Abstract
Objective
Quantify the effect of a continuous compared to discontinuous movement trajectory on parkinsonian rigidity and reflex responses to passive stretch and shortening.
Methods
Eighteen participants with Parkinson’s disease (PD) performed passive wrist flexion and extension movements through a 90° range of motion at 50 °/sec using continuous (CONT) and discontinuous (DISC) movement trajectories. Participants were tested in both the OFF-MED and ON-MED states. Rigidity was quantified by rigidity work score and slopes of the moment-angle plots during both flexion and extension. Reflex response was quantified by mean EMG amplitudes of forearm musculature.
Results
No differences were observed between CONT and DISC for rigidity (p = 0.18) or moment-angle plot slopes (Flexion: p = 0.97; Extension: p = 0.89). However, medication was associated with reductions in rigidity (p = 0.02) and increases in moment-angle plot slopes (Flexion: p = 0.03; Extension: p = 0.02). The CONT compared to DISC trajectory was associated with greater EMG amplitudes in the shortened muscles (p = 0.04) and smaller EMG ratios (p < 0.05) during flexion, and greater EMG amplitudes in the lengthened muscles (p = 0.02) during extension. Dopaminergic medication reduced EMG amplitudes in stretched muscles during extension (p < 0.05).
Conclusions
The nature of the movement trajectory (continuous vs. discontinuous) used during clinical assessment does not alter the presentation of rigidity in PD. Rigidity is reduced with the administration of dopaminergic medication, independent of movement trajectory.
Significance
These data suggest that the presentation of rigidity used in the determination of diagnosis, treatment and prognosis in PD will not be affected by the continuous nature of the movement trajectory used during clinical assessment.
Keywords: Parkinson’s disease, Rigidity, Dopaminergic medication, EMG, Kinetics
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease due to the loss of dopamine producing cells in the basal nuclei. Motor dysfunction associated with PD includes rigidity, bradykinesia, resting tremor and postural instability (14, 27). Rigidity is a cardinal symptom of PD and is a primary component of clinical diagnosis. Due to its responsiveness to dopaminergic medication and surgical interventions, rigidity is also used to assess the efficacy of anti-PD treatment (43, 45). Parkinsonian rigidity is described as an increased, uniform resistance to passive limb movement throughout the range of motion (19). A clinical rating scale, the Unified Parkinson’s Disease Rating Scale (UPDRS), is used to assess rigidity based on the examiner’s perception of resistance to passive movements of the patient’s wrist, elbow, neck and ankle joints (13, 20). Clinical assessment of rigidity is qualitative in nature, is highly subjective and is heavily dependent upon the examiner’s interpretations and experience (37).
The constant nature of parkinsonian rigidity has been shown to be the result of the interaction change in the physical properties of passive connective tissues (9, 10, 12, 32, 42, 46) as well as aberrant reflex responses to stretch and shortening (5, 6, 8, 30, 39, 44). Previous research investigating reflex responses to stretch in PD demonstrated that individuals with PD exhibit normal short-latency stretch reflexes (5, 8, 30, 39) while the long-latency stretch reflex was exaggerated when compared to healthy controls (6, 39, 44). It has also been demonstrated that individuals with PD exhibit enhanced shortening reactions, an anomalous muscle activation in response to passive shortening (1, 3, 47). It has been suggested that the exaggerated shortening reaction underlies the unique, constant, “lead-pipe” rigidity present in PD (2, 38, 48, 51).
Parkinsonian rigidity has been shown to be sensitive to a number of factors including dopaminergic medication (40, 45), movement amplitude (36), movement velocity (36) and contralateral muscle contractions (35). Previous investigations of mechanisms underlying PD-related rigidity have used discontinuous movement trajectories (DISC), or pairs of passive unidirectional movements separated by a quiescent period in which no movement was imposed (25, 26, 35, 36, 51). For example, one study investigating the effect of movement amplitude and velocity on parkinsonian rigidity (36) implemented a passive wrist flexion movement followed by a one second pause and then a passive wrist extension movement. However, due to the sensitivity of parkinsonian rigidity to background muscle activation (39), it could be postulated that a continuous movement trajectory (CONT) in which no quiescent period exists (i.e. a passive wrist flexion movement immediately followed by a passive wrist extension movement) could enhance reflex responses in individuals with PD altering the presentation of rigidity. Previous research has demonstrated that muscle force production is enhanced following a preliminary stretch (stretch-shortening cycle; SSC). Several underlying mechanisms have been suggested including passive input from connective tissues (15, 21, 23) and stretch reflex responses (11, 31). A recent investigation into the factors underlying enhanced force production as a result of the SSC revealed that pre-activation was a significant component in improving force production (16). This force potentiation associated with the SSC may, in part, explain previous research findings (36) describing the velocity-sensitive nature of PD-related rigidity (17). The available evidence suggests that the presence of a SSC may alter the presentation of rigidity in individuals with PD by enhancing the shortening reaction during a CONT compared to DISC movement trajectory, functionally reducing the presentation of rigidity. A perceived reduction in resistance to passive movement during a clinical assessment via the UPDRS may result in misdiagnosis or implementation of an inappropriate treatment regimen. Identifying the response of parkinsonian rigidity to CONT and DISC movement trajectories will inform clinical practitioners and scientists regarding current rigidity assessment techniques.
Therefore, the purpose of this study was to investigate the effect of CONT and DISC movement trajectories and dopaminergic medication on parkinsonian rigidity and reflex amplitudes of passively stretched and shortened muscles. It was hypothesized that (1) a CONT movement trajectory would be associated with reduced rigidity and greater shortening reactions compared to a DISC movement trajectory and that (2) medication would reduce rigidity, increase moment-angle plot slopes and reduce reflex responses in both movement trajectories (CONT and DISC).
