Abstract
Purpose
To test the association of androgen deprivation therapy (ADT) in the treatment of prostate cancer with subsequent Alzheimer’s disease risk.
Methods
We used a previously validated and implemented text-processing pipeline to analyze electronic medical record data in a retrospective cohort of patients at Stanford University and Mt. Sinai hospitals. Specifically, we extracted International Classification of Diseases-9th revision diagnosis and Current Procedural Terminology codes, medication lists, and positive-present mentions of drug and disease concepts from all clinical notes. We then tested the effect of ADT on risk of Alzheimer’s disease using 1:5 propensity score–matched and traditional multivariable-adjusted Cox proportional hazards models. The duration of ADT use was also tested for association with Alzheimer’s disease risk.
Results
There were 16,888 individuals with prostate cancer meeting all inclusion and exclusion criteria, with 2,397 (14.2%) receiving ADT during a median follow-up period of 2.7 years (interquartile range, 1.0-5.4 years). Propensity score–matched analysis (hazard ratio, 1.88; 95% CI, 1.10 to 3.20; P = .021) and traditional multivariable-adjusted Cox regression analysis (hazard ratio, 1.66; 95% CI, 1.05 to 2.64; P = .031) both supported a statistically significant association between ADT use and Alzheimer’s disease risk. We also observed a statistically significant increased risk of Alzheimer’s disease with increasing duration of ADT (P = .016).
Conclusion
Our results support an association between the use of ADT in the treatment of prostate cancer and an increased risk of Alzheimer’s disease in a general population cohort. This study demonstrates the utility of novel methods to analyze electronic medical record data to generate practice-based evidence.
INTRODUCTION
Androgen deprivation therapy (ADT) has been a mainstay of treatment of prostate cancer since the 1940s. Although its use has historically been limited to metastatic disease, randomized evidence supports the use of ADT in combination with external-beam radiation therapy for locoregional disease with high-risk features.1,2 Overall the use of ADT has increased dramatically over recent decades,3 with an estimated 500,000 men currently receiving ADT for prostate cancer in the United States.4
The goal of ADT is to profoundly lower male androgens, specifically testosterone, secondary to the androgen dependence of prostate cancer. Although most individuals return to normal testosterone levels post treatment, 20% to 30% have prolonged androgen suppression.5,6 Importantly, low testosterone levels have been linked to a number of adverse health effects, including cardiometabolic disease.7 Additionally, evidence supports an association between ADT and negative health consequences, including diabetes and cardiovascular disease.8,9
The use of ADT in the treatment of prostate cancer has also been associated with a number of cognitive deficits.10,11 Concerningly, ADT has been linked to impairments in visuomotor and executive functioning, which are cardinal features of Alzheimer’s disease.11 Additionally, men diagnosed with Alzheimer’s disease have demonstrated lower levels of circulating and brain testosterone, with low testosterone levels preceding disease onset.12-15 Among men with Alzheimer’s disease, testosterone supplementation has been shown to improve spatial and verbal memory.16 Finally, although the majority of cancers have an inverse association with Alzheimer’s disease risk, prostate cancer is associated with a significantly increased risk of Alzheimer’s disease.17
Despite these data, there have been limited and conflicting investigations examining the association of ADT with neurocognitive function10 and no known studies examining the association of ADT with risk of Alzheimer’s disease. In the current study we use a novel informatics approach, using electronic medical record data from more than 5 million patients, to examine the association of ADT with the subsequent development of Alzheimer’s disease among men with prostate cancer.
METHODS
Data Sources
We used data from Stanford University (1994 to 2013) and Mt. Sinai (2000 to 2013) health systems. There were 1.8 million patients at Stanford and 3.7 million patients at Mt. Sinai, representing 40 million patient encounters with transcriptions of all inpatient and outpatient clinical notes as well as pathology and radiology reports and structured medication lists. Both data sources were accessed under approved institutional review board protocols. Access to Mt. Sinai data was obtained via an institutional research agreement.
Electronic Medical Record Processing
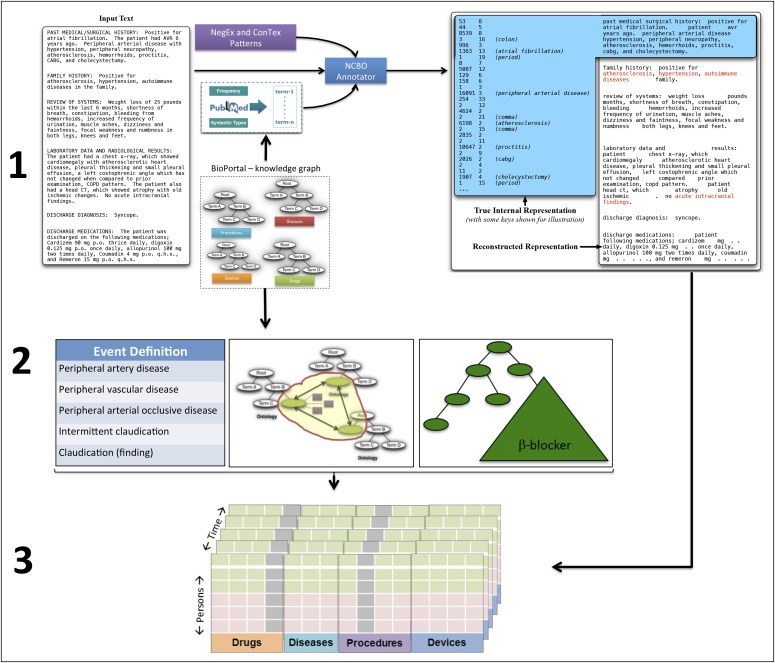
We used a previously validated18 and implemented19-21 text-processing pipeline22 to analyze clinical data. We extracted International Classification of Diseases-9th revision (ICD-9) diagnosis and Current Procedural Terminology codes, medication lists, and positive-present mentions of drug and disease concepts from all clinical notes. We removed uninformative phrases based on term frequency analysis of more than 50 million clinical documents23 and suppressed terms having fewer than four characters because the majority of these are ambiguous abbreviations. We used NegEx regular expressions to flag negative mentions (eg, “ruled out prostate cancer”) and to determine if a term was mentioned in the history or family history section of the note.24 The result is a list of present, positive mentions of biomedical concepts, which are converted into a patient-feature matrix for analysis (Appendix Fig A1, online only).
