Abstract
Purpose
The current dose-finding methodology for estimating the maximum tolerated dose of investigational anticancer agents is based on the cytotoxic chemotherapy paradigm. Molecularly targeted agents (MTAs) have different toxicity profiles, which may lead to more long-lasting mild or moderate toxicities as well as to late-onset and cumulative toxicities. Several approved MTAs have been poorly tolerated during long-term administration, leading to postmarketing dose optimization studies to re-evaluate the optimal treatment dose. Using data from completed bortezomib dose-finding trials, we explore its toxicity profile, optimize its dose, and examine the appropriateness of current designs for identifying an optimal dose.
Patients and Methods
We classified the toxicities captured from 481 patients in 14 bortezomib dose-finding studies conducted through the National Cancer Institute Cancer Therapy Evaluation Program, computed the incidence of late-onset toxicities, and compared the incidence of dose-limiting toxicities (DLTs) among groups of patients receiving different doses of bortezomib.
Results
A total of 13,008 toxicities were captured: 46% of patients’ first DLTs and 88% of dose reductions or discontinuations of treatment because of toxicity were observed after the first cycle. Moreover, for the approved dose of 1.3 mg/m2, the estimated cumulative incidence of DLT was > 50%, and the estimated cumulative incidence of dose reduction or treatment discontinuation because of toxicity was nearly 40%.
Conclusions
When considering the entire course of treatment, the approved bortezomib dose exceeds the conventional ceiling DLT rate of 20% to 33%. Retrospective analysis of trial data provides an opportunity for dose optimization of MTAs. Future dose-finding studies of MTAs should take into account late-onset toxicities to ensure that a tolerable dose is identified for future efficacy and comparative trials.
INTRODUCTION
In dose-finding cancer clinical trials, toxicities are assessed after each cycle of treatment using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE).1 Conventionally, only dose-limiting toxicities (DLTs) observed in the first cycle of treatment are considered for dose escalation decision making and estimation of the maximum tolerated dose (MTD). These assumptions and the current dose-finding methodology for estimating the MTD are based on the chemotherapy paradigm of cell cytotoxicity causing acute toxicity in the first cycle.2 However, these assumptions may not apply to the toxicity profile of molecularly targeted agents (MTAs) and immunotherapies for which the mechanism of action normally involves inhibition of a signaling pathway that may not cause acute toxicity during the first treatment cycle. Although newer noncytotoxic therapies have been thought to cause fewer and milder toxicities, not all are well tolerated by patients.3,4 The extended periods over which these therapies are intended to be administered increase the importance of late-onset toxicities, long-lasting mild or moderate toxicities, and cumulative toxicities.5
Several recently approved MTAs have been reported to be poorly tolerated by patients, leading to postmarketing dose optimization studies to re-evaluate the optimal dose.6 To address the need for better-designed trials, in May 2015, the Food and Drug Administration and the American Association of Cancer Research convened a meeting of researchers entitled, “Dose-finding of Small Molecule Oncology Drugs,” to evaluate alternative methods to optimize and improve dose finding for MTAs. Several case studies of MTAs approved at nonoptimal doses were presented at the meeting, indicating that case studies can help better illustrate the aspects of existing oncology drug development methods and designs that can be improved.
A recent review of the definition of DLT in a sample of 155 trials of MTAs reported that the median DLT assessment period was 28 days, with a range of 7 to 56 days.7 Although restricting attention to the first cycle may be adequate for agents for which toxicities generally happen shortly after the start of treatment, recent literature suggests that this practice may be insufficient for MTAs. In a study of 54 trials of MTAs, with 35 different agents, approximately one half of patients with a grade 3 or higher toxicity first presented with these toxicities after the first cycle, suggesting that the DLT assessment period should extend beyond the first cycle.8 A separate retrospective analysis of phase I trials of MTAs found that 57% of grade 3 or higher toxicities occurred after cycle 1.2 Moreover, 54% of corresponding authors of published phase I trials recommended using toxicities across all cycles of treatment to define a tolerable dose for MTAs.9
Previous studies of the toxicity profiles of MTAs have included a variety of MTAs with different toxicity profiles. Here, we examine the toxicity profile of one MTA, bortezomib, a reversible inhibitor of the ubiquitin-proteasome pathway poorly tolerated in the clinical setting, in the hope that we can optimize its dose by identifying a more tolerable dose that does not exceed the conventional DLT rate when administered over an extended number of cycles. We review and reanalyze the toxicities reported in 14 dose-finding bortezomib trials conducted through the NCI Cancer Therapy Evaluation Program between 1999 and 2008. The studies are useful for examining whether assessing toxicities beyond the first cycle is warranted, whether the currently approved dose for bortezomib is appropriate, and whether current trial designs are adequate for MTAs.