2. Methods
2.1 Participants
Eighteen subjects with idiopathic PD (7 men, 11 women) participated in this study. Subject anthropometrics, clinical characteristics and medication profiles are presented in Table 1. Each subject was assessed for inclusion using a verbal medical history and the Motor Section (Part III) of the UPDRS (13). Subjects were included if they (1) were between 40 and 80 years of age, (2) were treated using dopaminergic medication, (3) had the presence of clinical rigidity of 2 or 3 (mild to moderate or marked) in one or both arms when dopaminergic medication was temporarily withdrawn, and (4) had minimal tremor (≤1, slightly and infrequently present) in the tested arm in the untreated condition. Subjects were excluded if cognitive impairments prevented them from giving informed consent, understanding instructions or providing adequate feedback. Subjects were also excluded if they had insufficient wrist range of motion (less than 50° in either flexion or extension) or a history of upper extremity impairment that would affect wrist motion. The experimental protocol was approved by the Institutional Review Board of Creighton University, Omaha, Nebraska, USA. This study was conducted in accordance with the Declaration of Helsinki. All subjects provided written informed consent prior to participation in the study.
Table 1.
Patients’ clinical information
| Patient | Age (yrs) | Disease Duration (yrs) | Gender | Arm tested | Rigidity (UPDRS)a
|
Medication Informationb | |
|---|---|---|---|---|---|---|---|
| Off | On | ||||||
| 1 | 62 | 11 | F | R | 3 | 1 | C/L 50/200 (x4); C/L/E 100 mg (x2); Pra 1.5 mg (x3) |
| 2 | 67 | 13 | F | L | 3 | 2 | C/L 25/100 (x3); R 1 mg (x3); S 5 mg (x2) |
| 3 | 71 | 5 | F | R | 2 | 1 | C/L 25/100 (x3) |
| 4 | 69 | 3 | F | R | 2 | 2 | Pra 1.5 mg (x3) |
| 5 | 65 | 3 | M | L | 2 | 1 | C/L 25/100 mg (x3) |
| 6 | 59 | 8 | M | R | 2 | 1 | C/L 25/100 (x3); R 1.0 mg (x3) |
| 7 | 57 | 5 | F | R | 2 | 1 | Az 1 mg (x1); C/L 25/100 (x3); R 3.0 mg (x3) |
| 8 | 63 | 12 | M | R | 2 | 1 | Am 100 mg (x1); C/L 25/100 (x2); |
| 9 | 58 | 14 | M | L | 4 | 3 | Am 100 mg (x2); C/L 25/100 (x3); Ct 200 mg (x3) |
| 10 | 77 | 1 | F | L | 2 | 0 | C/L 25/100 (x3) |
| 11 | 67 | 10 | M | R | 2 | 1 | Az 1.0 mg (x1); C/L 100 mg (x1); Ct 200 mg (x1) |
| 12 | 63 | 7 | F | R | 2 | 1 | Pra 1.5 mg (x3); S 1.0 mg (x1) |
| 13 | 46 | 1 | F | L | 3 | 1 | C/L 25/100 (x1); R 1.0 mg (x3) |
| 14 | 73 | 2 | M | L | 3 | 2 | C/L 25/100 (x3) |
| 15 | 55 | 10 | M | L | 3 | 1 | C/L 25/250 mg (x4); R 1 mg (x4) |
| 16 | 72 | 4 | F | R | 2 | 1 | C/L 25/100 (x1); R 3.0 mg (x4) |
| 17 | 74 | 2 | F | L | 2 | 1 | C/L 25/100 (x3) |
| 18 | 48 | 2 | F | L | 2 | 1 | C/L 25/100 (x3); Pra 1.5 mg (x1) |
UPDRS (unified Parkinson’s disease rating scale). Rigidity: 0 - absent; 1 - slight; 2 - mild to moderate; 3 - marked; 4 - severe.
Am - amantadine; Az - azilect; C/L - carbidopa/levodopa; C/L/E - carbidopa/levodopa/entacapone; Ct - comtan; E - entacopone; Pra - pramipexole; R - ropinirole; S - selegiline.
2.2 Experimental protocol
Each subject was initially evaluated using the Motor Section (Part III) of the UPDRS (13); the subject was then seated in a height adjustable chair. The subject’s hand exhibiting greater rigidity according to the UPDRS was placed in a manipulandum attached to the shaft of a servomotor. With the subject’s shoulder and forearm in neutral position and the elbow in approximately 120° of flexion, the center of wrist joint rotation was aligned with the center of rotation of the servomotor. The forearm was stabilized using a vacuum bag splint which prevented forearm pronation and supination. The metacarpal restraints of the manipulandum restricted other motions of the wrist allowing only flexion and extension.
Subjects were instructed to remain relaxed during passive wrist flexion and extension movements generated by the servomotor. Each trial began with the wrist at approximately 45° of wrist extension and moved through a central range of motion of 90° (± 45°) at a constant velocity of 50 °/s. Two movement trajectories were applied to the passive wrist including CONT and DISC movement trajectories. The CONT was characterized by a passive wrist flexion movement of 90° followed by an immediate passive wrist extension movement of 90°. The DISC trajectory was characterized by a passive wrist flexion movement of 90° followed by a one second pause (no movement) then a passive wrist extension movement of 90°. Two trials were collected for each movement trajectory. Trajectory presentation order randomized and each trial was followed by a 30-second period of rest to minimize motor adaptation and fatigue.
