Abstract
Purpose
To compare incidence and survival of peripheral T-cell lymphoma (PTCL) subtypes among US racial/ethnic groups.
Methods
Patients with PTCL (age ≥ 15 years; 2000 to 2012) were identified in the Surveillance, Epidemiology, and End Results (SEER) registries. Race/ethnicity was categorized as non-Hispanic white, black, Asian/Pacific Islander, Hispanic white, or American Indian/Alaskan native. Age-standardized annual incidence rates and incidence rate ratios were estimated with 95% CIs, and case-case odds ratios were estimated by race/ethnicity using polytomous regression. Survival was estimated from SEER follow-up data with Cox regression.
Results
Thirteen thousand one hundred seven patients with PTCL were identified. Annual PTCL incidence was highest in blacks and lowest in Native Americans. Compared with non-Hispanic whites, blacks had a higher incidence of PTCL not otherwise specified (PTCL-NOS), anaplastic large-cell lymphoma, and adult T-cell leukemia/lymphoma (ATLL) and a lower incidence of angioimmunoblastic T-cell lymphoma (AITL); Asians/Pacific Islanders had a higher incidence of AITL, extranodal nasal-type natural killer/T-cell lymphoma and NK-cell leukemia (ENKCL), and ATLL and a lower incidence of anaplastic large-cell lymphoma; Hispanics had a higher incidence of AITL and ENKCL; and Native Americans had a lower incidence of PTCL-NOS (all P < .05). The ratio of ENKCL to PCTL-NOS among Native Americans, Asians/Pacific Islanders, and Hispanic whites was approximately three- to four-fold the same ratio among non-Hispanic whites. Survival varied significantly by race/ethnicity (P < .001), with blacks in particular experiencing shorter survival for most subtypes.
Conclusion
Striking variation in incidence, proportions of PTCL subtypes, and survival was observed. Aspects of these PTCL subtype patterns, such as for ENKCL and ATLL, were similar to corresponding global populations. Despite the small population size and limited number of Native American patients, PTCL subtype frequencies in this group were distinct but most similar to Hispanic whites. Survival disparities were evident, especially for blacks compared with non-Hispanic whites.
INTRODUCTION
The incidence of peripheral T-cell lymphoma (PTCL) and the relative frequencies of PTCL subtypes among patients display pronounced global variation.1,2 PTCL accounts for a larger fraction of all non-Hodgkin lymphoma cases in Asia than in the United States or Europe, and among patients with PTCL, adult T-cell leukemia/lymphoma (ATLL) and extranodal NK/T-cell lymphoma and natural killer (NK) –cell leukemia (ENKCL) are more common in Asia than in Western nations.1 The reasons for such geographic and ethnic predilections are not fully understood, but genetic predisposition to lifelong infection with human T-lymphotropic virus type 1 (HTLV-1) in the case of ATLL and Epstein-Barr virus in the case of ENKCL has been implicated.3,4
Within the diverse population of the United States, published studies describing non-Hodgkin lymphoma incidence and survival by race and ethnicity have typically grouped all PTCLs together or predate current WHO (2008) classification.5-8 Furthermore, inclusion of Native Americans and Hispanics in epidemiologic studies describing PTCL has been inconsistent.5,7,9 Therefore, questions remain regarding the overall and relative incidence of PTCL subtypes in US populations, and comparison with genetically and culturally related global populations has been hindered.
The International T-Cell Lymphoma Epidemiology and Outcomes Project demonstrated differences in survival between PTCL subtypes,1 which has also been reported in the United States.10 Biologic heterogeneity between PTCL entities is likely to be largely responsible for differences in survival in subtypes11; however, the impact of race and ethnicity has been less systematically evaluated. Possible mechanisms for such impact include differences in immunogenetics and pharmacogenomics between races and disparities in medical care received. Despite frequent reporting of racial composition of clinical trials, stratification of outcomes by race is rarely explored. Such analyses, if routinely performed, might provide useful information regarding differential dosing, efficacy, toxicity, and outcomes between races and ethnic groups.
In this study, we used data from the population-based US Surveillance, Epidemiology, and End Results (SEER) cancer registries to quantify PTCL incidence rates, and the proportions of patients with each PTCL subtype, by racial and ethnic groups. We also analyzed the survival differences between races in specific PTCL subtypes. We aimed to add to our understanding of patterns of PTCL incidence and survival in the United States, including the understudied American Indian/Alaskan native population, with the goal of facilitating comparisons to other global populations and introducing additional considerations for future clinical trials and allocation of health care resources.
