Abstract
Purpose
This multicenter study, to our knowledge, is the first phase III trial to compare trabectedin versus dacarbazine in patients with advanced liposarcoma or leiomyosarcoma after prior therapy with an anthracycline and at least one additional systemic regimen.
Patients and Methods
Patients were randomly assigned in a 2:1 ratio to receive trabectedin or dacarbazine intravenously every 3 weeks. The primary end point was overall survival (OS), secondary end points were disease control—progression-free survival (PFS), time to progression, objective response rate, and duration of response—as well as safety and patient-reported symptom scoring.
Results
A total of 518 patients were enrolled and randomly assigned to either trabectedin (n = 345) or dacarbazine (n = 173). In the final analysis of PFS, trabectedin administration resulted in a 45% reduction in the risk of disease progression or death compared with dacarbazine (median PFS for trabectedin v dacarbazine, 4.2 v 1.5 months; hazard ratio, 0.55; P < .001); benefits were observed across all preplanned subgroup analyses. The interim analysis of OS (64% censored) demonstrated a 13% reduction in risk of death in the trabectedin arm compared with dacarbazine (median OS for trabectedin v dacarbazine, 12.4 v 12.9 months; hazard ratio, 0.87; P = .37). The safety profiles were consistent with the well-characterized toxicities of both agents, and the most common grade 3 to 4 adverse effects were myelosuppression and transient elevation of transaminases in the trabectedin arm.
Conclusion
Trabectedin demonstrates superior disease control versus conventional dacarbazine in patients who have advanced liposarcoma and leiomyosarcoma after they experience failure of prior chemotherapy. Because disease control in advanced sarcomas is a clinically relevant end point, this study supports the activity of trabectedin for patients with these malignancies.
INTRODUCTION
After GI stromal tumors, leiomyosarcomas and liposarcomas are the most common subtypes of soft tissue sarcomas (STS), a heterogeneous group of malignancies that arise from tissues of mesenchymal origin and together compose approximately 1% of all solid tumors.1,2
The prognosis for patients with advanced or metastatic STS is poor, with an estimated median survival of 12 to 15 months.3-5 Treatment is palliative in nature, and the goal is delay of the progression and severe morbidity that can arise when tumor growth compromises organ function.6 Initial therapy for patients with STS that is unresectable for cure typically includes cytotoxic chemotherapy that is most commonly anthracycline based (mainly doxorubicin) or gemcitabine based.7,8 Other chemotherapeutic agents, including dacarbazine, ifosfamide, and unapproved analogs, have been investigated.9-13 In metastatic STS, combination chemotherapy with dose-intensive doxorubicin plus ifosfamide improves response rates and disease control compared with doxorubicin alone but with increased severe toxicities and without overall survival benefit.5 The activities of single-agent dacarbazine and ifosfamide in this clinical setting have been used by the European Organization for Research and Treatment of Cancer as a reference to assess the activity of novel agents in STS (3- and 6-month progression-free rates [PFRs] of 39% and 14%, respectively).14 These thresholds have been used to assess the activity of trabectedin in phase II studies15 and to identify the target population for the pivotal phase III study of pazopanib.16,17
Trabectedin, a marine-derived drug, has a complex mechanism of action that affects key cell biology processes in tumor cells and the tumor microenvironment through direct effects on tumor-associated macrophages and tissue-resident histiocytes.18-20 It binds to the minor groove of DNA, which thereby affects the function of DNA binding proteins, including transcription factors and DNA repair machinery, to result in perturbation of the cell cycle and induction of p53-independent apoptosis.21,22 In several phase II trials, trabectedin exhibited activity in patients with metastatic STS,23-25 and a randomized trial to test two different doses and schedules of trabectedin led to the first regulatory approval in 2007.15 The present multicenter study was conducted to compare the efficacy and safety of trabectedin versus dacarbazine in a randomized phase III trial in patients with advanced liposarcoma or leiomyosarcoma.
PATIENTS AND METHODS
Patients
Patients were eligible if they were age 15 years or older; had unresectable, locally advanced or metastatic liposarcoma or leiomyosarcomas; and were previously treated with at least either a combination of an anthracycline and ifosfamide or an anthracycline plus one or more additional cytotoxic chemotherapy regimen(s). Patients required adequate bone marrow, renal, and liver functions, and an Eastern Cooperative Oncology Group performance status score of 1 or lower. Exclusion criteria included known CNS metastasis, myocardial infarct within 6 months before enrollment, and New York Heart Association class II or greater heart failure. Review boards at all participating institutions approved the study, which was conducted according to the Declaration of Helsinki, the International Conference on Harmonisation, and the Guidelines for Good Clinical Practice. All patients provided written informed consent to participate on the study.
Study Design and Treatment
This phase III trial (NCT01343277, also coded as ET743-SAR-3007) is a randomized, open-label, active-controlled, parallel-group, multicenter study implemented at 85 sites in four countries. Patients were randomly assigned in a 2:1 ratio to receive either trabectedin at a starting dose of 1.5 mg/m2 as a 24-hour intravenous (IV) infusion or dacarbazine at a starting dose of 1 g/m2 as a 20- to 120-minute IV infusion. Study drug was administered on day 1 of each 21-day treatment cycle. All trabectedin doses were administered via central IV access after premedication with dexamethasone 20 mg IV. Criteria for dose reductions and dose delays, in case of treatment-associated toxicities, were standardized in the protocol.
The primary study end point was overall survival (OS); secondary end points assessed disease control and included progression-free survival (PFS), time to progression, objective response rate (ORR), and duration of response (DOR). The end points of clinical benefit rate26 (CBR; defined as the sum of complete responses + partial responses [PR] + stable disease for at least 18 weeks) and duration of stable disease were added to the statistical analysis plan as preplanned analyses to describe those patients who experienced prolonged disease control.
The study was designed with a preplanned interim analysis of the primary end point of OS, which was to occur concurrently with the final analysis of PFS, at 188 death events. The clinical cutoff date of September 16, 2013, was prospectively determined on the basis of anticipation of the required number of death events.