Resistance torque at the wrist was measured using a strain gauge torque transducer (TRT-200, Pacific Scientific, CA, USA) while angular position of the wrist joint was measured using emulated encoder output from the servomotor controller (SC904 series, Pacific Scientific, CA, USA). Torque and position signals were recorded at 1000 Hz and 100 Hz, respectively. To quantify reflex responses to passive muscle stretch and shortening, surface electromyography (EMG) signals were recorded from the extrinsic wrist and finger flexor and extensor muscles including: flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), flexor digitorum superficialis (FDS), extensor carpi radialis (ECR), extensor carpi ulnaris (ECU), extensor digitorum communis (EDC). EMG signals were recorded using a 16-channel surface EMG system (Delsys, Inc., MA, USA). Electrode placement followed previously published recommendations (34) and were confirmed using manual muscle testing. EMG signals were amplified (x10k) and band-pass filtered (20 – 450 Hz) before being sampled at 1000 Hz for each EMG channel. Torque, position and EMG data were captured using custom software (LabVIEW 2009, National Instruments, TX, USA).
Subjects with PD were tested in two medication states including Off- (OFF-MED) and On-medication (ON-MED). The OFF-MED condition occurred after a 12-hour withdrawal from dopaminergic medication (22) when a majority of the beneficial effects of dopamine replacement therapy was eliminated (7). After OFF-MED testing was completed, subjects with PD self-administered their regular dose of dopaminergic medication in the laboratory. Following a 45- to 60-minute period of rest, the effect of dopaminergic medication was validated verbally by the subject. Once the efficacy of medication was established, the participants repeated the experimental protocol in the ON-MED state.
2.3 Data analyses
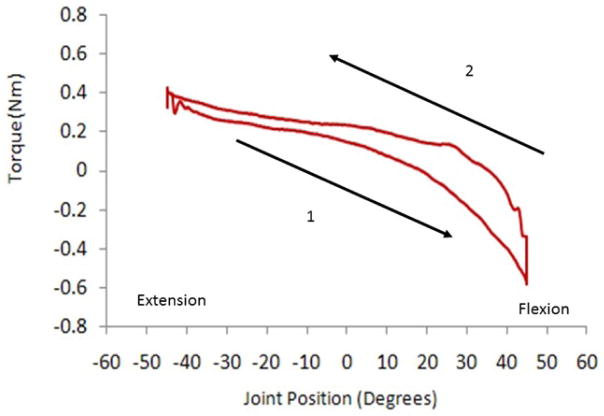
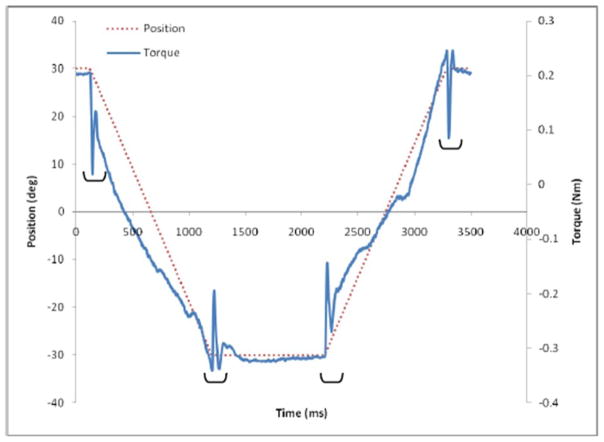
To quantify rigidity of the wrist, torque and EMG data were analyzed using custom software (MATLAB 2015, MathWorks, MA, USA). Rigidity was quantified using the rigidity work score (Figure 1) which is calculated as the torque signal integrated with respect to the joint angle (18, 35, 36, 49). Further, the slope of the torque-angle plot was assessed using linear regression analyses for the flexion and extension movements independently (35, 36, 49, 50). To remove calculation error associated with the inertial components of the torque signal, immediately following the onset of movement and associated with the inertia of the wrist and hand segment, the initial 5° of the motion were excluded from the rigidity analyses (Figure 2) (36).
Figure 1.
A sample moment-angle plot from a representative subject with Parkinson’s disease in the OFF-MED state during the passive wrist flexion and extension movements through the 90° range of motion at 50 °/sec.
Figure 2.
A representative sample of joint position (dotted) and joint torque (solid) signals recorded from one subject during an imposed flexion and extension movements at 50°/s. Rigidity work scores were calculated for the periods of flexion and extension while the inertial components of torque, denoted by brackets, were omitted from the analysis.
EMG signals were full-wave rectified and low-pass filtered with a cutoff frequency of 30 Hz. Mean EMG amplitudes were calculated for each muscle within the flexion and extension movement durations, respectively. Mean EMG values were normalized to background EMG amplitude and were calculated as the quotient of the mean EMG amplitudes during each independent movement phase (flexion and extension) divided by the mean background EMG amplitude during the 100 ms prior to the onset of movement as suggested in the literature (39). Normalized mean EMG values were grouped by function (flexors or extensors) and represented by the sum of the EMG amplitudes for each functional group (i.e. Flexors = FCR + FCU + FDS; Extensors = ECR + ECU+ EDC). For example, the mean EMG of stretched muscles was calculated as an average of normalized mean EMG of extensors during the flexion movement and the average of normalized mean EMG of flexors during the extension movement (35, 36, 51). Conversely, the mean EMG of shortened muscles was calculated as the average of normalized mean EMG signals of the flexors during flexion and the average of normalized mean EMG signals of the extensors during extension. In addition to EMG responses to passive muscle stretch and shortening, EMG ratios were calculated to represent the interaction between the stretch reflex and shortening reaction. The EMG ratio has been shown to be a robust index for the assessment of parkinsonian rigidity (35, 51). The EMG ratio was calculated as the normalized EMG activity of the stretched muscles divided by the normalized EMG activity of the shortened muscles during each phase of movement. For example, during the imposed flexion movement the extensor muscles were stretched while the flexor muscles were shortened. Thus the normalized mean EMG in the stretched extensor muscles was divided by the normalized mean EMG in the shortened muscles obtaining an EMG ratio for the flexion movement.