METHODS
Study Population
Data were retrieved from 18 SEER cancer registries in the United States12,13 describing all patients with T-cell lymphoma age ≥ 15 years diagnosed between 2000 and 2012 (N = 20,726). The National Cancer Institute SEER cancer registries assemble information on patients with cancer, including patient demographics, tumor site and histology, and outcomes, and capture approximately 97% of incident cancers within catchment areas covering approximately 28% of the US population.12,14,15
PTCL Subtypes
Patients with T-cell lymphoma (TCL) were categorized using International Classification of Diseases for Oncology, Third Edition codes,6 as follows: PTCL not otherwise specified (PTCL-NOS; 9702); anaplastic large-cell lymphoma (ALCL; 9714); angioimmunoblastic TCL (AITL; 9705); ENKCL (9719 [n = 655] and 9948 [n = 89]); ATLL (9827); and other PTCL. Other PTCL included cutaneous TCL not otherwise specified (9709 and 9726), enteropathy-associated TCL (9717), hepatosplenic TCL (9716), primary cutaneous anaplastic large-cell lymphoma (9718), subcutaneous panniculitis-like TCL (9708), T-cell large granular lymphocytic leukemia (9831), and T-cell prolymphocytic leukemia (9834). Patients diagnosed with mycosis fungoides/Sézary syndrome (9700 and 9701), precursor TCL (9729, 9727, 9835, and 9837), or TCL of unknown subtype (9591 and 9684) were excluded (n = 7,233) from these analyses. PTCL in persons with a prior PTCL were also excluded (n = 150).
Vital Status Follow-Up
SEER registries calculated survival time in months based on active follow-up for vital status until December 31, 2012,13 imputing for patients partially missing follow-up information.16 In total, 192 patients with insufficient follow-up information were excluded.
Race/Ethnicity
SEER registries classify race and ethnicity using 2000 US Census categories based on self-identification, medical records, and death certificates.17 American Indian individuals are additionally identified by SEER registries through linkage to Indian Health Service records. Hispanic ethnicity is not mutually exclusive of any race and is based on an identification algorithm that includes self-report, medical record, evaluation of surname, and birthplace.17,18 Hence, for these analyses, race/ethnicity was categorized as non-Hispanic white, Hispanic white, black, Asian/Pacific Islander, American Indian/Alaskan native, or unknown. Therefore, blacks, Asians/Pacific Islanders, and American Indians/Alaskan natives included both non-Hispanic and Hispanic persons (1.7%, 1.2%, and 4.8%, respectively). Persons of unknown race/ethnicity (n = 236) were excluded from primary statistical analyses.
For two subanalyses of ATLL, we used SEER database place of birth information for patients diagnosed through 2011 (n = 11,816; 37% with unknown birthplace) and additional ethnicity information among Asians.
Statistical Analysis
SEER*Stat software (version 8.2.1; Surveillance Research Program, National Cancer Institute, Bethesda, MD) was used to calculate age-standardized (to 2000 US population) incidence rates (IRs) and incidence rate ratios (IRRs) with 95% CIs.
Individual SEER patient records were downloaded for statistical analysis with Stata/SE version 14.0 (StataCorp, College Station, TX). We used χ2 tests to compare numbers of PTCL subtypes pairwise by race/ethnicity. Polytomous logistic regression was used to estimate case-case odds ratios (ORs) and 95% CIs for race/ethnic groups compared with non-Hispanic whites, adjusted for age, sex, geographic region, and diagnosis calendar year. PTCL-NOS was the reference outcome; therefore, case-case ORs for a PTCL subtype and racial/ethnic subpopulation are equivalent to the adjusted ratio
Kaplan-Meier product-limit estimates of overall survival probability were calculated for PTCLs together and for each subtype by race/ethnicity and compared with global log-rank tests.
Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% CIs of death from any cause, comparing each race/ethnicity to non-Hispanic whites. Baseline hazard functions were stratified by age category and HRs adjusted for sex, region, and diagnosis calendar year. For analysis of all PTCLs combined, the baseline hazard functions were also stratified by subtype. Consistent with the SEER program data use agreement, results for groups of five or fewer patients are not presented.
RESULTS
Patient Characteristics
A total of 13,107 patients with PTCL who were diagnosed between 2000 and 2012 and who met study inclusion criteria were identified, including 8,777 non-Hispanic whites (66%), 1,763 blacks (13%), 992 Asians/Pacific Islanders (8%), 1,492 Hispanic whites (11%), and 83 American Indians/Alaskan natives (0.6%). The median age of patients with PTCL was 62 years (interquartile range, 49 to 75 years) and varied by PTCL subtype (65 years for PTCL-NOS, 56 years for ALCL, 69 years for AITL, 54 years for ENKCL, 62 years for ATLL, and 63 years for other subtypes combined). The majority of non-Hispanic white (62%) and Asian/Pacific Islander (57%) patients were 60 years of age or older, whereas the majority of black (60%), Hispanic white (60%), and American Indian/Alaskan native (55%) patients were younger than 60 years at PTCL diagnosis (Table 1). There was male predominance for all ethnic groups (Table 1) and within each PTCL subtype except ATLL, which was nearly balanced (data not shown). More diagnoses were recorded during later study years than earlier years. Overall, patients were evenly distributed between the western region of the United States and other regions combined, although geographic location varied with race (Table 1).
Table 1.