These methods have been compared against advanced natural language–processing methods in a functional evaluation, which showed little impact on the accuracy of association detection in large data sets.22 As further validation, in drug safety studies, the sensitivity and specificity in detecting 25 different conditions via methods used in this study were 0.74 and 0.96, respectively.18
Definition of Outcomes and Covariates
Prostate cancer was defined as (1) ICD-9 code (185), (2) billing code for radical prostatectomy (ICD-9 60.5 or CPT code 55810-55815, 55840-55845) plus either ADT use (in medication lists or clinical text) or clinical text evidence of prostate cancer diagnosis (Appendix Table A1), or (3) clinical text evidence of prostate cancer diagnosis and ADT use (in medication lists or clinical text). In essence, in the absence of an ICD-9 code for prostate cancer, two data points specific for prostate cancer were required. This approach was used to identify prostate cancer diagnoses not reflected in billing codes (eg, only clinical notes were available or data were from visits not resulting in billing for prostate cancer) while limiting misclassification.
The use of ADT was defined using data from clinical notes and medication lists including pharmacy orders. Specific medication names are detailed in the Appendix. Duration of ADT was calculated using time-stamp data at each determined instance of ADT use.
New-onset Alzheimer’s disease was defined using terms from clinical notes (Appendix Table A1) and ICD-9 diagnostic code 331.0. Among ADT users, incident Alzheimer’s disease was ascertained after initiation of ADT and at least 180 days after prostate cancer diagnosis to limit inclusion of prevalent disease. Among non-ADT users, incident Alzheimer’s disease was ascertained starting 180 days after the time of prostate cancer diagnosis and after the median time to ADT use in our study.
Adjustment covariates included were age at prostate cancer diagnosis; race; smoking status; use of antiplatelet, anticoagulant, antihypertensive, and statin medications; and a history of cardiovascular disease, diabetes, or malignancy. Gleason score was included as a covariate in sensitivity analysis in those patients with available data (n = 4,373). We used ICD-9 diagnostic codes, clinical text data, medication lists, and pharmacy records to define each covariate with further details outlined in Appendix Table A2. Medication use and a history of diabetes or malignancy were determined using data from 365 days before through 180 days after prostate cancer diagnosis. A history of cardiovascular disease was determined using data from 365 days before through 180 days after prostate cancer diagnosis with the exception of myocardial infarction, which used data only before prostate cancer diagnosis.
Inclusion and Exclusion Criteria
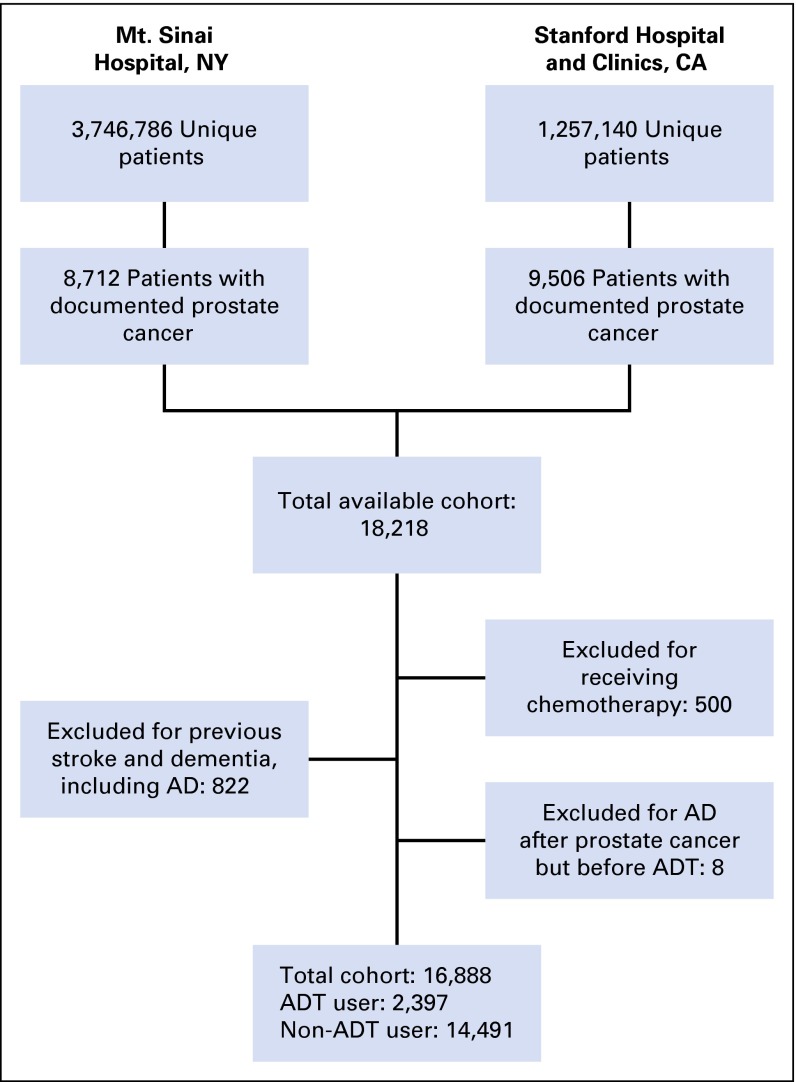
All individuals with prostate cancer and data on all defined covariates who had follow-up data for at least 180 days after prostate cancer diagnosis were eligible for study inclusion (Fig 1). Patients with exposure to ADT were only included if they had follow-up after initiation of ADT. Men who received chemotherapy were excluded, given evidence for chemotherapy-associated cognitive dysfunction and an expected high correlation between receipt of chemotherapy and ADT use.25 Those with a history of dementia, including Alzheimer’s disease, frontotemporal dementia, and Lewy body dementia, as well as stroke, were also excluded, given concern for misclassification of Alzheimer’s disease (see Appendix Table A2 for variable definition). A history of dementia was determined using data from 365 days before through 180 days after prostate cancer diagnosis to limit misclassification of prevalent disease. A history of stroke was determined using only data before prostate cancer diagnosis. Individuals who received ADT but developed Alzheimer’s disease before starting ADT were also excluded.