Assessments
Monitoring of study participant survival was performed and used to calculate OS. Investigators assessed tumor response by radiographic imaging of the chest, abdomen, and pelvis every 6 weeks for the first 36 weeks on study and every 9 weeks thereafter until disease progression, subsequent anticancer therapy, or patient death occurred. The PFS end point was validated through an audit by independent radiologists, who were blinded to treatment assignment and who assessed radiographic PFS (rPFS) in a subset of approximately 60% of the study population. Safety assessments were based on observed adverse events, clinical laboratory tests, vital sign measurement, physical examination, cardiac function (using multigated acquisition scan or echocardiogram), and concomitant medication use.
Statistical Analyses
The primary statistical methodology for comparison of treatment effectiveness for OS, PFS, and DOR was the unstratified log-rank test. A Cox proportional hazard model was used to examine the effect of the prognostic factors. The statistical methodology for ORR and CBR was Fisher's exact test. To detect a difference between a median OS of 10 months in the dacarbazine group and a median OS of 13.5 months in the trabectedin group (hazard ratio [HR], 0.74) at an overall two-sided significance level of .05 with a power of 80% required 376 events. A sample size of 570 patients was planned with an interim analysis for OS after 50% of events. The cumulative α spent was to be .003 and .047 for the interim and final OS analyses, respectively. This study used a group sequential method with the O'Brien-Fleming boundaries, as implemented by the Lan-DeMets α spending function.
One analysis of PFS was planned at the time of the OS interim analysis after a projected 331 PFS events, which provided at least 90% power to detect an HR of 0.667 (median PFS for dacarbazine v trabectedin arms, 2.50 v 3.75 months, respectively) with a two-sided significance level of .05. Final PFS and ORR data, after recommendations of the independent data monitoring committee, were submitted for regulatory consideration while the study continued toward the final analysis for OS. The results of the overall estimate of HR for rPFS, which were based on an independent audit, were calculated as proposed by the method of Dodd.27 The upper limit for the 95% CI was compared with 1.
RESULTS
Patients and Treatment
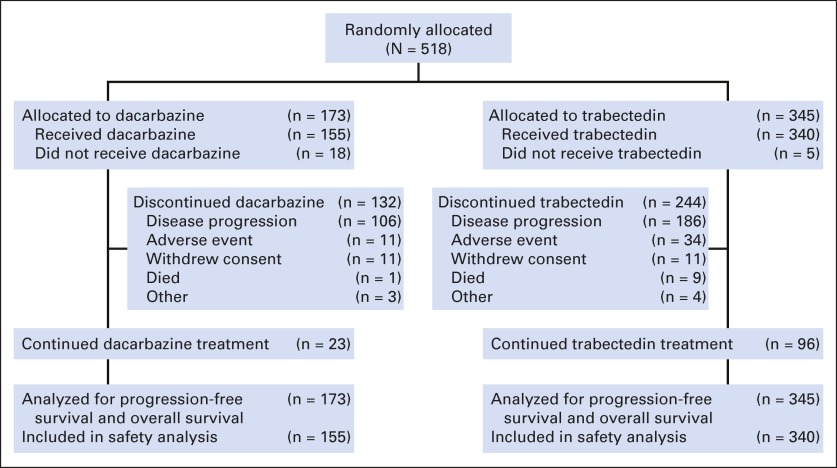
Between May 27, 2011, and the September 16, 2013, clinical cutoff for the interim analysis of the primary end point of OS, 518 of 570 planned patients were randomly assigned to receive either trabectedin (n = 345) or dacarbazine (n = 173; Fig 1) and are included in this interim analysis. Enrollment was ongoing at the time of the clinical cutoff. Baseline demographic and disease characteristics were well balanced (Table 1). The study population was heavily pretreated; 88% had at least two previous lines of systemic therapy, greater than 90% had previous surgery, and 50% had prior radiotherapy. The median time from last disease progression was less than 1 month in both treatment groups (0.85 v 0.82 months, respectively), and a majority (58%) reported progressive disease as the best response to their previous line of therapy.
Fig 1.
CONSORT diagram.
Table 1.
Baseline Demographic and Disease Characteristics
| Variable | No. (%) of Patients |
|
|---|---|---|
| Trabectedin (n = 345) | Dacarbazine (n = 173) | |
| Age, years | ||
| Median (range) | 57 (18.0-81.0) | 56 (17.0-79.0) |
| Sex | ||
| Male | 107 (31) | 47 (27) |
| Female | 238 (69) | 126 (73) |
| Baseline BMI, kg/m2 | ||
| Median (range) | 28.21 (14.5-78.1) | 27.05 (13.3-66.7) |
| Histology | ||
| Leiomyosarcoma | 252 (73) | 126 (73) |
| Uterine | 134 (39) | 78 (45) |
| Nonuterine | 118 (34) | 48 (28) |
| Liposarcoma | 93 (27) | 47 (27) |
| Myxoid ± round cell | 38 (11) | 19 (11) |
| Pleomorphic | 10 (3) | 3 (2) |
| Dedifferentiated | 45 (13) | 25 (15) |
| Baseline ECOG performance status score | ||
| 0 | 171 (50) | 86 (50) |
| 1 | 174 (50) | 87 (50) |
| Lines of prior chemotherapy | ||
| 1 | 38 (11) | 23 (13) |
| 2 | 160 (46) | 75 (43) |
| 3 | 87 (25) | 43 (25) |
| 4 | 37 (11) | 21 (12) |
| > 4 | 23 (7) | 11 (6) |
| Best response to last line of previous chemotherapy | ||
| Complete response | 4 (1) | 3 (2) |
| Partial response | 28 (8) | 14 (8) |
| No change (stable disease) | 114 (33) | 51 (30) |
| Progression of disease | 198 (57) | 103 (60) |
| Unknown/missing | 1 (0) | 2 (1) |
| Previous surgery for malignancy | ||
| Yes | 327 (95) | 158 (91) |
| No | 18 (5) | 15 (9) |
| Previous radiotherapy for malignancy | ||
| Yes | 176 (51) | 80 (46) |
| No | 169 (49) | 93 (54) |
| Time from initial diagnosis to random assignment, months | ||
| Median (range) | 33.94 (2.5-318.5) | 27.10 (1.6-267.1) |
| Time from last disease progression to random assignment, months | ||
| Median (range) | 0.85 (0.0-13.7) | 0.82 (0.1-9.8) |
NOTE. Percentages were calculated with the number of patients randomly assigned to each treatment group as the denominator.