Select Characteristics of Included Patients With Peripheral T-Cell Lymphoma (N = 13,107) by Race and Ethnicity, From SEER Cancer Registries, 2000 to 2012
| Characteristic | % of Patients | ||||
|---|---|---|---|---|---|
| Non-Hispanic White (n = 8,777) | Black (n = 1,763) | Asian/Pacific Islander (n = 992) | Hispanic White (n = 1,492) | American Indian/Alaskan Native (n = 83) | |
| Age at diagnosis, years | |||||
| < 50 | 20.6 | 37.4 | 25.4 | 42.6 | 43.4 |
| 50-59 | 17.6 | 22.8 | 17.2 | 17.2 | 12.0 |
| 60-69 | 21.3 | 17.8 | 19.7 | 16.3 | 22.9 |
| 70-79 | 22.9 | 14.0 | 23.5 | 14.4 | 16.9 |
| ≥ 80 | 17.7 | 8.0 | 14.2 | 9.5 | 4.8 |
| Female | 41.4 | 44.8 | 42.7 | 38.9 | 44.6 |
| Diagnosis year | |||||
| 2000-2002 | 20.2 | 19.3 | 18.0 | 16.0 | 19.3 |
| 2003-2005 | 23.4 | 23.0 | 22.7 | 20.8 | 13.3 |
| 2006-2008 | 24.1 | 22.9 | 23.0 | 25.7 | 22.9 |
| 2009-2011 | 32.2 | 34.8 | 36.3 | 37.5 | 44.6 |
| SEER region* | |||||
| West | 45.6 | 24.9 | 86.7 | 80.1 | 95.2 |
| Other | 54.4 | 75.1 | 13.3 | 19.9 | 4.8 |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
West includes Hawaii, New Mexico (and Arizona American Indians), Puget Sound/Seattle, Utah, Alaskan natives, and California registries; Other includes Detroit, Iowa, Connecticut, New Jersey, Atlanta, Rural Georgia, Kentucky, and Louisiana.
Incidence of PTCL Subtypes
The annual IR of all PTCLs was 1.56 (95% CI, 1.52 to 1.59) per 100,000 persons in non-Hispanic whites, and compared with this group, incidence was higher in blacks (IRR, 1.32; 95% CI, 1.25 to 1.39; P < .001), lower in Asians/Pacific Islanders (IRR, 0.89; 95% CI, 0.83 to 0.95; P < .001) and American Indians/Alaskan natives (IRR, 0.63; 95% CI, 0.49 to 0.79; P < .001), and nonsignificantly lower in Hispanic whites (IRR, 0.96; 95% CI, 0.90 to 1.01; P = .13).
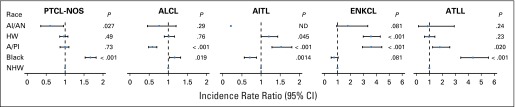
Incidence of PTCL-NOS, ALCL, AITL, ENKCL, and ATLL subtypes varied markedly by race/ethnicity (Fig 1). In blacks, compared with non-Hispanic whites, the incidence of PTCL-NOS (IRR, 1.67; 95% CI, 1.53 to 1.82), ALCL (IRR, 1.17; 95% CI, 1.03 to 1.32), and ATLL (IRR, 4.37; 95% CI, 3.42 to 5.56) was higher, whereas the incidence of AITL was lower (IRR, 0.71; 95% CI, 0.56 to 0.88). Among Asians/Pacific Islanders, the incidence of AITL (IRR, 1.53; 95% CI, 1.28 to 1.80), ENKCL (IRR, 3.61; 95% CI, 2.93 to 4.42), and ATLL (IRR, 1.81; 95% CI, 1.25 to 2.57) was higher and incidence of ALCL was lower (IRR, 0.59; 95% CI, 0.49 to 0.70) than in non-Hispanic whites. Hispanic whites had a higher ENKCL incidence (IRR, 3.59; 95% CI, 2.99 to 4.31) and marginally higher AITL incidence (IRR, 1.21; 95% CI, 1.00 to 1.44; P = .045) than non-Hispanic whites. Finally, among American Indians/Alaskan natives, few AITLs were observed, and the incidence of PTCL-NOS was 40% (95% CI, 4% to 64%) lower, whereas the incidence of ENKCL was 80% (95% CI, −7% to 234%) higher, than that in non-Hispanic whites, although the difference in ENKCL rates did not reach statistical significance.
Fig 1.
Incidence rate ratios (IRRs) of peripheral T-cell lymphoma (PTCL) subtypes by race/ethnicity compared with non-Hispanic whites (NHWs) in US Surveillance, Epidemiology, and End Results cancer registries (2000 to 2012). IRRs were calculated from age-standardized incidence rates. AI/AN, American Indian/Alaskan native; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; A/PI, Asian/Pacific Islander; ATLL, adult T-cell lymphoma/leukemia; ENKCL, extranodal nasal-type natural killer (NK)/T-cell lymphoma and NK-cell leukemia; HW, Hispanic white; ND, not determined because of small numbers; PTCL-NOS, PTCL not otherwise specified.