Fig 1.
Flow diagram of cohort selection. AD, Alzheimer’s disease; ADT, androgen deprivation therapy.
Statistical Methods
For all analyses, the start of the follow-up period was defined as either the initiation of ADT for ADT users or, for non-ADT users, the time of prostate cancer diagnosis plus the median time to ADT use in our study. The end of the follow-up period was that of the last available record, either inpatient or outpatient, or the time of Alzheimer’s disease diagnosis. Baseline patient characteristics were compared for ADT and non-ADT users using a t test or χ2 test. All included variables were binary except age.
Hazard ratios were calculated using propensity score–matched and traditional multivariable-adjusted Cox proportional hazards models to test the effect of ADT on risk of Alzheimer’s disease. Specifically, we used 1:5 nearest-neighbor propensity score matching with replacement. We then analyzed the propensity score–matched data set using weighted Cox proportional hazards models as previously described.26
Propensity score–matched analyses and traditional multivariable-adjusted Cox regression analyses were adjusted for age at prostate cancer diagnosis; race; smoking status; use of antiplatelet, anticoagulant, antihypertensive, and statin medications; and a history of cardiovascular disease, diabetes, or malignancy. We adjusted for cardiometabolic disease–associated variables and age, as they have been shown to increase Alzheimer’s disease risk27,28 and, along with prior malignancy, contribute to patients’ likelihood of receiving radiation therapy and therefore ADT, if, for example, they are poor surgical candidates. We additionally accounted for race given known racial disparities in stage at diagnosis and access to definitive cancer treatment.29,30 Further sensitivity analyses were conducted including individuals receiving chemotherapy, among only those with at least 5 years of follow-up, and using shared-frailty models to account for the matched nature of the data.
Results were compared between Stanford and Mt. Sinai. Kaplan-Meier curves were constructed to examine the cumulative probability of remaining Alzheimer’s disease–free in the full cohort and in the propensity score–matched cohort. Kaplan-Meier curves were compared in the full and propensity score–matched cohorts using log-rank and Cox regression–based tests for equality, respectively. The duration of ADT use was also tested for association with Alzheimer’s disease risk using Cox regression models. Specifically, we examined Alzheimer’s disease risk among those with less than 12 months ADT use and greater than or equal to 12 months ADT use compared with non-ADT users. We additionally conducted a test for trend of the risk of Alzheimer’s disease with increasing duration of ADT use across categories (ie, no ADT, < 12 months, ≥ 12 months). Finally, we evaluated whether an association between ADT and Alzheimer’s disease might be secondary to unmeasured physician or patient characteristics by using falsification analysis.31 Specifically, we selected five outcomes to test for association with ADT with no known or hypothesized association: anaphylaxis, rhabdomyolysis, tuberculosis, allergic rhinitis, and abdominal aortic aneurysm.
Proportional hazards assumptions were evaluated by Schoenfeld residuals tests. Tests were considered significant if the two-sided P value was less than .05. Analyses were performed using Stata version 12.0 (StataCorp, College Station, TX) and R version 3.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
There were 16,888 individuals with prostate cancer meeting all inclusion and exclusion criteria (Fig 1). Of these, 2,397 (14.2%) received ADT, with a median time from prostate cancer diagnosis to ADT use of 35.6 days. Before propensity score matching, individuals receiving ADT were statistically significantly older and more likely to be white, smokers, on antiplatelet, anticoagulant and antihypertensive medications, and have a history of cardiovascular disease and diabetes (Table 1). No statistically significant differences existed among measured baseline covariates in the propensity score–matched cohort. There were 125 new diagnoses of Alzheimer’s disease during a median follow-up period of 2.7 years (interquartile range, 1.0 to 5.4 years). The median time to Alzheimer’s disease diagnosis was 4.0 years (interquartile range, 2.0 to 7.4 years). Gleason score was available for a subset of patients (n = 4,373), of whom 58% had a Gleason score less than 7, 29% had a Gleason score of 7, and 13% had a Gleason score greater than 7 (Appendix Table A3).
Table 1.