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
Time on Study Treatments
At the time of interim analysis, the proportion of patients in the trabectedin arm still receiving study treatment was nearly double that in the dacarbazine arm (28% v 15%, respectively). Disease progression was the most common reason for discontinuation of study treatment regardless of treatment group (trabectedin v dacarbazine, 55% v 68%). Discontinuation resulting from toxicity, including adverse events and death, occurred in 12.6% and 7.7% of the trabectedin and dacarbazine groups, respectively. Withdrawal of consent occurred twice as frequently in the dacarbazine group, by 3.2% patients in the trabectedin group versus by 7.1% in the dacarbazine group.
The median number of cycles received in the trabectedin group was twice that of the dacarbazine group (4 v 2 cycles, respectively), and increased exposure rates were noted at both six cycles (34% v 17%, respectively) and 12 cycles (10% v 2%, respectively). Cycle delays or dose reductions were reported in 57% and 35% of patients in the trabectedin group, respectively, compared with 40% and 10% of patients in the dacarbazine group (Appendix Table A1, online only).
Efficacy
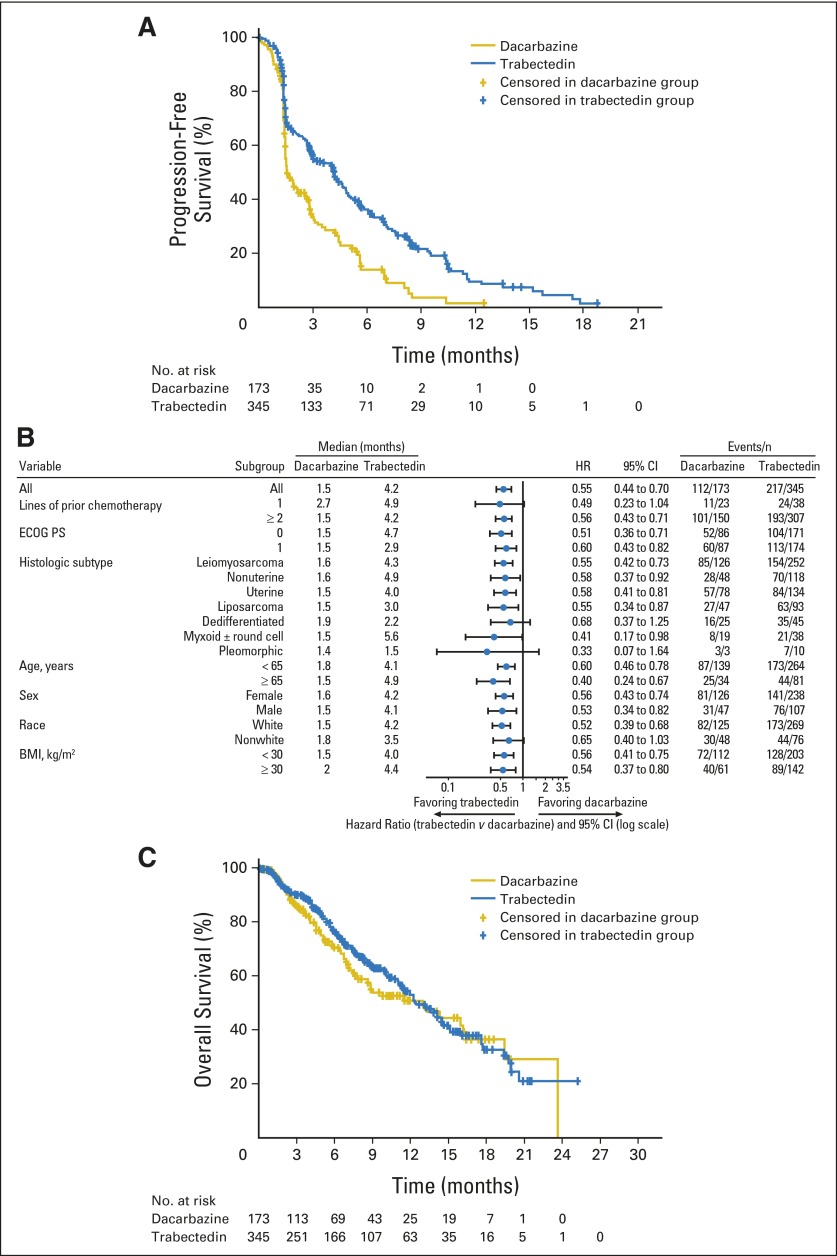
The final analysis of PFS, performed after 329 PFS events, showed that treatment with trabectedin resulted in a 45% reduction in the risk of disease progression or death compared with dacarbazine (HR, 0.55; 95% CI, 0.44 to 0.70; P < .001; Fig 2A). Improved disease control by trabectedin was discernible at the time of the first planned disease assessment (6 weeks), which led to an improved median PFS in the trabectedin group (4.2 months v 1.5 months with dacarbazine). PFRs at 3 and 6 months were 56% and 37% in the trabectedin arm versus 34% and 14% in the dacarbazine arm. The PFS treatment benefit with trabectedin was consistently observed across all 19 preplanned subgroups examined in sensitivity analyses (Fig 2B), and equivalent benefits were observed across the two sarcoma subtypes (HR, 0.55 each for patients with leiomyosarcoma and for patients with liposarcoma).
Fig 2.
Kaplan-Meier estimates of progression-free survival, subgroup analyses, and overall survival at the interim analysis. (A) Progression-free survival; (B) subgroup analysis (hazard ratios [HRs] and 95% CIs) of progression-free survival; and (C) overall survival. HR was calculated as the hazard in the trabectedin treatment group divided by the hazard in the dacarbazine treatment group. BMI, body mass index.
To validate the investigator assessment of PFS, an audit was conducted by independent radiologists who were blinded to treatment assignment. The rPFS of the entire study population, and of the audited and unaudited subgroups, was calculated by using investigator assessment; then, the rPFS was calculated for the audited subgroup by using the independent radiologists' assessments (Table 2). The independent assessment of the rPFS of the audited subset (HR, 0.55) was used to provide an estimate of the HR for the rPFS of the entire population by using an adaptation of a previously reported method27 (HR, 0.54; 95% CI, 0.41 to 0.71), which was consistent with the investigator assessment.
