total Recall | 22 | −0.13 | .40 | 9 | 51 | 32 |
| HVLT-R Delayed Recall | 22 | −0.59 | .04 | 5 | 59 | 36 |
| HVLT-R Delayed Recognition | 22 | 0.00 | .57 | 14 | 55 | 32 |
| Trail Making Test Part A | 22 | −0.38 | .26 | 18 | 64 | 18 |
| Trail Making Test Part B | 17 | −0.49 | .21 | 18 | 58 | 24 |
| COWA | 22 | −0.15 | .99 | 14 | 86 | 0 |
Abbreviations: COWA, Controlled Oral Word Association; HVLT-R, Hopkins Learning Verbal Test–Revised; SRT, signed rank test.
Data obtained by using the Reliable Change Index from baseline to off treatment.
DISCUSSION
In this phase II, single-arm study, we evaluated the efficacy and toxicity of single-agent neratinib in patients with progressive, HER2-positive brain metastases. The CNS ORR for neratinib monotherapy was low and did not meet its prespecified threshold for success among a highly treatment-refractory population, though clinical activity was observed and five patients (12.5%) remained on treatment for at least six cycles. Although diarrhea was initially common, rates substantially decreased with prophylactic loperamide. To lower diarrhea events further, loperamide prophylaxis has since been instituted in all neratinib protocols at higher doses than were used in our study (total, 16 mg/d).
Neratinib monotherapy has demonstrated promising clinical activity in HER2-positive extracranial disease in past trials,16 but it resulted only in a low CNS ORR in our study, similar to that seen with lapatinib monotherapy.21,37 Despite the low number of CNS responses observed with lapatinib and neratinib when used as monotherapy, studies of CNS tissue penetration of lapatinib in patients with glioblastoma multiforme and breast cancer CNS metastases have demonstrated significant concentrations of lapatinib in CNS surgical specimens, perhaps because of a disrupted blood-brain barrier or an inhibition of efflux transporters.19,38,39 Cohort 2 of our protocol is still enrolling and will provide important data on neratinib penetration in CNS tumors and CSF; patients in this cohort will receive neratinib pre- and postoperatively, and will have drug concentrations assessed in brain parenchyma, CSF, and plasma.
It is possible that even though lapatinib, and possibly neratinib, crosses the blood-tumor barrier, activity may be optimized when administered in combination with other agents. This may at least partially explain the higher CNS response rates observed when lapatinib is combined with capecitabine.20,21,40 A phase I and II protocol of neratinib-capecitabine in the setting of metastatic HER2-positive breast cancer demonstrated robust systemic activity41 and has provided the rationale for the NALA study (A Study of Neratinib Plus Capecitabine v Lapatinib Plus Capecitabine in Patients With HER2+ Metastatic Breast Cancer Who Have Received Two or More Prior HER2 Directed Regimens in the Metastatic Setting, ClinicalTrials.gov NCT01808573), which randomly assigns patients with metastatic HER2-positive disease to receive capecitabine-neratinib versus capecitabine-lapatinib. Although women with active brain metastases are not eligible for NALA, we are currently enrolling patients in a third cohort of our study, which is evaluating capecitabine-neratinib in patients with progressive CNS disease. We hypothesize that the combination may be more effective than single-agent neratinib, although this remains to be seen.
Although CTC count has been associated with survival in patients with metastatic breast cancer and in those with CNS metastases,32,42,43 our results were not conclusive. The lack of consistency in these findings may be related to differences in patient populations, varying disease burden, disease sites, and disease subtypes, and suggests the possibility that a CTC of five may not be a relevant threshold for all patient groups.
Our findings for worsened neurocognitive function test results for patients at end of treatment were not surprising given that most patients had experienced progression and most patients had received WBRT, a treatment known to impact learning and memory. Although direct comparisons with other brain metastasis populations are fraught with difficulty, the current study population exhibited better neurocognitive function than did those in most published samples.44-46 Given that neurocognitive function is a good prognostic factor for survival time, it is also not surprising that many patients remained alive longer than expected. Furthermore, our QOL results showed expected changes in worse global health status, increased fatigue, and increased diarrhea over time.