Demographic Characteristics
| Characteristic | Full Cohort | 1:5 Propensity Score–Matched Cohort | ||||
|---|---|---|---|---|---|---|
| ADT Users (n = 2,397) | Non-ADT Users (n = 14,491) | P for Difference | ADT Users (n = 2,397) | Non-ADT Users (n = 11,985) | P for Difference | |
| Age, mean (SD), years | 70.9 (10.8) | 66.7 (10.5) | < .001 | 70.9 (10.8) | 70.9 (12.6) | .974 |
| White | 1,243 (52) | 8,426 (58) | < .001 | 1,243 (52) | 6,487 (54) | .115 |
| Ever smoker | 890 (37) | 3,420 (24) | < .001 | 890 (37) | 4,553 (38) | .539 |
| Antiplatelet medication use | 802 (33) | 3,394 (23) | < .001 | 802 (33) | 3,871 (32) | .393 |
| Anticoagulant medication use | 420 (18) | 1,885 (13) | < .001 | 420 (18) | 1,950 (16) | .248 |
| Antihypertensive medication use | 1,205 (50) | 5,775 (40) | < .001 | 1,205 (50) | 6,015 (50) | .954 |
| Statin use | 559 (23) | 3,135 (22) | .064 | 559 (23) | 2,651 (22) | .321 |
| Prior cardiovascular disease | 679 (28) | 3,072 (21) | < .001 | 679 (28) | 3,288 (27) | .491 |
| Prior diabetes | 514 (21) | 2,295 (16) | < .001 | 514 (21) | 2,499 (21) | .616 |
| Prior malignancy | 166 (7) | 1,057 (7) | .519 | 166 (7) | 679 (6) | .073 |
NOTE. Data presented as No. (%) unless otherwise noted.
Abbreviations: ADT, androgen deprivation therapy; SD, standard deviation.
Validating Known Risk Factors
Age and a history of cardiovascular disease showed statistically significant associations with Alzheimer’s disease risk using traditional multivariable Cox regression models (Table 2). The results of the falsification analyses (Appendix Table A4) showed no statistically significant associations (P > .1)
Table 2.
Traditional Multivariable Cox Regression for the Association of Covariates With Alzheimer’s Disease
| Characteristic | HR (95% CI) | P |
|---|---|---|
| Age | 1.06 (1.04 to 1.08) | < .001 |
| White | 1.30 (0.88 to 1.91) | .182 |
| Ever smoker | 1.11 (0.72 to 1.73) | .635 |
| Antiplatelet medication use | 0.68 (0.43 to 1.10) | .114 |
| Anticoagulant medication use | 0.82 (0.47 to 1.45) | .499 |
| Antihypertensive medication use | 1.46 (0.94 to 2.26) | .093 |
| Statin use | 1.54 (0.93 to 2.53) | .091 |
| History of cardiovascular disease | 1.60 (1.04 to 2.44) | .031 |
| History of diabetes | 1.45 (0.91 to 2.30) | .121 |
| History of malignancy | 1.18 (0.60 to 2.31) | .627 |
Abbreviation: HR, hazard ratio.
Association of ADT Use and Development of Alzheimer’s Disease
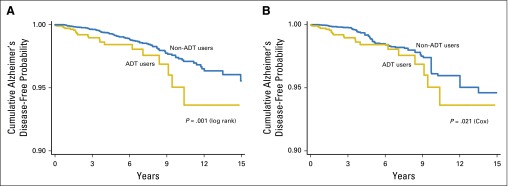
Kaplan-Meier curves demonstrated a lower cumulative probability of remaining Alzheimer’s disease–free among ADT users in the full (P = .001) and the propensity score–matched (P = .021) cohorts (Fig 2).
Fig 2.
Kaplan-Meier curves according to androgen deprivation therapy (ADT) use for the cumulative probability of remaining Alzheimer’s disease–free (y-axis) from the initiation of ADT, for ADT users, or from the time of prostate cancer diagnosis plus the median time to ADT use, for non-ADT users (x-axis), in the full (A) and propensity score–matched (B) cohorts. AD, Alzheimer’s disease; ADT, androgen deprivation therapy.
Propensity score–matched Cox regression analysis (Table 3) supported a statistically significant positive association between ADT use and Alzheimer’s disease risk (hazard ratio [HR], 1.88; 95% CI, 1.10 to 3.20; P = .021), as did traditional multivariable-adjusted Cox regression analysis (HR, 1.66; 95% CI, 1.05 to 2.64; P = .031). This association did not differ by location in the propensity score–matched Cox regression analysis (P = .700). Analyses stratified by duration of ADT use (Table 4) demonstrated that individuals with at least 12 months of ADT use had the greatest risk of subsequent Alzheimer’s disease (HR, 2.12; 95% CI, 1.11 to 4.03; P = .011). We also showed a statistically significant increased risk of Alzheimer’s disease by category of increasing ADT duration (P for trend = .016). Schoenfeld residuals tests demonstrated that the proportional hazards assumption was met for all models.
Table 3.
Propensity Score–Matched Cox Regression Analysis for the Association of ADT Use With Alzheimer’s Disease
| Exposure | HR (95% CI) | P |
|---|---|---|
| Propensity score–matched analysis | ||
| No ADT use | Ref | Ref |
| ADT use | 1.88 (1.10 to 3.20) | .021 |
| Traditional multivariable-adjusted analysis | ||
| No ADT use | Ref | Ref |
| ADT use | 1.66 (1.05 to 2.64) | .031 |
NOTE. Adjusted for age; race; smoking status; anticoagulant, antiplatelet, antihypertensive, and statin therapy; and history of cardiovascular disease, diabetes, or malignancy.
Abbreviations: ADT, androgen deprivation therapy; HR, hazard ratio; Ref, reference.
Table 4.
Propensity Score–Matched Cox Regression Analysis for the Association of ADT Use With Alzheimer’s Disease by Therapy Duration
| Duration of ADT Use (Months) | HR (95% CI) | P | P for Trend* |
|---|---|---|---|
| No ADT use | Ref | Ref | .016 |
| ADT users | |||
| < 12 months ADT use | 1.62 (0.82 to 3.21) | .165 | |
| ≥ 12 months ADT use | 2.12 (1.11 to 4.03) | .011 |
NOTE. Adjusted for age; race; smoking status; anticoagulant, antiplatelet, antihypertensive, and statin therapy; and history of cardiovascular disease, diabetes, or malignancy
Abbreviations: ADT, androgen deprivation therapy; HR, hazard ratio.
By category of ADT duration.