Table 2.
Summary of Key Progression-Free Survival/Radiographic Progression-Free Survival Results
| Variable | Survival Measure |
|||||
|---|---|---|---|---|---|---|
| PFS-INV (n = 518) | rPFS-INV (n = 518) | rPFS-INV (audited subset; n = 304) | rPFS-INV (unaudited subset; n = 214) | rPFS-IR (audited subset; n = 304) | rPFS-IR (overall estimate) | |
| HR* | 0.55 | 0.57 | 0.58 | 0.54 | 0.55 | 0.54 |
| 95% CI | 0.44 to 0.70 | 0.45 to 0.72 | 0.43 to 0.79 | 0.37 to 0.80 | 0.40 to 0.75 | 0.41 to 0.71 |
Abbreviations: HR, hazard ratio; INV, investigator assessed; IR, independent radiologist; PFS, progression-free survival; rPFS, radiographic progression-free survival.
The HR was calculated as the hazard in the trabectedin treatment group divided by the hazard in the dacarbazine treatment group.
Best responses experienced by both treatment groups, as well as their durations, are summarized in Table 3. There were no complete responses in either treatment group. PRs were more frequently observed within the trabectedin group (ORR, 9.9% v 6.9%; P = .33). The median DOR within the trabectedin group was approximately 50% greater than in the dacarbazine group (6.5 v 4.2 months, respectively; P < .14). Stable disease as best response was more frequently achieved in the trabectedin group (51% v 35%), and the median duration of stable disease was significantly improved in the trabectedin arm (6.0 v 4.2 months; P < .001). The clinical benefit rate, which reflects both objective disease response and durable stable disease, was significantly higher in the trabectedin group (34% v 19%; P < .001; Table 3).
Table 3.
End Points That Reflect Disease Control
| End Point | Trabectedin (n = 345) | Dacarbazine (n = 173) | HR/OR (95% CI)* | P |
|---|---|---|---|---|
| PFS, months | 4.2 | 1.5 | 0.55 (0.44 to 0.70) | < .001 |
| TTP, months | 4.2 | 1.5 | 0.52 (0.41 to 0.66) | < .001 |
| No. (%) of ORR | 34 (9.9) | 12 (6.9) | 1.47 (0.72 to 3.2) | .33 |
| DOR, months | 6.5 | 4.2 | 0.47 (0.17 to 1.32) | .14 |
| No. (%) with SD as best response | 177 (51) | 60 (35) | — | — |
| Duration of SD, months | 6.01 | 4.17 | 0.45 (0.30 to 0.67) | < .001 |
| % of CBR | 34 | 19 | 2.3 (1.45 to 3.7) | < .001 |
Abbreviations: CBR, clinical benefit rate; DOR, duration of response; HR, hazard ratio; OR, odds ratio; ORR, objective response rate; PFS, progression-free survival; SD, stable disease; TTP, time to progression..
The HR was calculated as the hazard in the trabectedin treatment group, divided by the hazard in the dacarbazine treatment group.
The interim analysis occurred as planned at 50% of the total events required for the final analysis of OS (ie, 189 death events). The median duration of survival follow-up time was 8.6 months. Although the OS data at this interim analysis were highly censored (64%), the results favored the trabectedin group, with an overall 13% reduction in risk of death (median OS of trabectedin v dacarbazine, 12.4 months v 12.9 months; HR, 0.87; P = .37; Fig 2C). Subsequent anticancer therapy was less frequently used in the trabectedin arm (47% v 56%, respectively) and occurred significantly later after study entry (median time to initiation from random assignment for trabectedin versus dacarbazine, 6.9 v 3.7 months; HR, 0.47; 95% CI, 0.36 to 0.61; P < .001). The most frequently used subsequent therapy in both arms (trabectedin v dacarbazine) was pazopanib (18% v 28%, respectively), which was followed by radiation (10% v 15%, respectively), gemcitabine (9% v 15%, respectively), and dacarbazine (17% v 6%, respectively; Table 4).
Table 4.
Selected Subsequent Anticancer Therapy
| Therapy | No. (%) of Patients |
|
|---|---|---|
| Trabectedin (n = 345) | Dacarbazine (n = 173) | |
| Total with subsequent anticancer chemotherapy | 162 (47) | 97 (56) |
| Pazopanib | 63 (18) | 48 (28) |
| Dacarbazine | 60 (17) | 11 (6) |
| Radiation | 35 (10) | 25 (15) |
| Gemcitabine | 30 (9) | 25 (15) |
| Surgery | 23 (7) | 17 (10) |
| Docetaxel | 19 (6) | 21 (12) |
| Ifosfamide | 7 (2) | 10 (6) |
| Doxorubicin | 9 (3) | 5 (3) |
| Eribulin | 9 (3) | 1 (1) |
| Trabectedin | 1 (< 1) | 4 (2) |
NOTE. Subsequent anticancer therapies that were used for at least 5% of patients in either treatment group were included. In addition, doxorubicin, eribulin, and trabectedin were also included on the basis of their previously demonstrated activities.
Safety
The adverse events of the study were consistent with the well-characterized safety and toxicity profiles of both study drugs. The most common adverse events were predominantly of grade 1 to 2 severity (Table 5). Grade 3 to 4 toxicities were primarily observed in laboratory-based measures of myelosuppressive toxicity (in both treatment groups) and transient transaminase elevations (in the trabectedin group). Less often, grade 3 to 4 creatine phosphokinase elevations were seen with trabectedin treatment (5.3% v 0.6% in the dacarbazine group), and 1.2% of patients who received trabectedin experienced rhabdomyolysis.
Table 5.