We recognize several study limitations. First, this was a small, phase II study without a comparison arm, and patients had a diverse set of past CNS-directed treatments. Our study population was heavily pretreated with a high burden of systemic disease, and several patients also had longstanding CNS disease. Second, because of the low number of responders, an examination of predictors of response is not possible. In addition, although it is possible that previous treatments, such as lapatinib, may limit responses, all of the responders in our study received previous lapatinib. Although these clinical issues may have led to an underestimation of the CNS activity of neratinib, our results did not meet the threshold to prompt continued investigation of neratinib monotherapy in this clinical context. In a currently enrolling cohort (cohort 3), neratinib and capecitabine are being administered in combination, and separate cohorts will enroll patients who have either not received (cohort 3a) or received (cohort 3b) previous lapatinib.
Beyond these specific trial results, the rapid accrual of this trial and others focused on brain metastases highlights the unmet medical need for new therapies for brain metastases as well as the feasibility of conducting trials in this patient population. Further study of potentially active CNS agents is crucial to improve QOL and duration of life for patients with progressive metastatic breast cancer of the brain. Results from ongoing treatment cohorts will be forthcoming.
Supplementary Material
Acknowledgment
We thank all the patients who generously volunteered to participate in this study. We thank the Translational Breast Cancer Research Consortium (TBCRC) investigators, research nurses, and study coordinators for their efforts on behalf of the patients.
Appendix
Table A1.
Summary of Baseline Data for Neurocognitive Function Analyses for Patients With Baseline Data
| Test | No. of Patients With Data Available | Median (Range) |
|---|---|---|
| HVLT-R Total Recall | 37 | −1.5 (−6.3-1.1) |
| HVLT-R Delayed Recall | 36 | −1.4 (−6.1-1.2) |
| HVLT-R Delayed Recognition | 36 | −0.2 (−11.1-0.9) |
| Trail Making Test Part A | 35 | 0.1 (−31.6-1.4) |
| Trail Making Test Part B | 33 | −1.1 (−169-1.4) |
| COWA | 33 | −0.7 (−3.4-0.8) |
NOTE. HVLT-R assessed learning and memory; Trail Making Test Parts A and B assessed processing speed and executive function; and COWA assessed executive function.
Abbreviations: COWA, Controlled Oral Word Association; HVLT-R, Hopkins Verbal Learning Test–Revised.
Table A2.
Quality of Life From Baseline to End of Treatment
| Test | No. of Patients With Paired Data Available* | Median Difference | Wilcoxon SRT P |
|---|---|---|---|
| Global health status† | 22 | −12.5 | .029 |
| Physical functioning | 24 | 0.0 | .37 |
| Cognitive functioning | 23 | 0.0 | .31 |
| Diarrhea‡ | 23 | 0.0 | .017‡ |
| Fatigue§ | 22 | 11.1 | .0016 |
| Nausea and vomiting | 23 | 0.0 | .46 |
| Emotional functioning | 22 | 0.0 | .61 |
NOTE. Paired data available for 22 to 24 patients using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30/BN20.
Abbreviation: SRT, signed rank test.
For seven patients, C2 data were used for end-of-treatment data.
For global health status, larger values are better; a negative difference indicates that a patient got worse. The differences were somewhat larger in patients whose end of treatment was beyond two cycles (median, −16.7) compared with patients with only one or two cycles (median, −12.5), but this difference was not statistically significant (P = .44).
Although the median difference for diarrhea is 0.0, of the 23 patients with data, 10 patients got worse, whereas two got better and 11 stayed the same.
For fatigue, larger values are worse; a positive difference indicates that a patient got worse.
Footnotes
Written on behalf of the Translational Breast Cancer Research Consortium.