2.4 Statistical analyses
A series of 2 × 2 repeated measures analysis of variance (ANOVAs) were performed to determine the main and interaction effects of movement trajectory (CONT vs. DISC) and medication (Off-Med vs. On-Med) on dependent variables associated with the flexion and extension phases of wrist movement as well as the total movement cycle (flexion and extension). In the presence of a significant interaction effect, post hoc t-tests were performed as tests of simple effects to determine the source of the significant interaction. Dependent variables included rigidity work score, torque-angle slope, EMG of stretched muscles, EMG of shortened muscles and EMG ratio of stretched-shortened muscles. For all statistical tests, significance was set at p ≤ 0.05. All statistical analyses were conducted using SPSS 21.0 (IBM, Armonk, NY, USA).
3. Results
3.1 Rigidity
Table 2 shows the summary Rigidity Work Scores for the total movement as well as the flexion and extension components of each movement across all subjects. The repeated measures ANOVA revealed no significant differences between the CONT and DISC conditions in rigidity work scores across the total movement (F = 2.019, p = 0.177), flexion movement (F = 0.662, p = 0.441) or extension movement (F = 1.152, p = 0.300).
Table 2.
Measures of rigidity including rigidity torque scores and moment-angle plot slopes. Rigidity torque scores are presented for the overall movement (TOTAL) as well as flexion and extension components of the overall movement. Moment-angle plot slopes are presented for the flexion and extension components of the passive movement. Presented as mean (SD)
| Rigidity Work Score | Slope | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Trajectory | Medication | Total | Flexion | Extension | Flexion | Extension |
| CONT | OFF-MED | 9.0 (4.2) | 5.8 (3.0) | 4.0 (1.4) | 2.9 (0.6) | 2.2 (1.5) |
| ON-MED | 4.5 (2.1)† | 2.1 (2.4)† | 3.1 (2.0) | 7.4 (4.9)† | 7.6 (5.9)† | |
|
| ||||||
| DISC | OFF-MED | 9.4 (3.0) | 4.7 (4.2) | 3.5 (3.1) | 3.5 (1.4) | 2.6 (1.1) |
| ON-MED | 6.4 (3.6)† | 2.2 (2.3)† | 3.2 (2.7) | 6.8 (3.3)† | 7.0 (4.0)† | |
Note:
denotes significant difference compared to DISC trajectory;
denotes significant difference compared to OFF-MED state.
The administration of dopaminergic medication was associated with significant reductions in total rigidity work scores (F = 6.753, p = 0.021). The smaller total rigidity work scores in the ON- compared to OFF-MED conditions were dominated by significant reductions in rigidity work scores during the flexion movement (F = 5.848, p = 0.027). Rigidity work scores in the extension movement were not significantly reduced in the ON-MED state (F = 0.169, p = 0.687). No trajectory by medication interactions were observed for the rigidity work scores across the total movement (F = 0.863, p = 0.369), flexion movement (F = 0.957, p = 0.342) or extension movement (F = 0.969, p = 0.341).
The slope of the torque-angle plot quantifies the uniform nature of rigidity in Parkinson’s disease. Table 2 presents slopes of the flexion and extension movements for all subjects. No significant differences in torque-angle slopes were observed between the CONT and DISC trajectories for the flexion movement (F = 0.001, p = 0.972) or extension movement (F = 0.019, p = 0.892). Dopaminergic medication was associated with significant increases in the slope of the torque-angle plot for both the flexion movement (F = 6.204, p = 0.030) and the extension movement (F = 7.911, p = 0.016). No significant trajectory by medication interactions were present for the slopes of the torque-angle plots in either the flexion movement (F = 1.972, p = 0.188) or extension movement (F = 0.719, p = 0.413).
3.2 Reflex Electromyography (EMG)
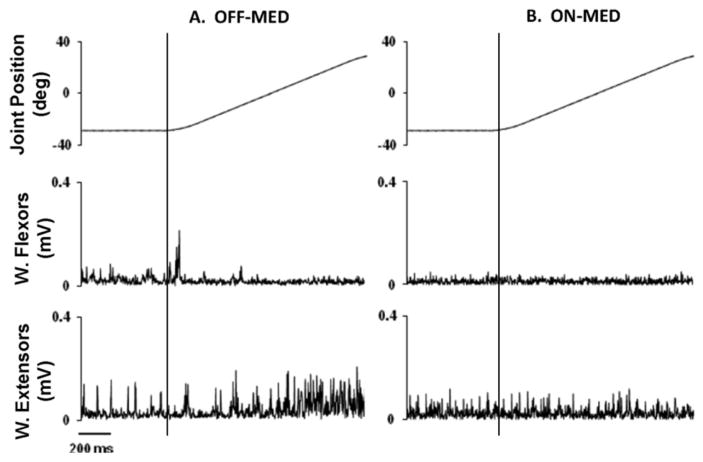
Joint position and EMG activity of the wrist flexors and extensors during the imposed extension movement are shown in Figure 3. In this example from a representative subject, a stretch reflex and shortening reaction are visualized in the OFF-MED (A) and ON-MED (B) conditions. The stretch reflex was observed in the wrist flexors while the shortening reaction was exhibited in the wrist extensors during the presented extension movement. In Figure 3, both the stretch reflex and shortening reaction were pronounced in the OFF-MED condition, but substantially reduced in the ON-MED condition. In the current study, muscle activation was quantified using the mean EMG values of the stretched and shortened muscles as well as EMG ratios of the stretched-shortened muscles for the flexion and extension movements. Table 3 presents muscle activation levels and EMG ratios during the flexion and extension movements.