Results were similar in propensity score–matched analyses that included individuals who received chemotherapy (HR, 1.96; 95% CI, 1.13 to 3.40; P = .016) and when accounting for within–propensity score–matched group correlation (HR, 1.73; 95% CI, 1.09 to 2.75; P = .019). Among those with at least 5 years of follow-up we observed a consistent proportion of ADT use (15.6%) and a similar risk of Alzheimer’s disease among ADT users (HR, 1.89; 95% CI, 1.11 to 3.21; P = .018). Subgroup analysis adjusted for Gleason score demonstrated a similar magnitude of effect (HR, 1.84; 95% CI, 0.78 to 4.32; P = .161) but had significantly decreased power secondary to few patients with Alzheimer’s disease (n = 47).
DISCUSSION
We used a novel informatics approach to demonstrate an association between the use of ADT in the treatment of prostate cancer and an increased future risk of Alzheimer’s disease. We support this association using both propensity score–matched and traditional multivariable regression models adjusted for a wide range of potential confounding factors. We also show an association between greater duration of ADT use and increased risk of Alzheimer’s disease, which is significant given that length of ADT use is associated with a longer period of testosterone suppression.5 Our findings did not differ across two separate electronic medical record data sets in a large patient population consisting of a diverse group of individuals. Additionally, we support the validity of our study by showing no association between ADT and five distinct outcomes as falsification tests31 as well as replicating known associations between age and cardiovascular disease with Alzheimer’s disease. Use of the electronic medical record in this way allows rapid investigation of a rich data source to study a broad range of postmarketing outcomes, including those unlikely to be seen in smaller clinical trials.19
There are a number of plausible mechanisms to explain a neuropathic effect of androgen deficiency in the etiology of Alzheimer’s disease. Androgens have been shown to aid in neuron growth and axonal regeneration.32 Androgens also modulate β-amyloid protein accumulation, the main component of amyloid plaques.32 ADT use in the treatment of prostate cancer has been shown to result in elevated circulating β-amyloid protein levels.33 Additionally, low testosterone levels and ADT have been associated with a number of cardiometabolic derangements, including subsequent diabetes, coronary heart disease, myocardial infarction, and peripheral arterial disease.8,34-36 Interestingly, cardiometabolic diseases have been shown to increase Alzheimer’s disease risk,27,28 which we demonstrate in the current analysis with a positive association between cardiovascular disease and future Alzheimer’s disease. Furthermore, the combination of atherosclerosis and the possession of the Alzheimer’s disease risk genotype, apolipoprotein-E ε4, may interact to further increase Alzheimer’s disease risk.37
Despite these data, limited direct research has been conducted regarding the association of ADT with cognitive impairment, and existing studies have yielded mixed results. Individual analyses have shown that 47% to 69% of men receiving ADT develop deficits in at least one cognitive area characteristic of Alzheimer’s disease, including visuospatial abilities and executive functioning.11 A 2014 meta-analysis examined the literature across seven cognitive domains and found that patients receiving ADT had a statistically significant impairment of visuomotor ability compared with control participants.10 No known studies have specifically examined the association of ADT use in the treatment of prostate cancer with future Alzheimer’s disease risk.
The current study has a number of limitations that warrant discussion. Our criteria for Alzheimer’s disease were limited to billing codes and clinical note documentation. The use of billing records in the diagnosis of Alzheimer’s disease introduces a potential for bias,38 as may the incorporation of clinical text data. Even in a controlled setting, the diagnosis of Alzheimer’s disease remains clinical and susceptible to misclassification. However, data support a high degree of accuracy in the clinical diagnosis of Alzheimer’s disease compared with autopsy results.39,40 Although we cannot exclude the presence of prevalent Alzheimer’s disease in this study, we have no reason to suspect a differential misclassification that would introduce bias into our analysis.
Additionally, individuals may be more likely to receive definitive radiation therapy and ADT if they are poor surgical candidates secondary to a high number of comorbidities, such as cardiovascular disease, which are also risk factors for Alzheimer’s disease.27 We therefore adjusted for a broad range of comorbidities and conducted propensity score–matched analyses. However, given the retrospective nature of this study and the limitations of data available from the electronic medical record, we were unable to account for all possible risk factors, such as laboratory values (eg, lipids), family history, and other biometric data (eg, systolic blood pressure). Similarly, we were only able to extract Gleason score for a minority of patients; despite demonstrating a similar magnitude of effect, the analysis was underpowered, and therefore no conclusion can be drawn about the effect of disease stage based on our data. Future studies may be needed to fully account for stage of disease and to characterize the nature of this association across prostate cancer risk groups.
Finally, we calculated ADT duration from clinical notes as well as medication and pharmacy records. It is probable that some individuals received a different duration of ADT than was documented. We therefore conducted ADT duration analysis using broad categories of ADT duration (ie, greater than or less than 12 months use) to limit the potential influence of any misclassification. Additionally, misclassification of ADT duration would likely bias the significant result we observed toward the null, as compared groups would become more similar.
In conclusion, we provide support for an association between the use of ADT in the treatment of prostate cancer and an increased risk of Alzheimer’s disease in a general population cohort of 16,888 individuals with prostate cancer. Our study further demonstrates the utility of novel methods of analyzing electronic medical record data to generate practice-based evidence.20 Future prospective studies in traditional cohorts are needed to confirm this finding.
Supplementary Material
Appendix
Medication names queried to identify individuals on androgen deprivation therapy: leuprolide, goserelin, triptorelin, histrelin, degarelix, flutamide, bicalutamide, nilutamide, enzalutamide, cyproterone acetate, Lupron, Zoladex, Trelstar, Vantas, Firmagon, Eulexin, Casodex, Nilandron, Xtandi, Nizoral, Androcur, Eligard, Cyprostat, Anandron, Flutamin, Cytomid, Cosudex, Calutide, and Kalumid.