Most Common Adverse Events
| Adverse Event | No. (%) Adverse Events by Treatment and Grade |
|||||
|---|---|---|---|---|---|---|
| Trabectedin (n = 340) |
Dacarbazine (n = 155) |
|||||
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
| Nausea | 247 (73) | 18 (5) | 0 | 76 (49) | 3 (2) | 0 |
| Fatigue | 228 (67) | 20 (6) | 0 | 79 (51) | 2 (1) | 1 (1) |
| Neutropenia | 165 (49) | 70 (21) | 56 (16) | 45 (29) | 17 (11) | 15 (10) |
| Alanine aminotransferase increased | 154 (45) | 85 (25) | 4 (1) | 9 (6) | 1 (1) | 0 |
| Vomiting | 149 (44) | 16 (5) | 0 | 33 (21) | 2 (1) | 0 |
| Anemia | 134 (39) | 49 (14) | 0 | 45 (29) | 17 (11) | 1 (1) |
| Constipation | 121 (36) | 3 (1) | 0 | 44 (28) | 0 | 0 |
| Aspartate aminotransferase increased | 120 (35) | 40 (12) | 4 (1) | 8 (5) | 0 | 0 |
| Decreased appetite | 116 (34) | 7 (2) | 0 | 31 (20) | 0 | 1 (1) |
| Diarrhea | 115 (34) | 6 (2) | 0 | 35 (23) | 0 | 0 |
| Thrombocytopenia | 101 (30) | 27 (8) | 31 (9) | 56 (36) | 15 (10) | 13 (8) |
| Dyspnea | 84 (25) | 12 (4) | 1 (< 1) | 30 (19) | 1 (1) | 0 |
| Peripheral edema | 83 (24) | 3 (1) | 0 | 21 (14) | 1 (1) | 0 |
| Headache | 78 (23) | 1 (< 1) | 0 | 29 (19) | 0 | 0 |
| Blood alkaline phosphatase increased | 69 (20) | 5 (1) | 0 | 11 (7) | 0 | 0 |
| Cough | 61 (18) | 1 (< 1) | 0 | 32 (21) | 0 | 0 |
NOTE. Most common adverse events occurred with ≥ 20% frequency.
The incidence of patients who died within 60 days of the first dose of study drug was similar in both treatment arms (7.1% v 5.8% in trabectedin v dacarbazine arms, respectively), whereas treatment-related deaths (n = 7; 2.1%) were only reported in the trabectedin group. These deaths were related to sepsis/septic shock (n = 3), rhabdomyolysis/sepsis (n = 1), renal failure (n = 1), renal failure/cardiac arrest (n = 1), or multiorgan failure (n = 1).
DISCUSSION
This multicenter, randomized, international, phase III clinical trial confirms prior experience in smaller trials that documented the efficacy of trabectedin in the control of advanced STS after failure of prior cytotoxic chemotherapy. The patients studied were heavily pretreated, having experienced failure of previous systemic therapy, surgery, and radiation therapy, and they had rapidly progressing disease. Within this high-risk population, trabectedin administration resulted in a statistically significant 45% reduction in the risk of disease progression or death versus the active control therapy, dacarbazine (P < .001). This benefit in disease control was observed regardless of disease histology; previous lines of systemic therapy; or clinical considerations, such as age, sex, ethnicity, or baseline performance status. Of note, the greatest increase in median PFS (5.6 months v 1.5 months with trabectedin v dacarbazine, respectively) was observed within the myxoid/round cell liposarcoma subgroup (Fig 2B), which is consistent with the early identification of this uniquely sensitive STS subtype25,28 and similar to a recent report of trabectedin activity in patients with translocation-related sarcomas.29,30 This is also highly consistent with the proposed action of trabectedin as a direct inhibitor of the chimeric FUS-CHOP translocation-generated oncoprotein that has transcriptional regulatory activity in these tumors.31-33
The benefit of trabectedin versus dacarbazine treatment was also supported by other secondary end points, with improvements in both the ORR (9.9% v 6.9%, respectively), and the median duration of responses (6.5 v 4.2 months, respectively). Importantly, the majority of patients who benefited from trabectedin experienced stable disease as their best response, at an increased rate than that observed with dacarbazine (51% v 35%) and for significantly increased durations. The CBR, which included objective disease shrinkage (which consisted of PRs in this trial) and durable stable disease (ie, > 18 weeks in duration), also documents the anticancer activity of trabectedin, because nearly twice the proportion of patients who received trabectedin versus those who received dacarbazine achieved this end point in this randomized study (34% v 19%, respectively). The therapeutic benefit of continued disease control with extended trabectedin dosing beyond six cycles has been reported in a recent study as well.34
The safety and tolerability of these study drugs were consistent with extensive prior experience and reports.11,12,15,35 Laboratory abnormalities were the most frequently reported grade 3 to 4 adverse events in this study; were generally transient and noncumulative; and were managed by dose delays, reductions, supportive care, and, if required, treatment discontinuations. Although this study demonstrates that toxicity was increased in the trabectedin arm, trabectedin is regarded as a generally well-tolerated agent relative to other sarcoma treatment options, with low rates of mucositis and alopecia and no cumulative toxicities that limit treatment duration. Consistent with this profile, a greater proportion of patients in the trabectedin arm than in the dacarbazine arm were able to receive prolonged courses of therapy. Extensive clinical experience with trabectedin outside the United States in standard practice has supported its reputation as a generally well-tolerated agent with no cumulative toxicities, which was again demonstrated in this study.35-38
The 3- and 6-month PFRs observed in this study provide historical context to these results, which are consistent with earlier studies with both agents. The 3- and 6-month PFRs of dacarbazine (34% and 14%, respectively) were consistent with the European Organization for Research and Treatment of Cancer criteria established to define active agents in unselected STS (39% and 14%, respectively) and with a recent phase II study in patients with unselected STS.12 This level of activity across multiple studies suggests that the activity of dacarbazine is similar across sarcoma study populations (ie, liposarcoma or leiomyosarcoma with selected and unselected histologies) and justifies its selection as the active comparator in this study.