Distribution of PTCL Subtypes by Race
In blacks, the proportions of patients with PTCL-NOS and ATLL were higher and proportions of patients with AITL or ENKCL were lower than in non-Hispanic whites (Table 2). A larger percentage of Asians/Pacific Islanders and Hispanic whites were diagnosed with ATLL compared with non-Hispanic whites. Among Asians/Pacific Islanders, Hispanic whites, and American Indians/Alaskan natives, approximately 14% of patients had ENKCL, compared with 3.3% of patients among non-Hispanic whites and 2.0% of patients among blacks. Pairwise tests of the distribution of PTCL subtypes between race/ethnicities showed highly significant differences for all pairs of race/ethnic groups (each P < .001) except for Hispanic whites compared with American Indians/Alaskan natives (P = .22).
Table 2.
Number and Percentage of Patients With Each PTCL Subtype by Race/Ethnic Group and Case-Case ORs for Each PTCL Subtype Compared With PTCL-NOS, SEER Cancer Registries, 2000 to 2012
| PTCL Subtype | Non-Hispanic White | Black | Asian/Pacific Islander | Hispanic White | American Indian/Alaskan Native | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | OR* | 95% CI | No. (%) | OR* | 95% CI | No. (%) | OR* | 95% CI | No. (%) | OR* | 95% CI | |
| PTCL-NOS | 2,689 (30.6) | 661 (37.5) | Ref | 322 (32.5) | Ref. | 418 (28.0) | Ref | 20 (24.1) | Ref | ||||
| ALCL | 1,608 (18.3) | 312 (17.7) | 0.66 | 0.56 to 0.77 | 130 (13.1) | 0.62 | 0.49 to 0.77 | 335 (22.5) | 1.03 | 0.87 to 1.22 | 23 (27.7) | 1.51 | 0.83 to 2.76 |
| AITL | 944 (10.8) | 94 (5.3) | 0.48 | 0.38 to 0.61 | 166 (16.7) | 1.22 | 0.99 to 1.50 | 161 (10.8) | 1.02 | 0.83 to 1.25 | ≤ 5 | NE | 0.10 to 1.22 |
| ENKCL | 304 (3.5) | 37 (2.1) | 0.42 | 0.30 to 0.61 | 146 (14.7) | 3.29 | 2.59 to 4.19 | 244 (16.4) | 3.55 | 2.90 to 4.35 | 13 (15.7) | 3.92 | 1.98 to 7.76 |
| ATLL | 180 (2.1) | 118 (6.7) | 2.58 | 2.01 to 3.31 | 40 (4.0) | 1.92 | 1.31 to 2.78 | 34 (2.3) | 1.23 | 0.83 to 1.82 | ≤ 5 | NE | |
| Other† | 3,052 (34.8) | 541 (30.7) | 0.66 | 0.58 to 0.75 | 188 (19.0) | 0.52 | 0.43 to 0.63 | 300 (20.1) | 0.61 | 0.52 to 0.71 | 22 (26.5) | 0.94 | 0.51 to 1.73 |
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ATLL, adult T-cell lymphoma/leukemia; ENKCL, extranodal nasal-type natural killer (NK)/T-cell lymphoma and NK leukemia; NE, not estimable as a result of small numbers of patients; OR, odds ratio; PTCL, peripheral T-cell lymphoma; PTCL-NOS, PTCL, not otherwise specified; Ref, reference; SEER, Surveillance, Epidemiology, and End Results.
Adjusted for age, sex, SEER registry region, and year of diagnosis. Case-case OR = (%subtype/%PTCL-NOS)group/(%subtype/%PTCL-NOS)NHW.
Other includes subcutaneous panniculitis-like T-cell lymphoma, hepatosplenic T-cell lymphoma, enteropathy-associated T-cell lymphoma, cutaneous T-cell lymphoma, not otherwise specified, primary cutaneous anaplastic large-cell lymphoma, T-cell large granular lymphocytic leukemia, and T-cell prolymphocytic leukemia.
With adjustment for age, sex, year of diagnosis, and geographical region, case-case ORs showed that among American Indians/Alaskan natives, the ratio of ENKCLs to PTCL-NOSs was 3.92-fold (95% CI, 1.98- to 7.76-fold) the same ratio in non-Hispanic whites; results for Asians/Pacific Islanders and Hispanic whites were similar. In contrast, among blacks, the same ratio was 0.42 (95% CI, 0.30 to 0.61; Table 2). Differences in the same comparative OR were significant for several other racial/ethnic groups and PTCL subtypes, notably for ATLL among Asians/Pacific Islander compared with non-Hispanic whites (OR, 1.92; 95% CI, 1.31 to 2.78). Although the percentage of both PTCL-NOSs and ATLLs among blacks was higher than among non-Hispanic whites, the case-case OR was significantly different from 1 (OR, 2.58; 95% CI, 2.01 to 3.31), indicating a disproportionately higher percentage of ATLLs among blacks.