Fig A1.
Three steps of the text processing workflow: (1) A custom dictionary derived from BioPortal ontologies and supplemented with trigger terms from NegEx and ConText is used to index (recognize) disease and drug term mentions in narratives as well as recognize contextual cues such as negation or family history. Patient and temporal meta-data are also indexed in this step. The output of this step is indexed positive present mentions of drugs and conditions. (2) Specification of the definition for events (indications and outcomes) and drugs of interest along with normalization schemes for grouping and aggregating terms by synonymy or class. (3) Using the temporal information, the aggregated event and drug mentions, and contextual filters to create a patient-feature matrix and construct patient cohorts for further statistical analysis. Using the temporal information is crucial; for example, a case in which mentions of a drug temporally follows an outcome is used differently in the downstream analysis than a case where a drug precedes the outcome. NCBO, National Center for Biomedical Ontology.
Table A1.
The Concepts and Terms Defining Prostate Cancer and Alzheimer’s Disease in Clinical Text Analysis
| String | Drug/Event | Concept Unique Identifier |
|---|---|---|
| Alzheimer dementia | Alzheimer’s disease | C0002395 |
| Alzheimer disease | Alzheimer’s disease | C0002395 |
| Alzheimer disease 1 | Alzheimer’s disease | C2931257 |
| Alzheimer disease 10 | Alzheimer’s disease | C1864828 |
| Alzheimer disease 11 | Alzheimer’s disease | C1853360 |
| Alzheimer disease 12 | Alzheimer’s disease | C1970209 |
| Alzheimer disease 3 | Alzheimer’s disease | C1843013 |
| Alzheimer disease 4 | Alzheimer’s disease | C1847200 |
| Alzheimer disease 8 | Alzheimer’s disease | C1846735 |
| Alzheimer disease type 1 β | Alzheimer’s disease | C2931257 |
| Alzheimer disease type 3 | Alzheimer’s disease | C1843013 |
| Alzheimer disease type 4 | Alzheimer’s disease | C1847200 |
| Alzheimer disease, early onset | Alzheimer’s disease | C0750901 |
| Alzheimer disease, late onset | Alzheimer’s disease | C0494463 |
| Alzheimer type dementia | Alzheimer’s disease | C0002395 |
| Alzheimer’s dementia | Alzheimer’s disease | C0002395 |
| Alzheimer’s disease | Alzheimer’s disease | C0002395 |
| Alzheimer’s disease with early onset | Alzheimer’s disease | C0750901 |
| Alzheimer’s disease with late onset | Alzheimer’s disease | C0494463 |
| Alzheimers dis | Alzheimer’s disease | C0002395 |
| Dementia Alzheimer’s type | Alzheimer’s disease | C0002395 |
| Dementia in Alzheimer’s disease | Alzheimer’s disease | C0002395 |
| Dementia in Alzheimer’s disease with early onset | Alzheimer’s disease | C0750901 |
| Dementia in Alzheimer’s disease with late onset | Alzheimer’s disease | C0494463 |
| Dementia, Alzheimer type | Alzheimer’s disease | C0002395 |
| Disease, Alzheimer | Alzheimer’s disease | C0002395 |
| Disease, Alzheimer’s | Alzheimer’s disease | C0002395 |
| Early onset Alzheimer disease | Alzheimer’s disease | C0750901 |
| Late onset Alzheimer disease | Alzheimer’s disease | C0494463 |
| Senile dementia, Alzheimer type | Alzheimer’s disease | C0002395 |
| Adenocarcinoma of prostate | Prostate cancer | C0007112 |
| Adenocarcinoma of the prostate | Prostate cancer | C0007112 |
| CA - cancer of prostate | Prostate cancer | C0376358 |
| CA prostate | Prostate cancer | C0376358 |
| Cancer of prostate | Prostate cancer | C0376358 |
| Cancer of the prostate | Prostate cancer | C0376358 |
| Cancer, prostate | Prostate cancer | C0376358 |
| Cancer, prostatic | Prostate cancer | C0376358 |
| Cancers, prostate | Prostate cancer | C0376358 |
| Carcinoma of prostate | Prostate cancer | C0600139 |
| Carcinoma of the prostate | Prostate cancer | C0600139 |
| Carcinoma prostate | Prostate cancer | C0600139 |
| Carcinoma prostatic | Prostate cancer | C0600139 |
| Ductal adenocarcinoma of the prostate | Prostate cancer | C0349672 |
| Embryonal rhabdomyosarcoma of the prostate | Prostate cancer | C1335508 |
| Endometrioid carcinoma of the prostate | Prostate cancer | C0349672 |
| Hormone refractory prostate cancer | Prostate cancer | C1328504 |
| Hormone-refractory prostate cancer | Prostate cancer | C1328504 |
| Leiomyosarcoma of prostate | Prostate cancer | C1335511 |
| Leiomyosarcoma of the prostate | Prostate cancer | C1335511 |
| Malignant neoplasm of prostate | Prostate cancer | C0376358 |