In this study, 3- and 6-month PFRs of trabectedin (56% and 37%, respectively) confirm the higher level of anticancer activity of trabectedin in patients with leiomyosarcoma and liposarcoma, which was also observed in a prior randomized, phase II study (52% and 36%, respectively).15 The confirmation of activity within this patient population provides an additional treatment option for patients who have limited systemic options, given the lower ifosfamide efficacy in patients with leiomyosarcoma5,39 and the relative lack of efficacy of pazopanib in patients with liposarcoma.16 Furthermore, the favorable efficacy observed in patients older than 65 years, with a safety profile similar to that observed in younger patients (data not shown), confirms the results of earlier analyses40 that suggest that trabectedin is an important treatment option for a group of patients who are often excluded from treatment with doxorubicin-based regimens.5
The interim analysis of OS, the primary end point of this study, demonstrated a statistically nonsignificant 13% reduction in risk of death that favored the trabectedin group. Of note, the median OS in the dacarbazine arm was statistically equivalent to the trabectedin group and exceeded the predefined statistical assumption of 10 months; this may reflect the use of subsequent therapies, including pazopanib, which was approved by regulatory agencies for STS during the conduct of this trial. Although the study continued per protocol for the final analysis of OS, the results of two recently reported phase III studies have illustrated the difficulty in prolonging OS, despite robust improvements in PFS, even when the control arm involves a placebo.5,17 Given the historical difficulty in demonstrating OS improvement, the clinical documentation of disease control, measured as PFS and CBR, has been proposed as a measure of clinically relevant efficacy in advanced sarcomas.26 The results of this large, randomized trial support the activity of trabectedin as an effective anticancer agent in this population of patients who have rare but life-threatening malignancies.
Supplementary Material
Acknowledgment
We thank the investigators and coordinators at each of the clinical sites; the patients who volunteered to participate in this study and their families; the sponsor staff involved in data collection and analyses; Namit Ghildyal, PhD, for editorial assistance in the development of the manuscript; and the Adelson Medical Research Foundation for partial support of this research (to G.D.D.). The academic authors had complete access to all data and maintained control over the manuscript, including final wording and conclusions.
Appendix
The following additional investigators, listed in alphabetical order, participated in the SAR-3007 study: Australia—W. Joubert (Woolloongabba, Queensland), C. Lewis (Randwick, New South Wales), G. Richardson (Malvern, Victoria); Brazil—V. Dybal (Salvador, Bahia), R. Schmerling (São Paulo, São Paulo), S.V. Serrano (Barretos, São Paulo), L. Viola (Porto Alegre, Rio Grande do Sul); New Zealand—A. O'Donnell (Wellington), D. Porter (Auckland); United States—A. Aboulafia (Baltimore, MD), M. Agulnik (Chicago, IL), M. Auber (Morgantown, WV), J. Ayers formerly L. Horvath (Warrenville, IL), M. Batus (Chicago, IL), A. Bhinder (Columbus, OH), J. Burke (Savannah, GA), J. Charlson (Milwaukee, WI), C. Cowey (Dallas, TX), L. Cranmer (Tucson, AZ), S. Dakhil (Wichita, KS), G. D'Amato (Atlanta, GA), T. Davis (Lebanon, NH), H.M.D. Deshpande (New Haven, CT), G. Fernandez-Castro (Miami, FL), C. Forscher (Los Angeles, CA), S. Ghamande (Augusta, GA), J. Gibbons (Cleveland, OH), R. Gollard (Las Vegas, NV), R. Govindarajan (Little Rock, AR), S. Gupta-Burt (Overland Park, KS), J. Hamm (Louisville, KY), W. Hanna (Knoxville, TN), W.G. Harker (Salt Lake City, UT), R. Harris (Johnson City, NY), H. Harvey (Hershey, PA), I. Hinshaw (Denver, CO), R. Holloway (Orlando, FL), K. Hotten-Leu (Omaha, NE), A. Hussein (Hollywood, FL), S. Jaggernauth (Tulsa, OK), P. Kaiser (Park Ridge, IL), K. Kalinsky (New York, NY), A. Karnad (San Antonio, TX), S. Karri (Dallas, TX), V. Keedy (Nashville, TN), H. Koh (Bellflower, CA), A. Kraft (Charleston, SC), N. Le-Lindqwister (Peoria, IL), M. Livingston (Charlotte, NC), P. Mansky (Green Bay, WI), S. McMeekin (Oklahoma City, OK), C. Meyer (Baltimore, MD), B. Monk (Phoenix, AZ), K. Mulvey (Post Falls, ID), M. Myron (Overland Park, KS), B. Piperdi (New York, NY), L. Pliner (Newark, NJ), D. Reed (Tampa, FL), G. Rosen (New York, NY), D. Rushing (Indianapolis, IN), C. Ryan (Portland, OR), J. Sandbach (Austin, TX), M. Seetharam (Phoenix, AZ), R. Seth (Syracuse, NY), M. Shaheen (Albuquerque, NM), R. Siegel (Hartford, CT), A. Singh (Santa Monica, CA), G. Srkalovic (Lansing, MI), S. Tejwani (Detroit, MI), S. Thomas (Orlando, FL), S. Undevia (Naperville, IL).
Table A1.
Treatment Cycle Delays and Dose Reductions
| Cycle or Dose Variable | No. (%) of Patients |
|
|---|---|---|
| Trabectedin (n = 340) | Dacarbazine (n = 155) | |
| Total with at least two cycles | 298 (88) | 125 (81) |
| Cycle delay | ||
| Yes | 193 (57) | 62 (40) |
| No | 105 (31) | 63 (41) |
| No. of cycle delays | ||
| 1 | 96 (28) | 35 (23) |
| 2 | 37 (11) | 13 (8) |
| 3 | 27 (8) | 8 (5) |
| 4 | 12 (4) | 1 (1) |
| ≥ 5 | 21 (6) | 5 (3) |
| Dose reduction | ||
| Yes | 119 (35) | 15 (10) |
| No | 179 (53) | 110 (71) |
| No. of dose reductions | ||
| 1 | 83 (24) | 13 (8) |
| 2 | 36 (11) | 2 (1) |
NOTE. Cycle delays and dose reductions were tabulated for patients who received at least two cycles of treatment.
Footnotes
See accompanying editorial on page 769
Written on behalf of the SAR-3007 investigators.
Processed as a Rapid Communication manuscript.
Supported by Janssen Pharmaceuticals, and supported in part by the Adelson Medical Research Foundation (G.D.D.).
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01343277.