Support provided to the Translational Breast Cancer Research Consortium by the AVON Foundation, the Breast Cancer Research Foundation, and Susan G. Komen for the Cure. Additional funding support provided by the Breast Cancer Research Foundation (N.U.L.), the Dana-Farber Women’s Cancer Program Executive Council Personalized Medicine Award (N.U.L.), Dana-Farber/Harvard Cancer Center P30 CA006516 (R.S.G.), and Puma Biotechnology.
Presented in part at the 50th Annual American Society of Clinical Oncology Meeting, Chicago, IL, June 2, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01494662.
AUTHOR CONTRIBUTIONS
Conception and design: Rachel A. Freedman, Rebecca S. Gelman, Jeffrey S. Wefel, Michelle E. Melisko, Roisin M. Connolly, Catherine H. Van Poznak, Polly A. Niravath, Shannon L. Puhalla, Nuhad Ibrahim, Kimberly L. Blackwell, Christina Herold, Minetta C. Liu, Alarice Lowe, Nathalie Y.R. Agar, Nicole Ryabin, Elizabeth Lawler. Mothaffar F. Rimawi, Ian E. Krop, Antonio C. Wolff, Eric P. Winer, Nancy U. Lin
Financial support: Nancy U. Lin
Administrative support: Rachel A. Freedman, Nicole Ryabin, Sarah Farooq, Elizabeth Lawler, Mothaffar F. Rimawi, Ian E. Krop, Antonio C. Wolff, Nancy U. Lin
Provision of study materials or patients: Rachel A. Freedman, Michelle E. Melisko, Roisin M. Connolly, Catherine H. Van Poznak, Polly A. Niravath, Shannon L. Puhalla, Kimberly L. Blackwell, Beverly Moy, Alarice Lowe, Nicole Ryabin, Mothaffar F. Rimawi, Antonio C. Wolff, Eric P. Winer, Nancy U. Lin
Collection and assembly of data: Rachel A. Freedman, Rebecca S. Gelman, Jeffrey S. Wefel, Kimberly L. Blackwell, Christina Herold, Alarice Lowe, Nicole Ryabin, Sarah Farooq, Elizabeth Lawler, Nancy U. Lin
Data analysis and interpretation: Rachel A. Freedman, Rebecca S. Gelman, Jeffrey S. Wefel, Michelle E. Melisko, Kenneth R. Hess, Roisin M. Connolly, Catherine H. Van Poznak, Polly A. Niravath, Shannon L. Puhalla, Nuhad Ibrahim, Kimberly L. Blackwell, Beverly Moy, Christina Herold, Minetta C. Liu, Alarice Lowe, Nathalie Y.R. Agar, Elizabeth Lawler, Mothaffar F. Rimawi, Ian E. Krop, Antonio C. Wolff, Eric P. Winer, Nancy U. Lin
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Translational Breast Cancer Research Consortium (TBCRC) 022: A Phase II Trial of Neratinib for Patients With Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Rachel A. Freedman
Research Funding: Puma Biotechnology (Inst), Genentech (Inst), Eisai (Inst)
Rebecca S. Gelman
No relationship to disclose
Jeffrey S. Wefel
Consulting or Advisory Role: Genentech, Roche, Juno Therapeutics, Adelphi Values
Michelle E. Melisko
Stock or Other Ownership: Merrimack (I)
Honoraria: Agendia, Genentech (I), Genentech, Nektar
Consulting or Advisory Role: Genentech (I)
Speakers' Bureau: Genentech (I), Agendia
Research Funding: Genentech (Inst), Galena Biopharma (Inst), Celldex (Inst), Puma Biotechnology (Inst)
Patents, Royalties, Other Intellectual Property: Multiple patents related to immunoliposomal drugs being studied and potentially brought to market by Merrimack (I)
Kenneth R. Hess
Travel, Accommodations, Expenses: Angiochem
Roisin M. Connolly
Research Funding: Genentech (Inst), Puma Biotechnology (Inst), Novartis (Inst), Merrimack (Inst), Clovis Oncology (Inst)
Catherine H. Van Poznak
Research Funding: Bayer AG (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate, royalties for writing updates
Polly A. Niravath
Research Funding: Puma Biotechnology (Inst)
Shannon L. Puhalla
Consulting or Advisory Role: Celldex, MedImmune, Pfizer
Research Funding: AbbVie (Inst), Pfizer (Inst), Genentech (Inst), Eli Lilly (Inst), AstraZeneca (Inst), Biomarin (Inst)
Nuhad Ibrahim
Honoraria: Celgene, Roche, Novartis
Speakers' Bureau: Celgene, Roche, Novartis
Research Funding: GenomeDx, Newlink Genetics, Archer Biosciences