PATIENTS AND METHODS
Patient and Trial Characteristics
This study is a secondary analysis of toxicity data from 14 bortezomib dose-finding trials; a total of 481 patients were administered bortezomib (range, 13 to 62 across studies). Five of the trials were bortezomib-only trials. In the others, bortezomib was administered in combination with either chemotherapy (n = 8) or another MTA (n = 1). Three trials were for hematologic malignancies, six were for solid tumors, and five were for either hematologic malignancies or solid tumors. The study designs, patient characteristics, and results of all but one10 of these studies have been published. Summary details of these studies are presented in Tables 1 and 2. Patients were treated at doses ranging from 0.25 mg/m2 to 2.0 mg/m2. Cycle length varied from 2 to 6 weeks. The most common cycle length was 3 weeks, with eight trials following the currently approved dosing schedule for bortezomib, with dose administrations on days 1, 4, 8, and 11 of each 3-week cycle. The median number of treatment cycles patients received before treatment discontinuation ranged from 1 to 4. The most common reason for discontinuation of treatment was disease progression, followed by toxicity.
Table 1.
Summary Description of the 14 Bortezomib Dose-Finding Trials
| First Author | Protocol ID | No. of Patients Administered Bortezomib | Drug | Bortezomib Dose* (No. of patients started at each dose) | Median Follow-Up (No. of cycles) | Cycle Length (weeks) |
|---|---|---|---|---|---|---|
| Cortes11 | 94 | 15 | Bortezomib | 0.75, 1.25, 1.5 (3, 7, 5) | 1 | 6 |
| Walker12 | 6520 | 13 | Bortezomib 17-AAG | 0.7 (13) | 1 | 3 |
| LoRusso13 | 6432 | 61 | Bortezomib | 0.5, 0.7, 1.0, 1.3 (5, 15, 15, 26) | 1 | 3 |
| Leal14 | 5874 | 62 | Bortezomib | 0.7, 1.0, 1.3, 1.5 (10, 8, 43, 1) | 2 | 3 |
| Dy15 | T99-0071 | 52 | Bortezomib | 0.5, 0.9, 1.25, 1.5, 1.6, 1.7 (4, 4, 5, 24, 8, 7) | 2 | 3 or 6 |
| Iqbal10 | T99-0048 | 35 | Bortezomib Fluorouracil Leucovorin | 0.5, 0.7, 1.0, 1.3 (2, 7, 15, 11) | 2 | 6 |
| Messersmith16 | 1858 | 14 | Bortezomib Docetaxel | 0.6, 0.8, 1.0 (1, 6, 7) | 2 | 3 |
| LoConte17 | 3771 | 26 | Bortezomib Docorubicin | 1.0, 1.3, 1.5 (4, 13, 9) | 2 | 3 |
| Aghajanian18 | 5326 | 15 | Bortezomib Carboplatin | 0.75, 1.0, 1.3, 1.5 (3, 3, 7, 2) | 3 | 3 |
| Ma19 | 1860 | 53 | Bortezomib Paclitaxel Carboplatin | 0.7, 0.9, 1.2, 1.5 (6, 6, 38, 3) | 3 | 3 |
| Ramaswamy20 | 1857 | 45 | Bortezomib Paclitaxel | 0.6, 0.8, 1.2, 1.6, 1.8, 2.0 (10, 12, 3, 8, 9, 3) | 3 | 3 |
| Hamilton21 | T99-0047 | 40 | Bortezomib | 0.25, 0.8, 1.0, 1.2, 1.45, 1.75,1.9 (3, 3, 3, 6, 5, 13, 7) | 4 | 2 |
| Davies22 | 5856 | 26 | Bortezomib Gemcitabine Carboplatin | 1.0, 1.3 (19, 7) | 4 | 3 |
| Barr23 | 6126 | 24 | Bortezomib Fludarabine Rituximab | 0.7, 1.0, 1.3 (3, 6, 15) | 4 | 3 |
Bortezomib dose in milligrams per meter squared.