Survival
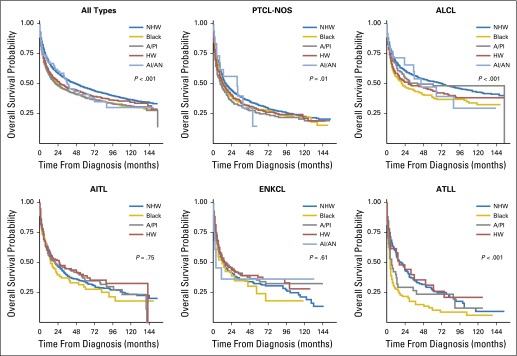
Follow-up information was available for 12,915 patients with PTCL of known race, and 6,875 deaths occurred over a median of 19 months of follow-up (interquartile range, 4 to 59 months). Survival curves showed significant variation by race; median survival time of patients with any PTCL was 49, 24, 22, 28, and 36 months for non-Hispanic whites, blacks, Asians/Pacific Islanders, Hispanic whites, and American Indians/Alaskan natives, respectively (global log-rank P < .001; Fig 2). Separately, survival after PTCL-NOS, ALCL, and ATLL diagnosis varied significantly by race/ethnicity (global log-rank P = .01, P < .001, and P < .001, respectively), in contrast to AITL and ENKCL (P = .75 and P = .61, respectively). Accounting for age, sex, geographic region, and calendar year of diagnosis, compared with non-Hispanic whites, survival was shorter for each minority racial/ethnic group when all PTCLs were considered together (Table 3). By subtype and race, compared with non-Hispanic whites, blacks had shorter survival after diagnosis with PTCL-NOS (HR, 1.34; 95% CI, 1.20 to 1.49), ALCL (HR, 1.70; 95% CI, 1.45 to 2.00), AITL (HR, 1.58; 95% CI, 1.25 to 1.99), or ATLL (HR, 2.13; 95% CI, 1.60 to 2.84; Table 3). Similar results were observed for Asian/Pacific Islander patients with PTCL-NOS (HR, 1.33; 95% CI, 1.15 to 1.55); for Hispanic white patients with PTCL-NOS (HR, 1.33; 95% CI, 1.16 to 1.51) or ALCL (HR, 1.50; 95% CI, 1.27 to 1.79); and for American Indian/Alaskan native patients with ALCL (HR, 1.55; 95% CI, 1.00 to 2.41).
Fig 2.
Overall survival of patients with peripheral T-cell lymphoma (PTCL) according to subtype and race/ethnicity, from US Surveillance, Epidemiology, and End Results cancer registries (2000 to 2012). P values are from global log-rank tests of the survival curves. AI/AN, American Indian/Alaskan native; AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; A/PI, Asian/Pacific Islander; ATLL, adult T-cell lymphoma/leukemia; ENKCL, extranodal nasal-type natural killer (NK)/T-cell lymphoma and NK-cell leukemia; HW, Hispanic white; NHW, non-Hispanic white; PTCL-NOS, PTCL not otherwise specified.
Table 3.
Hazard Ratios for Mortality Among Patients With PTCL Comparing Each Race/Ethnicity to Non-Hispanic White, SEER Cancer Registries, 2000 to 2012
| Race/Ethnicity | All PTCLs* (n = 12,915; deaths, n = 6,875) | PTCL-NOS (n = 4,017; deaths, n = 2,617) | ALCL (n = 2,376; deaths, n = 1,213) | AITL (n = 1,360; deaths, n = 834) | ENKCL (n = 736; deaths, n = 449) | ATLL (n = 362; deaths, n = 279) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR†‡ (95% CI) | P | HR† (95% CI) | P | HR† (95% CI) | P | HR† (95% CI) | P | HR† (95% CI) | P | HR† (95% CI) | P | |
| Non-Hispanic white | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Black | 1.49 (1.39 to 1.60) | < .001 | 1.34 (1.20 to 1.49) | < .001 | 1.70 (1.45 to 2.00) | < .001 | 1.58 (1.25 to 1.99) | < .001 | 1.19 (0.81 to 1.75) | .37 | 2.13 (1.66 to 2.51) | < .001 |
| Asian/Pacific Islander | 1.20 (1.10 to 1.32) | < .001 | 1.33 (1.15 to 1.55) | < .001 | 1.12 (0.85 to 1.49) | .42 | 1.06 (0.86 to 1.31) | .56 | 0.94 (0.71 to 1.23) | .85 | 1.33 (0.87 to 2.04) | .18 |
| Hispanic white | 1.25(1.15 to 1.36) | < .001 | 1.33 (1.16 to 1.51) | < .001 | 1.50 (1.27 to 1.79) | < .001 | 1.12 (0.90 to 1.40) | .30 | 0.98 (0.77 to 1.24) | .44 | 1.16 (0.72 to 1.87) | .55 |
| American Indian/Alaskan native | 1.28 (0.97 to 1.70) | .08 | 1.07 (0.67 to 1.69) | .79 | 1.55 (1.00 to 2.41) | .049 | NE | 1.40 (0.59 to 3.32) | NE | |||
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ATLL, adult T-cell lymphoma/leukemia; ENKCL, extranodal nasal-type natural killer (NK)/T-cell lymphoma and NK leukemia; HR, hazard ratio; NE, not estimable as a result of small numbers of patients; PTCL, peripheral T-cell lymphoma; PTCL-NOS, PTCL, not otherwise specified; Ref, reference; SEER, Surveillance, Epidemiology, and End Results.