Travel, Accommodations, Expenses: Roche, Novartis
Kimberly L. Blackwell
Consulting or Advisory Role: Celgene, Hospira, Novartis, Pfizer, Rockwell Medical, Roche, Genentech, Sandoz, AstraZeneca
Research Funding: Celgene (Inst), Genentech (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Celgene, Hospira, Novartis, Pfizer, Roche, Rockwell Medical, Sandoz, Genentech
Beverly Moy
No relationship to disclose
Christina Herold
No relationship to disclose
Minetta C. Liu
Research Funding: Eisai (Inst), Seattle Genetics (Inst), Celgene (Inst), Veridex (Inst), Clearbridge Biomedics (Inst), Novartis (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Genentech
Alarice Lowe
Stock or Other Ownership: La Jolla Pharmaceuticals, Illumina, CVS Health Corp (I), Peregrine Pharma (I), Biopath Holdings (I)
Nathalie Y.R. Agar
Honoraria: BayesianDx
Consulting or Advisory Role: Bayesian Dx
Patents, Royalties, Other Intellectual Property: Intellectual property portfolio at Partners Healthcare for surgical margin delineation and intraoperative diagnosis largely on the basis of mass spectrometry
Nicole Ryabin
No relationship to disclose
Sarah Farooq
No relationship to disclose
Elizabeth Lawler
No relationship to disclose
Mothaffar F. Rimawi
Consulting or Advisory Role: Celgene
Research Funding: GlaxoSmithKline (Inst)
Ian E. Krop
Employment: AMAG Pharmaceuticals (I)
Leadership: AMAG Pharmaceuticals (I)
Stock or Other Ownership: AMAG Pharmaceuticals (I)
Research Funding: Genentech
Travel, Accommodations, Expenses: Bayer AG
Antonio C. Wolff
Honoraria: Medscape
Consulting or Advisory Role: Mersana
Research Funding: Myriad Genetics (Inst), Pfizer (Inst)
Patents, Royalties, Other Intellectual Property: Johns Hopkins University Reference C12014: A Quantitative Multiplex Methylation Specific PCR Method-cMethDNA, Reagents, and Its Use
Eric P. Winer
No relationship to disclose
Nancy U. Lin
Consulting or Advisory Role: Novartis
Research Funding: Genentech, GlaxoSmithKline, Array BioPharma, Novartis, Geron, Kadmon
REFERENCES
- 1.Lin NU, Winer EP. Brain metastases: The HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 2.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 3.Pestalozzi BC. Brain metastases and subtypes of breast cancer. Ann Oncol. 2009;20:803–805. doi: 10.1093/annonc/mdp246. [DOI] [PubMed] [Google Scholar]
- 4.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 5.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 6.Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: A retrospective substudy of the HERA trial (BIG 1-01) Lancet Oncol. 2013;14:244–248. doi: 10.1016/S1470-2045(13)70017-2. [DOI] [PubMed] [Google Scholar]
- 7.Olson EM, Najita JS, Sohl J, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22:525–531. doi: 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmoud-Ahmed AS, Suh JH, Lee SY, et al. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: A retrospective study. Int J Radiat Oncol Biol Phys. 2002;54:810–817. doi: 10.1016/s0360-3016(02)02967-x. [DOI] [PubMed] [Google Scholar]
- 9.Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: The importance of HER-2 status. Cancer. 2008;112:2359–2367. doi: 10.1002/cncr.23468. [DOI] [PubMed] [Google Scholar]
- 10.Gori S, Rimondini S, De Angelis V, et al. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: Incidence, survival, and risk factors. Oncologist. 2007;12:766–773. doi: 10.1634/theoncologist.12-7-766. [DOI] [PubMed] [Google Scholar]