AUTHOR CONTRIBUTIONS
Conception and design: George D. Demetri, Margaret von Mehren, Nushmia Z. Khokhar, Youn Choi Park, Roland E. Knoblauch, Trilok V. Parekh, Robert G. Maki, Shreyaskumar R. Patel
Provision of study materials or patients: George D. Demetri, Margaret von Mehren, Robin L. Jones, Martee L. Hensley, Scott M. Schuetze, Arthur Staddon, Mohammed Milhem, Anthony Elias, Kristen Ganjoo, Hussein Tawbi, Brian A. Van Tine, Alexander Spira, Andrew Dean, Shreyaskumar R. Patel
Collection and assembly of data: George D. Demetri, Margaret von Mehren, Robin L. Jones, Martee L. Hensley, Scott M. Schuetze, Arthur Staddon, Mohammed Milhem, Anthony Elias, Kristen Ganjoo, Hussein Tawbi, Brian A. Van Tine, Alexander Spira, Andrew Dean, Shreyaskumar R. Patel
Data analysis and interpretation: George D. Demetri, Margaret von Mehren, Nushmia Z. Khokhar, Youn Choi Park, Roland E. Knoblauch, Trilok V. Parekh, Robert G. Maki, Shreyaskumar R. Patel
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
George D. Demetri
Stock or Other Ownership: Blueprint Medicines, Kolltan Pharmaceuticals, G1 Therapeutics, Caris Life Sciences, N-of-One
Consulting or Advisory Role: Bayer, Janssen Pharmaceuticals, GlaxoSmithKline, Sanofi, EMD Serono, Blueprint Medicines, Kolltan Pharmaceuticals, G1 Therapeutics, Caris Life Sciences, ARIAD Pharmaceuticals, Threshold Pharmaceuticals, Champions Oncology, ZIOPHARM Oncology, WIRB-Copernicus Group, Polaris Group, Pfizer, Novartis
Research Funding: Janssen Pharmaceuticals (Inst), Novartis (Inst), Pfizer (Inst), Bayer (Inst), Threshold Pharmaceuticals (Inst), EMD Serono (Inst)
Expert Testimony: Janssen Pharmaceuticals
Margaret von Mehren
Consulting or Advisory Role: Janssen Pharmaceuticals
Robin L. Jones
Consulting or Advisory Role: Pfizer (Inst), IMS Consulting Group (Inst), The Dominion Group (Inst), EMD Serono (Inst), Novartis (Inst), Compass Oncology (Inst)
Research Funding: Janssen Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: EMD Serono, Pfizer, Novartis, Compass Oncology
Martee L. Hensley
Employment: Sanofi (I)
Consulting or Advisory Role: EMD Serono, INSYS Therapeutics, Janssen Pharmaceuticals, Natera
Research Funding: Janssen Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Scott M. Schuetze
Consulting or Advisory Role: Janssen Pharmaceuticals, EMD Serono
Research Funding: Janssen Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Janssen Pharmaceuticals, EMD Serono
Arthur Staddon
Research Funding: Janssen Pharmaceuticals (Inst)
Mohammed Milhem
Consulting or Advisory Role: EMD Serono, Novartis
Travel, Accommodations, Expenses: EMD Serono, Novartis
Anthony Elias
Consulting or Advisory Role: Genentech
Research Funding: Medivation (Inst), Astellas Pharma (Inst), Genentech (Inst), Incyte (Inst), CytRx (Inst), Eisai (Inst), Johnson & Johnson (Inst), Imclone Systems (Inst)
Travel, Accommodations, Expenses: Genentech
Kristen Ganjoo
No relationship to disclose
Hussein Tawbi
No relationship to disclose
Brian A. Van Tine
Consulting or Advisory Role: Advaxis, GlaxoSmithKline, Eli Lilly, ImClone Systems, EMD Serono, Johnson & Johnson, Caris Life Sciences, DFINE, Threshold Pharmaceuticals, Novartis
Speakers' Bureau: GlaxoSmithKline, Caris Life Sciences, DFINE
Research Funding: Polaris Group, Morphotek
Alexander Spira
No relationship to disclose
Andrew Dean
Honoraria: Gilead Sciences, Specialised Therapeutics Australia
Consulting or Advisory Role: AstraZeneca (Inst)
Speakers' Bureau: Novartis
Travel, Accommodations, Expenses: Boehringer Ingelheim
Nushmia Z. Khokhar
Employment: Janssen Pharmaceuticals
Stock or Other Ownership: Johnson & Johnson
Youn Choi Park
Employment: Janssen Pharmaceuticals
Stock or Other Ownership: Johnson & Johnson
Roland E. Knoblauch
Employment: Janssen Pharmaceuticals
Stock or Other Ownership: Johnson & Johnson
Patents, Royalties, Other Intellectual Property: Use of phenformin for the treatment of cancer
Trilok V. Parekh
Employment: Janssen Pharmaceuticals
Stock or Other Ownership: Johnson & Johnson
Robert G. Maki
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals, Eisai, Eli Lilly, Gem Pharmaceuticals, GlaxoSmithKline, SARC: Sarcoma Alliance for Research through Collaboration, TRACON Pharmaceuticals
Speakers' Bureau: Bayer
Research Funding: Eisai (Inst), TRACON Pharmaceuticals (Inst), Eli Lilly (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Travel, Accommodations, Expenses: Bayer, Onyx Pharmaceuticals, Janssen Pharmaceuticals
Shreyaskumar R. Patel
Consulting or Advisory Role: Janssen Pharmaceuticals, Novartis, CytRx, EMD-Serono
Research Funding: Janssen Pharmaceuticals (Inst), Morphotek (Inst), Eisai (Inst)
REFERENCES
- 1.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens—A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 2.Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 3.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: A randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537–1545. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, Vadhan-Raj S, Burgess MA, et al. Results of two consecutive trials of dose-intensive chemotherapy with doxorubicin and ifosfamide in patients with sarcomas. Am J Clin Oncol. 1998;21:317–321. doi: 10.1097/00000421-199806000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomized controlled phase III trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 6.Schoffski P, Cornillie J, Wozniak A, et al. Soft tissue sarcoma: An update on systemic treatment options for patients with advanced disease. Oncol Res Treat. 2014;37:355–362. doi: 10.1159/000362631. [DOI] [PubMed] [Google Scholar]
- 7.Hensley ML, Miller A, O'Malley DM, et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2015;33:1180–1185. doi: 10.1200/JCO.2014.58.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Cesne A, Judson I, Crowther D, et al. Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: A trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 2000;18:2676–2684. doi: 10.1200/JCO.2000.18.14.2676. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Guidelines for Treatment of Cancer. www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