Includes PTCL-NOS, ALCL, AITL, ENKCL, ATLL, and other subtypes (subcutaneous panniculitis-like T-cell lymphoma, hepatosplenic T-cell lymphoma, enteropathy-associated T-cell lymphoma, cutaneous T-cell lymphoma, not otherwise specified, primary cutaneous anaplastic large-cell lymphoma, T-cell large granular lymphocytic leukemia, and T-cell prolymphocytic leukemia).
Adjusted for sex, calendar year of diagnosis, and region; baseline hazards stratified by age group.
Baseline hazards additionally stratified by PTCL subtype: PTCL-NOS, ALCL, AITL, ENKCL, ATLL, or other.
DISCUSSION
PTCLs are a rare and diverse group of lymphoid malignancies with strong racial and geographic predilections and significant differences in subtype-specific outcomes.1 Given the diversity of the US population, we analyzed the incidence and proportions of PTCL subtypes and survival after diagnosis using data from the population-based SEER cancer registries. To our knowledge, this is the most complete report of its kind. Several striking features are evident in our results that have received limited previously published attention.
First, blacks were diagnosed with PTCL at a higher rate than non-Hispanic whites or any other group; this has been observed previously.5,7 Our results, from a more recent period of time, add detail showing that the higher rate of PTCL in blacks was in large measure a result of higher PTCL-NOS incidence; ATLL incidence was also much higher in blacks than non-Hispanic whites but accounts for few patients. Conversely, the incidence of AITL was significantly lower and the incidence of ENKCL was somewhat lower in this group than in non-Hispanic whites, which are differences that have not been previously reported and, therefore, are poorly understood. Our findings also highlight the need for stronger efforts to increase recruitment of blacks into clinical trials of PTCL therapies.
Second, ENKCL rates were much higher in Asians/Pacific Islanders and Hispanic whites and nonsignificantly higher in American Indians/Alaskan natives than in non-Hispanic whites and blacks. Correspondingly, a larger proportion of PTCL in these populations was ENKCL (approximately 14%) than in non-Hispanic whites (approximately 3%). This is similar to but lower than previously published data from Asia, where approximately 22% of PTCLs are ENKCL.1,19 ENKCL is also reported to be more common in Latin America than in the United States and Europe,20 although data are sparse. Although information on place of birth in SEER registries is of limited validity,21,22 35% of patients with ENKCL were born outside of the United States compared with 16% of all patients with PTCL (data not shown). Thus, differences in ENKCL rates and in the proportion of patients with PTCL who have ENKCL between global populations and US Hispanic and Asian/Pacific Islander populations may reflect the mixture of immigrants and US-born persons in the US populations, differences in the specific Asian and Hispanic subpopulations in our study compared with others, and the influence of both genetic and environmental risk factors.
In addition, the similar ENKCL incidence among Hispanic whites, Asians/Pacific Islanders, and American Indians/Alaskan natives suggests shared risk factors. Epstein-Barr virus infection is closely associated with ENKCL,23-25 and risk of developing ENKCL seems to depend on immunogenetic background and environmental factors.3
Third, we observed a lower incidence of ALCL among Asians/Pacific Islanders compared with non-Hispanic whites. As with ENKCL, these differences resemble global variation between populations.1 In contrast to ENKCL, the incidence of ALCL in Asians/Pacific Islanders was not similar to the incidence of ALCL in Hispanic whites and American Indians/Alaskan natives. The underlying reasons for global variation in ALCL incidence remain unknown,1 and risk factors for ALCL have not been clearly identified.26,27 ALK status of patients with ALCL is not available in the SEER database and could be an area for further research with respect to racial variations.
Fourth, incidence of ATLL was higher among blacks and Asians/Pacific Islanders than among non-Hispanic whites, corresponding to a higher prevalence of HTLV-1 in these minority populations.28 Given global epidemiology of ATLL that corresponds to the prevalence of HTLV-1 in populations, it is reasonable to speculate that there will be differences in ATLL incidence between Caribbean-born and other blacks. Our exploratory analysis showed that the proportion of ATLLs was nearly three times higher for Caribbean-born blacks than for US-born blacks (data not shown). However, because of limitations in identifying birthplace of the patients in the SEER registry,22 these calculations should be viewed with caution. Similarly, although Japanese patients comprised only 1.1% of all patients with PTCL, 21 (5.6%) of 374 patients with ATLL were Japanese. The same was not true for other Asian populations.