- 11.Karam I, Hamilton S, Nichol A, et al. Population-based outcomes after brain radiotherapy in patients with brain metastases from breast cancer in the pre-trastuzumab and trastuzumab eras. Radiat Oncol. 2013;8:12. doi: 10.1186/1748-717X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melisko ME, Moore DH, Sneed PK, et al. Brain metastases in breast cancer: Clinical and pathologic characteristics associated with improvements in survival. J Neurooncol. 2008;88:359–365. doi: 10.1007/s11060-008-9578-5. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 15.Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 16.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Bonneterre J, Geyer CE, Jr, et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer. 2013;49:3763–3772. doi: 10.1016/j.ejca.2013.07.142. [DOI] [PubMed] [Google Scholar]
- 18.Puma Biotechnology . Neratinib investigator brochure. Puma Biotechnology: Los Angeles, CA; 2012. [Google Scholar]
- 19.Kuhn JG, Robbins H, Mehta M, et al. Tumor sequestration of lapatinib (NABTC 04-01) Neuro-oncol. 2008;10(abstr ET-05):783. [Google Scholar]
- 20.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 21.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond EH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz LH, Bogaerts J, Ford R, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–267. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 26.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 27.Sprangers MA, Cull A, Bjordal K, et al. EORTC Study Group on Quality of Life The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: Guidelines for developing questionnaire modules. Qual Life Res. 1993;2:287–295. doi: 10.1007/BF00434800. [DOI] [PubMed] [Google Scholar]
- 28.King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 29.Cocks K, King MT, Velikova G, et al. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 data in randomised controlled trials. Eur J Cancer. 2008;44:1793–1798. doi: 10.1016/j.ejca.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 31.Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastates: Proposal from the RANO group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 32.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 33.Ruff RM, Light RH, Parker SB, et al. Benton Controlled Oral Word Association Test: Reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338. [PubMed] [Google Scholar]
- 34.Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 35.Benedict RHB, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 36.Wefel JS, Cloughesy T, Zazzali JL, et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro-oncol. 2011;13:660–668. doi: 10.1093/neuonc/nor024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polli JW, Humphreys JE, Harmon KA, et al. The role of efflux and uptake transporters in [N-3-chloro-4-[(3-fluorobenzyl)oxy]phenyl-6-[5-([2-(methylsulfonyl)ethyl]aminomethyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 39.Morikawa A, Peereboom DM, Thorsheim HR, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: A prospective study. Neuro-oncol. 2015;17:289–295. doi: 10.1093/neuonc/nou141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin NU, Eierman W, Greil R, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;105:613–620. doi: 10.1007/s11060-011-0629-y. [DOI] [PubMed] [Google Scholar]
- 41.Saura C, Martin M, Moroose R, et al. Safety of neratinib (HKI-272) in combination with capecitabine in patients with solid tumors:; A phase 1/2 study. Presented at the San Antonio Breast Cancer Symposium San Antonio, TX; December 10-13, 2009; doi: 10.1158/0008-5472.SABCS-09-5108. [Google Scholar]
- 42.Pierga JY, Bidard FC, Cropet C, et al. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: The LANDSCAPE trial. Ann Oncol. 2013;24:2999–3004. doi: 10.1093/annonc/mdt348. [DOI] [PubMed] [Google Scholar]
- 43.Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32:3483–3489. doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 45.Brown PD, Pugh S, Laack NN, et al. Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: Results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.