Figure 3.
Representative joint position and EMG tracings of the wrist flexors and extensors from a subject with Parkinson’s disease during the wrist extension movement at 50°/s with 60° range of motion. In the OFF-MED condition (A), a shortening reaction was observed in the extensors while a stretch reflex was recorded in the wrist flexors. Dopaminergic medication greatly diminished the amplitude of these phenomena in the ON-MED condition (B). The onset of movement is indicated by the vertical line. Top panel: joint position (°); middle panel: average EMG of wrist flexor muscles; lower panel: averaged EMG of wrist extensor muscles.
Table 3.
Normalized EMG measurements of reflex responses to stretch and shortening during the passive wrist flexion and extension movements in the shortened muscles, lengthened muscles and the EMG ratio. Presented as mean (SD)
| Rigidity Work Score | Slope | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Trajectory | Medication | Total | Flexion | Extension | Flexion | Extension |
| CONT | OFF-MED | 9.0 (4.2) | 5.8 (3.0) | 4.0 (1.4) | 2.9 (0.6) | 2.2 (1.5) |
| ON-MED | 4.5 (2.1)† | 2.1 (2.4)† | 3.1 (2.0) | 7.4 (4.9)† | 7.6 (5.9)† | |
|
| ||||||
| DISC | OFF-MED | 9.4 (3.0) | 4.7 (4.2) | 3.5 (3.1) | 3.5 (1.4) | 2.6 (1.1) |
| ON-MED | 6.4 (3.6)† | 2.2 (2.3)† | 3.2 (2.7) | 6.8 (3.3)† | 7.0 (4.0)† | |
Note:
denotes significant difference compared to DISC trajectory;
denotes significant difference compared to OFF-MED state.
During the flexion movement, the CONT trajectory was associated with significantly greater EMG amplitudes in shortened muscles (F = 4.817, p = 0.040) and significantly smaller EMG ratios (F = 4.389, p = 0.048) than the DISC trajectory. Post-hoc t-tests revealed that the CONT trajectory was associated with significantly greater reflex responses EMG amplitudes than the DISC in the shortened muscles in the ON-MED state (p = 0.032) while no differences were observed between the movement trajectories (CONT vs. DISC) in the OFF-MED state (p = 0.175). No significant differences were observed between CONT and DISC trajectories in EMG amplitudes of the stretched muscles during the flexion movement (F = 0.208, p = 0.652). Administration of dopaminergic medication was not associated with significant changes in EMG amplitudes of the stretched (F = 0.024, p = 0.879) or shortened muscles (F = 0.155, p = 0.698), or EMG ratios (F = 0.007, p = 0.933).
During the extension movement, a significant trajectory by medication interaction was observed for the stretched muscles (F = 4.721, p = 0.041) and EMG ratios (F = 6.924, p = 0.016). Post-hoc t-tests revealed that in the OFF-MED state, the CONT trajectory was associated with significantly greater EMG amplitudes in the stretched muscles than the DISC trajectory (p = 0.018), while no differences were observed between movement trajectories in the ON-MED state. Post-hoc analyses also revealed that in the CONT trajectory, the administration of dopaminergic medication (OFF- vs. ON-MED) was associated with significantly smaller EMG amplitudes of the stretched muscles during the extension movement (p = 0.047). Conversely, no differences were observed between the OFF- and ON-MED states in the stretched muscles during the DISC trajectory (p = 0.051).
A significant trajectory by medication interaction was also observed for EMG ratios during the extension movement (F = 6.924, p = 0.016). Post-hoc t-tests revealed no significant differences between the CONT and DISC trajectories in either the OFF- (p = 0.066) or ON-MED states (p = 0.166). The post-hoc analyses did not reveal a significant effect of medication in either the CONT (p = 0.106) or DISC movement trajectories (p = 0.055). Though no significant differences were present, it should be noted that administration of dopaminergic medication was associated with non-significant decreases in EMG ratios in the CONT trajectory while a small (non-significant) increase in EMG ratios were observed in the DISC movement trajectory.
4. Discussion
The purpose of this study was to investigate the effect of movement trajectory and dopaminergic medication on parkinsonian rigidity and underlying reflex responses in individuals with PD. The current findings revealed no differences in measures of rigidity including rigidity work score or slopes of the moment-angle plot when comparing between CONT and DISC movement trajectories. Dopaminergic medication was associated with significant reductions in rigidity work scores and increased slopes of the moment-angle plots suggesting an improvement in clinical rigidity symptoms. EMG data revealed that reflex responses were greater in the CONT compared to DISC movements in shortened muscles during flexion and stretched muscles during extension. These data present novel findings suggesting that reflex responses may be enhanced when immediately following passive shortening in individuals with PD.