Finally, as with incidence, substantial variation in survival was observed between races. Our results are broadly consistent with, but more detailed than, previous reports.7,9,10,29 Survival with PTCL-NOS was shorter for every minority group compared with non-Hispanic whites, and blacks had worse prognoses for almost every histology. The reasons behind these survival differences are poorly understood. Potentially relevant biologic differences between races include variations in pharmacokinetics or pharmacodynamics of therapeutic agents and genomic variations of the tumors. Disparities in health care delivery and socioeconomic status might also be contributing factors. Over the time period of our study, first-line treatment of each PTCL type would have been fairly homogeneous in the United States. Nonetheless, receipt of therapy, second-line and further treatment of relapse, and supportive care may vary between ethnic groups. Clearly, future studies in the United States would benefit from including detailed information on treatment and socioeconomic factors to better understand racial disparities in survival.
Our study had several strengths, most importantly, its use of the population-based SEER cancer registries, which ascertain more than 95% of patients with cancer within their geographically dispersed catchment areas that cover approximately 28% of the US population.12-15 Therefore, our results are representative of PTCL in the United States, and estimates on minority populations are robust. In addition, because our analysis of case-case ORs and survival HRs were adjusted for age, sex, calendar year of diagnosis, and region, our results are not likely to be a result of differences in these factors between racial/ethnic populations in the United States. For example, although Hispanics and Asians are typically younger than non-Hispanic whites, and risk of PTCL subtypes varies with age,5 this cannot explain our observations.
Our study has some limitations that must be considered when interpreting the results. For some racial groups, especially Native Americans, the number of patients with PTCL is modest because of the rarity of PTCL and the small size of the population. Nonetheless, we observed statistically significant differences in IRs and cancer subtype proportions. In addition, misclassification of race or ethnicity in cancer registry records could have affected our results; for example, if Native Americans were misclassified as non-Hispanic white or unknown, IRs and IRRs for American Indians/Alaskan natives would be underestimates.30 However, the case-case ORs and survival analyses use data only from patient cases and, therefore, should be less influenced by misclassification of race. Therefore, although the IRs and IRRs for American Indians/Alaskan natives and possibly other minorities may be interpreted with caution, we believe that when taken together with the comparisons of case proportions and survival by subtype, our results are robust and informative.
In summary, pronounced differences were observed in the incidence, proportions of PTCL subtypes, and survival between racial/ethnic subpopulations in the United States. Our results support further studies to identify factors contributing to variability in PTCL incidence and survival between racial and ethnic groups and suggest future advances may occur by combining epidemiologic data with molecular and genomic profiling of patients and tumors.
Footnotes
Listen to the podcast by Dr Amengual at www.jco.org/podcasts
Supported by the Yolanda Willis Memorial Fund, University of Washington, Seattle, WA.
The views expressed do not represent those of the Surveillance, Epidemiology, and End Results cancer registries or its funders.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Presented in part at the 7th Annual T-Cell Lymphoma Forum, San Francisco, CA, January 29-31, 2015.
AUTHOR CONTRIBUTIONS
Conception and design: Scott V. Adams, Andrei R. Shustov
Administrative support: Polly A. Newcomb
Collection and assembly of data: Scott V. Adams
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial Patterns of Peripheral T-Cell Lymphoma Incidence and Survival in the United States
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Scott V. Adams
No relationship to disclose
Polly A. Newcomb
No relationship to disclose
Andrei R. Shustov
Honoraria: Celgene, Spectrum Pharmaceuticals, Bristol-Myers Squibb
Consulting or Advisory Role: Celgene, Spectrum Pharmaceuticals, Bristol-Myers Squibb
Research Funding: Spectrum Pharmaceuticals, Celgene, Millennium, Gilead Sciences, Seattle Genetics, Pfizer
REFERENCES
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: Distributions of the major subtypes differ by geographic locations—Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1998;9:717–720. doi: 10.1023/a:1008265532487. [DOI] [PubMed] [Google Scholar]
- 3.Kanno H, Kojya S, Li T, et al. Low frequency of HLA-A*0201 allele in patients with Epstein-Barr virus-positive nasal lymphomas with polymorphic reticulosis morphology. Int J Cancer. 2000;87:195–199. [PubMed] [Google Scholar]
- 4. Tajima K, Hinuma Y: Epidemiology of HTLV-I/II in Japan and the world. Gann Monogr Canc Res 39:129-149, 1992.