| Malignant neoplasm of the prostate | Prostate cancer | C0376358 |
| Metastatic prostate cancer | Prostate cancer | C0936223 |
| Metastatic prostate carcinoma | Prostate cancer | C0936223 |
| Neuroendocrine tumor of the prostate | Prostate cancer | C1335515 |
| pN0 prostate cancer | Prostate cancer | C1709722 |
| pN1 prostate cancer | Prostate cancer | C1709723 |
| Prostate adenocarcinoma | Prostate cancer | C0007112 |
| Prostate cancer | Prostate cancer | C0376358 |
| Prostate cancer metastatic | Prostate cancer | C0936223 |
| Prostate cancer recurrent | Prostate cancer | C0278838 |
| Prostate cancer stage I | Prostate cancer | C0278834 |
| Prostate cancer stage II | Prostate cancer | C0278835 |
| Prostate cancer stage IV | Prostate cancer | C0278837 |
| Prostate cancers | Prostate cancer | C0376358 |
| Prostate carcinoma | Prostate cancer | C0600139 |
| Prostate carcinoma metastatic | Prostate cancer | C0936223 |
| Prostate ductal adenocarcinoma | Prostate cancer | C0349672 |
| Prostate embryonal rhabdomyosarcoma | Prostate cancer | C1335508 |
| Prostate rhabdomyosarcoma | Prostate cancer | C1335518 |
| Prostate sarcoma | Prostate cancer | C0238393 |
| Prostatic cancer | Prostate cancer | C0376358 |
| Prostatic cancer metastatic | Prostate cancer | C0936223 |
| Prostatic cancers | Prostate cancer | C0376358 |
| Prostatic carcinoma | Prostate cancer | C0600139 |
| pT2 prostate cancer | Prostate cancer | C1709725 |
| pT2a prostate cancer | Prostate cancer | C1709726 |
| pT2b prostate cancer | Prostate cancer | C1709727 |
| pT2c carcinoma of prostate | Prostate cancer | C1709728 |
| pT2c prostate cancer | Prostate cancer | C1709728 |
| pT3 prostate cancer | Prostate cancer | C1709729 |
| pT3b prostate cancer | Prostate cancer | C1709731 |
| Recurrent cancer of the prostate | Prostate cancer | C0278838 |
| Recurrent prostate cancer | Prostate cancer | C0278838 |
| Recurrent prostate carcinoma | Prostate cancer | C0278838 |
| Rhabdomyosarcoma of prostate | Prostate cancer | C1335518 |
| Rhabdomyosarcoma of the prostate | Prostate cancer | C1335518 |
| Sarcoma of prostate | Prostate cancer | C0238393 |
| Sarcoma of the prostate | Prostate cancer | C0238393 |
| Small cell carcinoma of the prostate | Prostate cancer | C1300585 |
| Stage I prostate cancer | Prostate cancer | C0278834 |
| Stage II prostate cancer | Prostate cancer | C0278835 |
| Stage III prostate cancer | Prostate cancer | C0278836 |
| Stage IV prostate cancer | Prostate cancer | C0278837 |
Table A2.
Variable ICD-9 Diagnostic Codes
| Term | ICD-9 Code | CPT Code | Notes |
|---|---|---|---|
| Prostate cancer | 185 | ||
| Radical prostatectomy | 60.5 | 55810-55815, 55840-55845 | |
| Alzheimer’s disease | 331.0 | ||
| Cardiovascular disease | 443.9, 440.2, 428, 413, 414 (excluding 414.1x), 412, 411, 410 | 410 and 412 (myocardial infarction) included only if before prostate cancer diagnosis | |
| Diabetes | 250; 357.2; 362.0-362.0x; 366.41 | ||
| Stroke | 430, 431, 432, 433, 434, 436 | ||
| Frontotemporal dementia | 331.1 | ||
| Lewy body dementia | 331.82 | ||
| Malignancy | 140-172; 174; 175; 179-184; 186-209.3 |
Abbreviations: CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases-9th revision.
Table A3.
Gleason Score Distribution in Available Patients (n = 4,373)
| Gleason Score | No. | % |
|---|---|---|
| 3 | 1 | 0.0 |
| 4 | 31 | 0.7 |
| 5 | 103 | 2.4 |
| 6 | 2,413 | 55.2 |
| 7 | 1,248 | 28.5 |
| 8 | 374 | 8.6 |
| 9 | 184 | 4.2 |
| 10 | 19 | 0.4 |
Table A4.
Traditional Multivariable Cox Regression for the Association of Androgen Deprivation Therapy With Negative Controls
| Outcome | HR (95% CI) | P | No.* |
|---|---|---|---|
| Anaphylaxis | 0.21 (0.03 to 1.52) | .120 | 32 |
| Rhabdomyolysis | 1.27 (0.27 to 5.90) | .764 | 14 |
| Tuberculosis | 1.30 (0.75 to 2.25) | .346 | 101 |
| Allergic rhinitis | 1.27 (0.90 to 1.80) | .178 | 290 |
| Abdominal aortic aneurysm | 0.92 (0.57 to 1.48) | .729 | 178 |
NOTE. Adjusted for age; race; smoking status; anticoagulant, antiplatelet, antihypertensive, and statin therapy; and history of cardiovascular disease, diabetes, or malignancy
Abbreviation: HR, hazard ratio.
Number of relevant failure events.
Footnotes
See accompanying editorial on page 530
Supported by Grant U54HG004028 from the National Institutes of Health for the National Center for Biomedical Ontology, Grant R01 LM011369 from the National Library of Medicine, and Grant R01 GM101430 from the National Institute of General Medical Sciences (N.H.S.).