Parkinsonian rigidity is the mechanical outcome of changes in the intrinsic properties of muscle and connective tissues as well as aberrant reflexes (4, 9, 10, 28). A unique characteristic of parkinsonian rigidity is the constant and uniform nature of the resistance to passive motion (13). This hallmark symptom is the result of exaggerated reflex responses to passive stretch and shortening (25, 26, 50). Parkinsonian rigidity during passive movements has been the focus of many research investigations which have demonstrated that the magnitude of rigidity is sensitive to a variety of movement parameters including movement velocity and amplitude, and the performance of a contralateral activation maneuver (35, 36). However, no previous investigation has compared the rigidity response to a CONT versus DISC movement trajectory. In the current study, no differences were observed in rigidity work scores or moment-angle plot slopes in the CONT compared to DISC movement trajectories. These findings do not support the hypothesis that a CONT would be associated with reduced rigidity compared to DISC movement trajectories. Existing research has demonstrated that torque and power output are enhanced during an active stretch-shortening cycle compared to an isolated concentric contraction (16, 33). Moreover, it has been suggested that the presence of background neural drive potentiates muscle force (11, 31). Though muscular performance was enhanced during the stretch-shortening cycle in these studies, it has been shown that individuals with PD exhibit less efficient neuromuscular drive as evidenced by greater EMG-torque ratios (33). However, the study by Pedersen et al. (1997) investigated muscular performance during voluntary muscular activation rather than a reflex response to passive movement. In the present study, resistance to independent passive stretch and shortening movements were compared to passive stretch-shortening cycles at the wrist. The present findings reveal no differences in rigidity between the two experimental movement trajectories suggesting that a CONT stretch-shortening cycle does not attenuate parkinsonian rigidity compared to independent movements (DISC). These findings are clinically relevant in that they inform physician’s that the movement pattern (CONT vs. DISC) used to assess clinical rigidity will not significantly alter clinical diagnosis or prognosis.
Though movement trajectory was not revealed to significantly alter the amplitude or characteristics of parkinsonian rigidity, these data clearly show that dopaminergic medication was associated with reductions in PD-related rigidity. It has been demonstrated that dopaminergic medication improves both the direct and indirect striatal pathways in individuals with PD reducing rigidity and enhancing motor coordination (24, 35, 52, 53). Therefore, these findings partially support the second hypothesis by demonstrating that dopaminergic medication was associated with reductions in rigidity work score and increases in moment-angle plots slopes. Re-testing to assess the effect of medication on rigidity was conducted 45 to 60 minutes following the administration of anti-PD medication which has been suggested to provide sufficient time for dopaminergic medication to become efficacious (22) which was verbally confirmed by participants. Further, these data demonstrating the effectiveness of dopaminergic medication in reducing parkinsonian rigidity is supported by previous research findings that have implemented a similar timeline for re-testing (24, 35, 36, 52, 53).
In contrast to the measures of rigidity, EMG data demonstrated a significant effect of movement trajectory. Specifically, the shortened muscles had significantly greater activation intensity during passive flexion while the stretched muscles had greater activation intensity during passive extension in the CONT compared to DISC movement trajectory. Functionally these findings suggest that the flexor musculature exhibits greater reflex responses to a CONT trajectory compared to a DISC trajectory. However, the initial conditions of the CONT and DISC were similar and thus no differences due to movement trajectory should be observed during the initial flexion movement. Further, as the order of trials were randomized, no systematic differences in reflex responses should have been observed due to habituation (29). One potential explanation for these findings pertains to the level of background muscle activation prior to the onset of the passive wrist movements. In the present study, EMG signals were normalized to the resting background EMG prior to the onset of movement (4). It is possible that resting background EMG signals were smaller in the CONT compared to DISC movement trajectories, potentially due to the “smoother” nature of the movement pattern. However, no evidence exists to support this postulation. The methodology by which reflex responses are quantified should be further investigated as preliminary research has suggested that the current method may mask differences in reflex amplitudes in response to stretch and shortening (41). Therefore, the methodology used in the current study may underlie these distinct differences in reflex responses to CONT versus DISC movement trajectories.
While the current study presents novel, clinically applicable findings, the authors recognize several limitations to the current study. One limitation of the current study pertains to the methods used to quantify EMG amplitudes in the OFF- versus ON-MED conditions. It has been previously suggested that one underlying mechanism of parkinsonian rigidity is the patient’s inability to complete relax as evidenced by increased background EMG amplitudes at rest. Previous research has demonstrated that decreases of 11% in background EMG amplitudes associated with dopaminergic medication which may contribute to difficulty interpreting changes in EMG-related variables of aberrant reflex responses in the OFF- compared to ON-MED states (36). A second limitation of this study pertains to the period of time between the OFF- and ON-MED testing. While previous research has suggested that 45 to 60 minutes is sufficient for dopaminergic medication to become efficacious (22) and the findings of the current study demonstrated medication-induced improvements in parkinsonian rigidity, it is possible that a greater period of time would have resulted in greater differences in response to anti-PD treatment.
Conclusions
The current study is the first to directly investigate the effect of a CONT versus DISC movement trajectory on parkinsonian rigidity and reflex responses to stretch and shortening. The findings of this study demonstrate that movement trajectory does not significantly alter parkinsonian rigidity and would therefore not affect clinical diagnoses, treatments or prognoses. These data also give rise to questions regarding the methods by which we quantify the amplitudes of reflex responses.
Highlights.
No differences in measures of rigidity (rigidity work score or moment-angle plot slopes) were attributed to movement trajectory
Dopaminergic medication significantly reduced rigidity and increased moment-angle plot slopes
Flexors exhibited greater EMG amplitudes during continuous versus discontinuous movement trajectories
Dopaminergic medication did not systematically alter EMG amplitudes regardless of trajectory
Acknowledgments
This study was funded by the National Institutes of Health under grants R15-HD061022 and R15-HD061022-S1.
Footnotes
Conflict of interest: No conflicts declared.