- 10.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: Results of Sarcoma Alliance for Research Through Collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 11.Zucali PA, Bertuzzi A, Parra HJ, et al. The old drug dacarbazine as a second/third line chemotherapy in advanced soft tissue sarcomas. Invest New Drugs. 2008;26:175–181. doi: 10.1007/s10637-007-9086-z. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: A Spanish Group for Research on Sarcomas study. J Clin Oncol. 2011;29:2528–2533. doi: 10.1200/JCO.2010.33.6107. [DOI] [PubMed] [Google Scholar]
- 13.Ryan C, Schoffski P, Merimsky O, et al. PICASSO 3: A phase III international, randomized, double-blind, placebo-controlled study of doxorubicin (dox) plus palifosfamide (pali) vs. dox plus placebo for patients (pts) in first-line for metastatic soft tissue sarcoma (mSTS) Eur J Cancer. 2013:S876. (abstr 3802) [Google Scholar]
- 14.Van Glabbeke M, Verweij J, Judson I, et al. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–549. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: Results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–4196. doi: 10.1200/JCO.2008.21.0088. [DOI] [PubMed] [Google Scholar]
- 16.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: A phase II study from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 17.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomized, double-blind, placebo-controlled, phase III trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 18.Allavena P, Signorelli M, Chieppa M, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): Inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 19.Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Germano G, Frapolli R, Simone M, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70:2235–2244. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 21.Zewail-Foote M, Hurley LH. Ecteinascidin 743: A minor groove alkylator that bends DNA toward the major groove. J Med Chem. 1999;42:2493–2497. doi: 10.1021/jm990241l. [DOI] [PubMed] [Google Scholar]
- 22.D'Incalci M, Jimeno J. Preclinical and clinical results with the natural marine product ET-743. Expert Opin Investig Drugs. 2003;12:1843–1853. [PubMed] [Google Scholar]
- 23.Yovine A, Riofrio M, Blay J, et al. Phase II study of Ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol. 2004;22:890–900. doi: 10.1200/JCO.2004.05.210. [DOI] [PubMed] [Google Scholar]
- 24.Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: A European Organisation for the Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group trial. J Clin Oncol. 2005;23:576–584. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Carbonero R, Supko J, Manola J, et al. Phase II and pharmacokinetic study of Ecteinascidin-743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol. 2004;22:1480–1490. doi: 10.1200/JCO.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 26.Chmielowski B, Federman N, Tap WD. Clinical trial end points for assessing efficacy of novel therapies for soft tissue sarcomas. Expert Rev Anticancer Ther. 2012;12:1217–1228. doi: 10.1586/era.12.100. [DOI] [PubMed] [Google Scholar]
- 27.Dodd LE, Korn EL, Freidlin B, et al. An audit strategy for progression-free survival. Biometrics. 2011;67:1092–1099. doi: 10.1111/j.1541-0420.2010.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (Ecteinascidin-743) in advanced pretreated myxoid liposarcomas: A retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S, Araki N, Sugiura H, et al. A randomized phase II study comparing trabectedin (T) and best supportive care (BSC) in patients (pts) with translocation-related sarcomas (TRS) J Clin Oncol. 2014;32:674s. (abstr 10524) [Google Scholar]
- 30.Yonemoto T, Takahashi S, Araki N, et al. Intra-and inter-patient comparison of efficacy between two phase II studies of trabectedin (T) in patients (PTS) with translocation-related sarcomas (TRS); A randomized comparative study (Study-C) and a single arm study (Study-S) Ann Oncol. 2014;25:iv498. [Google Scholar]
- 31.Forni C, Minuzzo M, Virdis E, et al. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol Cancer Ther. 2009;8:449–457. doi: 10.1158/1535-7163.MCT-08-0848. [DOI] [PubMed] [Google Scholar]
- 32.Di Giandomenico S, Frapolli R, Bello E, et al. Mode of action of trabectedin in myxoid liposarcomas. Oncogene. 2014;33:5201–5210. doi: 10.1038/onc.2013.462. [DOI] [PubMed] [Google Scholar]
- 33.Grohar PJ, Segars LE, Yeung C, et al. Dual targeting of EWS-FLI1 activity and the associated DNA damage response with trabectedin and SN38 synergistically inhibits Ewing sarcoma cell growth. Clin Cancer Res. 2014;20:1190–1203. doi: 10.1158/1078-0432.CCR-13-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Cesne A, Blay JY, Domont J, et al. Interruption versus continuation of trabectedin in patients with soft-tissue sarcoma (T-DIS): A randomized phase II trial. Lancet Oncol. 2015;16:312–319. doi: 10.1016/S1470-2045(15)70031-8. [DOI] [PubMed] [Google Scholar]
- 35.Le Cesne A, Yovine A, Blay J-Y, et al. A retrospective pooled analysis of trabectedin safety in 1,132 patients with solid tumors treated in phase II clinical trials. Invest New Drugs. 2012;30:1193–1202. doi: 10.1007/s10637-011-9662-0. [DOI] [PubMed] [Google Scholar]
- 36.Le Cesne A, Ray-Coquard I, Duffaud F, et al. Trabectedin in patients with advanced soft tissue sarcoma: A retrospective national analysis of the French Sarcoma Group. Eur J Cancer. 2015;51:742–750. doi: 10.1016/j.ejca.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Liberal J, Judson I. Safety evaluation of trabectedin in treatment of soft-tissue sarcomas. Expert Opin Drug Saf. 2013;12:905–911. doi: 10.1517/14740338.2013.829037. [DOI] [PubMed] [Google Scholar]
- 38.Leporini C, Patanè M, Saullo F, et al. A comprehensive safety evaluation of trabectedin and drug-drug interactions of trabectedin-based combinations. BioDrugs. 2014;28:499–511. doi: 10.1007/s40259-014-0100-7. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen OS, Judson I, van Hoesel Q, et al. Effect of high-dose ifosfamide in advanced soft tissue sarcomas: A multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2000;36:61–67. doi: 10.1016/s0959-8049(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 40.Le Cesne A, Judson I, Maki R, et al. Trabectedin is a feasible treatment for soft tissue sarcoma patients regardless of patient age: A retrospective pooled analysis of five phase II trials. Br J Cancer. 2013;109:1717–1724. doi: 10.1038/bjc.2013.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.