N.H.S. is an inventor on patents owned by Stanford University that enable the use of clinical text for data-mining: Methods for Ontology based Analytics and numbers: US13/273038, US13/420402, US13/424375, and US13/424376.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Kevin T. Nead, Greg Gaskin, Cariad Chester, Nicholas J. Leeper, Nigam H. Shah
Financial support: Nigam H. Shah
Administrative support: Nigam H. Shah
Provision of study materials or patients: Nigam H. Shah
Collection and assembly of data: Kevin T. Nead, Greg Gaskin, Joel T. Dudley, Nigam H. Shah
Data analysis and interpretation: Kevin T. Nead, Samuel Swisher-McClure, Nicholas J. Leeper, Nigam H. Shah
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Androgen Deprivation Therapy and Future Alzheimer’s Disease Risk
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Kevin T. Nead
No relationship to disclose
Greg Gaskin
No relationship to disclose
Cariad Chester
No relationship to disclose
Samuel Swisher-McClure
No relationship to disclose
Joel T. Dudley
Stock or Other Ownership: LAM Therapeutics, NuMedii
Honoraria: Janssen Pharmaceuticals
Consulting or Advisory Role: LAM Therapeutics, NuMedii
Research Funding: AstraZeneca, GlaxoSmithKline, Janssen Pharmaceuticals, LEO Pharma
Patents, Royalties, Other Intellectual Property: US Patent 8700337
Travel, Accommodations, Expenses: Boehringer Ingelheim, LEO Pharma
Nicholas J. Leeper
No relationship to disclose
Nigam H. Shah
Stock or Other Ownership: Kyron, Apixio
Consulting or Advisory Role: Kyron, Apixio
REFERENCES
- 1.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 3.Shahinian VB, Kuo YF, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 4.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042–2049. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 5.Yoon FH, Gardner SL, Danjoux C, et al. Testosterone recovery after prolonged androgen suppression in patients with prostate cancer. J Urol. 2008;180:1438–1443. doi: 10.1016/j.juro.2008.06.029. discussion 1443-1444. [DOI] [PubMed] [Google Scholar]
- 6.Pickles T, Agranovich A, Berthelet E, et al. British Columbia Cancer Agency, Prostate Cohort Outcomes Initiative Testosterone recovery following prolonged adjuvant androgen ablation for prostate carcinoma. Cancer. 2002;94:362–367. doi: 10.1002/cncr.10219. [DOI] [PubMed] [Google Scholar]
- 7.Brand JS, van der Tweel I, Grobbee DE, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome: A systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40:189–207. doi: 10.1093/ije/dyq158. [DOI] [PubMed] [Google Scholar]
- 8.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 9.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 10.McGinty HL, Phillips KM, Jim HS, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: A systematic review and meta-analysis. Support Care Cancer. 2014;22:2271–2280. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson CJ, Lee JS, Gamboa MC, et al. Cognitive effects of hormone therapy in men with prostate cancer: A review. Cancer. 2008;113:1097–1106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogervorst E, Bandelow S, Combrinck M, et al. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Hogervorst E, Williams J, Budge M, et al. Serum total testosterone is lower in men with Alzheimer’s disease. Neuroendocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- 14.Moffat SD, Zonderman AB, Metter EJ, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- 15.Rosario ER, Chang L, Stanczyk FZ, et al. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292:1431–1432. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- 16.Cherrier MM, Matsumoto AM, Amory JK, et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 17.Frain L, Swanson D, Betensky R, et al. A reduced risk of Alzheimer’s disease is associated with the majority of cancers in a national cohort of veterans. Alzheimers Dement, 9:4, 2013 (suppl; abstr P617) [Google Scholar]
- 18.LePendu P, Iyer SV, Bauer-Mehren A, et al. Pharmacovigilance using clinical notes. Clin Pharmacol Ther. 2013;93:547–555. doi: 10.1038/clpt.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leeper NJ, Bauer-Mehren A, Iyer SV, et al. Practice-based evidence: Profiling the safety of cilostazol by text-mining of clinical notes. PLoS One. 2013;8:e63499. doi: 10.1371/journal.pone.0063499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole TS, Frankovich J, Iyer S, et al. Profiling risk factors for chronic uveitis in juvenile idiopathic arthritis: A new model for EHR-based research. Pediatr Rheumatol Online J. 2013;11:45. doi: 10.1186/1546-0096-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung K, LePendu P, Iyer S, et al. Functional evaluation of out-of-the-box text-mining tools for data-mining tasks. J Am Med Inform Assoc. 2015;22:121–131. doi: 10.1136/amiajnl-2014-002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu ST, Liu H, Li D, et al. Unified Medical Language System term occurrences in clinical notes: A large-scale corpus analysis. J Am Med Inform Assoc. 2012;19:e149–e156. doi: 10.1136/amiajnl-2011-000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harkema H, Dowling JN, Thornblade T, et al. ConText: An algorithm for determining negation, experiencer, and temporal status from clinical reports. J Biomed Inform. 2009;42:839–851. doi: 10.1016/j.jbi.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger JA, Tang MX, Stern Y, et al. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 29.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 30.Morton RA., Jr Racial differences in adenocarcinoma of the prostate in North American men. Urology. 1994;44:637–645. doi: 10.1016/s0090-4295(94)80196-7. [DOI] [PubMed] [Google Scholar]
- 31.Frakt AB. An observational study goes where randomized clinical trials have not. JAMA. 2015;313:1091–1092. doi: 10.1001/jama.2015.0544. [DOI] [PubMed] [Google Scholar]
- 32.Vest RS, Pike CJ. Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav. 2013;63:301–307. doi: 10.1016/j.yhbeh.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandy S, Almeida OP, Fonte J, et al. Chemical andropause and amyloid-beta peptide. JAMA. 2001;285:2195–2196. doi: 10.1001/jama.285.17.2195-a. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34:1669–1675. doi: 10.2337/dc10-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai HK, D’Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 36.Hu JC, Williams SB, O’Malley AJ, et al. Androgen-deprivation therapy for nonmetastatic prostate cancer is associated with an increased risk of peripheral arterial disease and venous thromboembolism. Eur Urol. 2012;61:1119–1128. doi: 10.1016/j.eururo.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 38.Kukull WA, Bowen JD. Dementia epidemiology. Med Clin North Am. 2002;86:573–590. doi: 10.1016/s0025-7125(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 39.Snowden JS, Thompson JC, Stopford CL, et al. The clinical diagnosis of early-onset dementias: Diagnostic accuracy and clinicopathological relationships. Brain. 2011;134:2478–2492. doi: 10.1093/brain/awr189. [DOI] [PubMed] [Google Scholar]
- 40.Beach TG, Monsell SE, Phillips LE, et al. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.