References
- 1.Andrews CJ, Burke D, Lance JW. The response to muscle stretch and shortening in Parkinsonian rigidity. Brain. 1972;95:795–812. doi: 10.1093/brain/95.4.795. [DOI] [PubMed] [Google Scholar]
- 2.Angel RW. Muscular contractions elicited by passive shortening. Adv Neurol. 1983;39:555–563. [PubMed] [Google Scholar]
- 3.Berardelli A, Hallett M. Shortening reaction of human tibialis anterior. Neurology. 1984;34:242–245. doi: 10.1212/wnl.34.2.242. [DOI] [PubMed] [Google Scholar]
- 4.Berardelli A, Sabra AF, Hallett M. Physiological mechanisms of rigidity in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1983;46:45–53. doi: 10.1136/jnnp.46.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergui M, Lopiano L, Paglia G, Quattrocolo G, Scarzella L, Bergamasco B. Stretch reflex of quadriceps femoris and its relation to rigidity in Parkinson’s disease. Acta Neurol Scand. 1992;86:226–229. doi: 10.1111/j.1600-0404.1992.tb05075.x. [DOI] [PubMed] [Google Scholar]
- 6.Cody FW, MacDermott N, Matthews PB, Richardson HC. Observations on the genesis of the stretch reflex in Parkinson’s disease. Brain. 1986;109(Pt 2):229–249. doi: 10.1093/brain/109.2.229. [DOI] [PubMed] [Google Scholar]
- 7.Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD) Mov Disord. 1999;14:572–584. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Delwaide PJ, Sabbatino M, Delwaide C. Some pathophysiological aspects of the parkinsonian rigidity. J Neural Transm Suppl. 1986;22:129–139. [PubMed] [Google Scholar]
- 9.Dietz V. Changes of inherent muscle stiffness in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1987;50:944. doi: 10.1136/jnnp.50.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104:431–449. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- 11.Dietz V, Schmidtbleicher D, Noth J. Neuronal mechanisms of human locomotion. J Neurophysiol. 1979;42:1212–1222. doi: 10.1152/jn.1979.42.5.1212. [DOI] [PubMed] [Google Scholar]
- 12.Endo T, Okuno R, Yokoe M, Akazawa K, Sakoda S. A novel method for systematic analysis of rigidity in Parkinson’s disease. Mov Disord. 2009;24:2218–2224. doi: 10.1002/mds.22752. [DOI] [PubMed] [Google Scholar]
- 13.Fahn S, Elton RL . Members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, et al., editors. Recent developments in Parkinson’s disease. Florham, NJ: Macmillian Healthcare; 1987. [Google Scholar]
- 14.Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finni T, Ikegawa S, Komi PV. Concentric force enhancement during human movement. Acta Physiol Scand. 2001;173:369–377. doi: 10.1046/j.1365-201X.2001.00915.x. [DOI] [PubMed] [Google Scholar]
- 16.Fukutani A, Kurihara T, Isaka T. Factors of force potentiation induced by stretch-shortening cycle in plantarflexors. PLoS One. 2015;10:e0120579. doi: 10.1371/journal.pone.0120579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukutani A, Kurihara T, Isaka T. Influence of joint angular velocity on electrically evoked concentric force potentiation induced by stretch-shortening cycle in young adults. Springerplus. 2015;4:82. doi: 10.1186/s40064-015-0875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung VS, Burne JA, Morris JG. Objective quantification of resting and activated parkinsonian rigidity: a comparison of angular impulse and work scores. Mov Disord. 2000;15:48–55. doi: 10.1002/1531-8257(200001)15:1<48::aid-mds1009>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Fung VS, Thompson PD. Rigidity & Spasticity. Philadelphia: Lippencott Williams & Wilkens; 2002. [Google Scholar]
- 20.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa M, Komi PV, Finni T, Kuitunen S. Contribution of the tendinous tissue to force enhancement during stretch-shortening cycle exercise depends on the prestretch and concentric phase intensities. J Electromyogr Kinesiol. 2006;16:423–431. doi: 10.1016/j.jelekin.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Jahanshahi M, Jones CR, Zijlmans J, Katzenschlager R, Lee L, Quinn N, Frith CD, Lees AJ. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain. 2010;133:727–745. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- 23.Kawakami Y, Muraoka T, Ito S, Kanehisa H, Fukunaga T. In vivo muscle fibre behaviour during counter-movement exercise in humans reveals a significant role for tendon elasticity. J Physiol. 2002;540:635–646. doi: 10.1113/jphysiol.2001.013459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon Y, Kim JW, Ho Y, Jeon HM, Bang MJ, Eom GM, Koh SB. Analysis of antagonistic co-contractions with motorized passive movement device in patients with Parkinson’s disease. Biomed Mater Eng. 2014;24:2291–2297. doi: 10.3233/BME-141042. [DOI] [PubMed] [Google Scholar]
- 26.Kwon Y, Kim JW, Kim JS, Koh SB, Eom GM, Lim TH. Comparison of EMG during passive stretching and shortening phases of each muscle for the investigation of parkinsonian rigidity. Biomed Mater Eng. 2015;26(Suppl 1):S2155–2163. doi: 10.3233/BME-151521. [DOI] [PubMed] [Google Scholar]
- 27.Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 28.Lee RG, Tatton WG. Motor responses to sudden limb displacements in primates with specific CNS lesions and in human patients with motor system disorders. Can J Neurol Sci. 1975;2:285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto H, Noro H, Kaneshige Y, Chiba S, Miyano N, Motoi Y, Yanada Y. A correlation study between blink reflex habituation and clinical state in patients with Parkinson’s disease. J Neurol Sci. 1992;107:155–159. doi: 10.1016/0022-510x(92)90283-q. [DOI] [PubMed] [Google Scholar]