- 5.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. (ed 4). Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 7.Abouyabis AN, Shenoy PJ, Lechowicz MJ, et al. Incidence and outcomes of the peripheral T-cell lymphoma subtypes in the United States. Leuk Lymphoma. 2008;49:2099–2107. doi: 10.1080/10428190802455867. [DOI] [PubMed] [Google Scholar]
- 8.Shiels MS, Engels EA, Linet MS, et al. The epidemic of non-Hodgkin lymphoma in the United States: Disentangling the effect of HIV, 1992-2009. Cancer Epidemiol Biomarkers Prev. 2013;22:1069–1078. doi: 10.1158/1055-9965.EPI-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai WZ, Chang ET, Fish K, et al. Racial patterns of extranodal natural killer/T-cell lymphoma, nasal type, in California: A population-based study. Br J Haematol. 2012;156:626–632. doi: 10.1111/j.1365-2141.2011.08982.x. [DOI] [PubMed] [Google Scholar]
- 10.Petrich AM, Helenowski IB, Bryan LJ, et al. Factors predicting survival in peripheral T-cell lymphoma in the USA: A population-based analysis of 8802 patients in the modern era. Br J Haematol. 2015;168:708–718. doi: 10.1111/bjh.13202. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal J, Wright G, Wang C, et al. Lymphoma Leukemia Molecular Profiling Project and the International Peripheral T-Cell Lymphoma Project Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123:2915–2923. doi: 10.1182/blood-2013-11-536359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (2000-2012) <Katrina/Rita Population Adjustment> - Linked to County Attributes - Total U.S., 1969-2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission. http://seer.cancer.gov/
- 13.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review 1975-2012. Bethesda, MD,: National Cancer Institute; 2015. [Google Scholar]
- 14.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Clegg LX, Feuer EJ, Midthune DN, et al. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 16. National Cancer Institute, Surveillance, Epidemiology, and End Results Program: Months survived based on complete dates. Updated 2013. http://seer.cancer.gov/survivaltime/
- 17. Adamo MB, Johnson CH, Ruhl JL, et al: 2011 SEER Program Coding and Staging Manual. Bethesda, MD, National Cancer Institute, NIH Publication 11-5581, 2011. [Google Scholar]
- 18. North American Association of Central Cancer Registries: North American Association of Central Cancer Registries (NAACCR) Guideline for Enhancing Hispanic/Latino Identification. Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. Springfield, IL, North American Association of Central Cancer Registries, 2011. [Google Scholar]
- 19.Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: A single center experience of 10 years. Ann Oncol. 2005;16:206–214. doi: 10.1093/annonc/mdi037. [DOI] [PubMed] [Google Scholar]
- 20.Quintanilla-Martinez L, Franklin JL, Guerrero I, et al. Histological and immunophenotypic profile of nasal NK/T cell lymphomas from Peru: High prevalence of p53 overexpression. Hum Pathol. 1999;30:849–855. doi: 10.1016/s0046-8177(99)90147-8. [DOI] [PubMed] [Google Scholar]
- 21.Montealegre JR, Zhou R, Amirian ES, et al. Uncovering nativity disparities in cancer patterns: Multiple imputation strategy to handle missing nativity data in the Surveillance, Epidemiology, and End Results data file. Cancer. 2014;120:1203–1211. doi: 10.1002/cncr.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinheiro PS, Bungum TJ, Jin H. Limitations in the imputation strategy to handle missing nativity data in the Surveillance, Epidemiology, and End Results program. Cancer. 2014;120:3261–3262. doi: 10.1002/cncr.28866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rüdiger T, Weisenburger DD, Anderson JR, et al. Non-Hodgkin’s Lymphoma Classification Project Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): Results from the Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 2002;13:140–149. doi: 10.1093/annonc/mdf033. [DOI] [PubMed] [Google Scholar]
- 24.Chan JK, Sin VC, Wong KF, et al. Nonnasal lymphoma expressing the natural killer cell marker CD56: A clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood. 1997;89:4501–4513. [PubMed] [Google Scholar]
- 25.Kanavaros P, Lescs MC, Brière J, et al. Nasal T-cell lymphoma: A clinicopathologic entity associated with peculiar phenotype and with Epstein-Barr virus. Blood. 1993;81:2688–2695. [PubMed] [Google Scholar]
- 26.Ferreri AJ, Govi S, Pileri SA, et al. Anaplastic large cell lymphoma, ALK-negative. Crit Rev Oncol Hematol. 2013;85:206–215. doi: 10.1016/j.critrevonc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Ferreri AJ, Govi S, Pileri SA, et al. Anaplastic large cell lymphoma, ALK-positive. Crit Rev Oncol Hematol. 2012;83:293–302. doi: 10.1016/j.critrevonc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Chang YB, Kaidarova Z, Hindes D, et al. Seroprevalence and demographic determinants of human T-lymphotropic virus type 1 and 2 infections among first-time blood donors–United States, 2000-2009. J Infect Dis. 2014;209:523–531. doi: 10.1093/infdis/jit497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crozier JA, Sher T, Yang D, et al. Persistent disparities among patients with T-cell non-Hodgkin lymphomas and B-cell diffuse large cell lymphomas over 40 years: A SEER database review. Clin Lymphoma Myeloma Leuk. 2015;15:578–585. doi: 10.1016/j.clml.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jim MA, Arias E, Seneca DS, et al. Racial misclassification of American Indians and Alaska Natives by Indian Health Service Contract Health Service Delivery Area. Am J Public Health. 2014;104(suppl 3):S295–S302. doi: 10.2105/AJPH.2014.301933. [DOI] [PMC free article] [PubMed] [Google Scholar]