- 30.Meara RJ, Cody FW. Stretch reflexes of individual parkinsonian patients studied during changes in clinical rigidity following medication. Electroencephalogr Clin Neurophysiol. 1993;89:261–268. doi: 10.1016/0168-5597(93)90105-x. [DOI] [PubMed] [Google Scholar]
- 31.Nichols TR, Houk JC. Reflex compensation for variations in the mechanical properties of a muscle. Science. 1973;181:182–184. doi: 10.1126/science.181.4095.182. [DOI] [PubMed] [Google Scholar]
- 32.Park BK, Kwon Y, Kim JW, Lee JH, Eom GM, Koh SB, Jun JH, Hong J. Analysis of viscoelastic properties of wrist joint for quantification of parkinsonian rigidity. IEEE Trans Neural Syst Rehabil Eng. 2011;19:167–176. doi: 10.1109/TNSRE.2010.2091149. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen SW, Oberg B. Stretch-shortening contraction in Parkinson patients: evidence of normal muscle contraction execution with low efficiency. Scand J Rehabil Med. 1997;29:251–255. [PubMed] [Google Scholar]
- 34.Perotto AO. Anatomical Guide for the Electromyographer: The Limb and Trunk. Springfield; Illinois, USA: 1994. [Google Scholar]
- 35.Powell D, Hanson N, Joseph Threlkeld A, Fang X, Xia R. Enhancement of parkinsonian rigidity with contralateral hand activation. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.1001.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell D, Joseph Threlkeld A, Fang X, Muthumani A, Xia R. Amplitude- and velocity-dependency of rigidity measured at the wrist in Parkinson’s disease. Clin Neurophysiol. 2012;123:764–773. doi: 10.1016/j.clinph.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prochazka A, Bennett DJ, Stephens MJ, Patrick SK, Sears-Duru R, Roberts T, Jhamandas JH. Measurement of rigidity in Parkinson’s disease. Mov Disord. 1997;12:24–32. doi: 10.1002/mds.870120106. [DOI] [PubMed] [Google Scholar]
- 38.Rondot P, Metral S. Analysis of the shortening reaction in man. In: Desmedt JE, editor. New developments in electromyography and clinical neurophysiology. Basel: Karger; 1973. pp. 629–634. [Google Scholar]
- 39.Rothwell JC, Obeso JA, Traub MM, Marsden CD. The behaviour of the long-latency stretch reflex in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1983;46:35–44. doi: 10.1136/jnnp.46.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schapira AH, Emre M, Jenner P, Poewe W. Levodopa in the treatment of Parkinson’s disease. Eur J Neurol. 2009;16:982–989. doi: 10.1111/j.1468-1331.2009.02697.x. [DOI] [PubMed] [Google Scholar]
- 41.Scott LA, Ake KM, Muthumani A, Xia R, Powell DW. EMG normalization masks changes in reflex amplitude in response to dopaminergic medication in Parkinson’s disease. Med Sci Sports Exerc. 2015;47:470. [Google Scholar]
- 42.Sepehri B, Esteki A, Ebrahimi-Takamjani E, Shahidi GA, Khamseh F, Moinodin M. Quantification of rigidity in Parkinson’s disease. Ann Biomed Eng. 2007;35:2196–2203. doi: 10.1007/s10439-007-9379-6. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro MB, Vaillancourt DE, Sturman MM, Metman LV, Bakay RA, Corcos DM. Effects of STN DBS on rigidity in Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15:173–181. doi: 10.1109/TNSRE.2007.896997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatton WG, Bedingham W, Verrier MC, Blair RD. Characteristic alterations in responses to imposed wrist displacements in parkinsonian rigidity and dystonia musculorum deformans. Can J Neurol Sci. 1984;11:281–287. doi: 10.1017/s0317167100045546. [DOI] [PubMed] [Google Scholar]
- 45.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- 46.Watts RL, Wiegner AW, Young RR. Elastic properties of muscles measured at the elbow in man: II. Patients with parkinsonian rigidity. J Neurol Neurosurg Psychiatry. 1986;49:1177–1181. doi: 10.1136/jnnp.49.10.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westphal C. Uber eine Art paradoxer Muskel-contraction. Arch Psychiat und Nervenkr. 1880;10:243–248. [Google Scholar]
- 48.Xia R. Physiological and biomechanical analyses of rigidity in Parkinson’s disease. In: Rana A, editor. Parkinson’s Disease/Book 3. Vienna, Austria: InTech; 2011. pp. 1–16. In Press. [Google Scholar]
- 49.Xia R, Markopoulou K, Puumala SE, Rymer WZ. A comparison of the effects of imposed extension and flexion movements on Parkinsonian rigidity. Clin Neurophysiol. 2006;117:2302–2307. doi: 10.1016/j.clinph.2006.06.176. [DOI] [PubMed] [Google Scholar]
- 50.Xia R, Rymer WZ. The role of shortening reaction in mediating rigidity in Parkinson’s disease. Exp Brain Res. 2004;156:524–528. doi: 10.1007/s00221-004-1919-9. [DOI] [PubMed] [Google Scholar]
- 51.Xia R, Sun J, Threlkeld AJ. Analysis of interactive effect of stretch reflex and shortening reaction on rigidity in Parkinson’s disease. Clin Neurophysiol. 2009;120:1400–1407. doi: 10.1016/j.clinph.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Zhou FM. A transient receptor potential channel regulates basal ganglia output. Rev Neurosci. 2010;21:95–118. doi: 10.1515/revneuro.2010.21.2.95. [DOI] [PubMed] [Google Scholar]
- 53.Zhou FW, Jin Y, Matta SG, Xu M, Zhou FM. An ultra-short dopamine pathway regulates basal ganglia output. J Neurosci. 2009;29:10424–10435. doi: 10.1523/JNEUROSCI.4402-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]