Table 2.
Patient Characteristics of the 14 Bortezomib Dose-Finding Trials
| First Author | Protocol ID | Female (%) | Age (years) | Performance Status (% ≥ 2 or ≤ 60)† | Body Mass Index (kg/m2) | Patient Population Included |
|---|---|---|---|---|---|---|
| Cortes11 | 94 | 40 | 59 (18-71) | 21 | 25.82 (19.22-40.00) | Refractory or relapsed AML, ALL or MDS |
| Walker12 | 6520* | 46 | 61 (42-76) | 0 | 26.72 (19.40-42.22) | Refractory or relapsed AML |
| LoRusso13 | 6432* | 48 | 62 (30-85) | 30 | 24.93 (13.53-42.67) | Advanced solid tumors, non-Hodgkin lymphoma or hepatocellular carcinoma |
| Leal14 | 5874* | 40 | 62.50 (42-86) | 11 | 26.36 (14.64-45.20) | Advanced solid tumors, non-Hodgkin lymphoma or multiple myeloma |
| Dy15 | T99-0071* | 40 | 62 (32-83) | 6 | 27.17 (19.53-40.89) | Advanced solid tumors, non-Hodgkin lymphoma or multiple myeloma |
| Iqbal10 | T99-0048 | 49 | 59 (39-84) | 11 | 26.26 (16.02-38.22) | Advanced solid tumors |
| Messersmith16 | 1858 | 29 | 64 (40-76) | 0 | 27.85 (17.81-34.60) | Advanced solid tumors |
| LoConte17 | 3771* | 27 | 65 (37-79) | 4 | 28.85 (22.40-43.02) | Advanced solid tumors or lymphoma |
| Aghajanian18 | 5326* | 100 | 53 (33-68) | 0 | 27.02 (19.60-47.75) | Recurrent or progressive epithelial ovarian or primary peritoneal carcinoma |
| Ma19 | 1860 | 47 | 54 (23-76) | 6 | 25.06 (15.64-38.22) | Metastatic or locally advanced solid tumors |
| Ramaswamy20 | 1857 | 56 | 57 (36-79) | 13 | 26.40 (15.81-55.99) | Metastatic or locally advanced solid tumors |
| Hamilton21 | T99-0047 | 40 | 58 (25-78) | 5 | 25.82 (19.15-39.30) | Advanced solid tumors or lymphoma |
| Davies22 | 5856* | 44 | 59 (34-75) | 19 | 27.78 (20.35-36.76) | Advanced or recurrent non–small-cell lung cancer |
| Barr23 | 6126* | 42 | 63 (36-86) | 4 | 27.95 (23.16-42.26) | Relapsed and refractory non-Hodgkin lymphoma |
NOTE. Data are presented as median (range) unless indicated otherwise.
Dose-finding trials on the approved dosing schedule of days 1, 4, 8, and 11 of each 21-day cycle.
Percentage of patients in each trial with either Eastern Cooperative Oncology Group performance status ≥ 2 or Karnofsky performance score ≤ 60.
Toxicity Data and Statistical Analysis
Toxicity data for the 14 trials were captured using the CTCAE (versions 2 and 3), in the form of descriptions and, for most toxicity events, codes. These descriptions were used to resolve all the toxicities to match terms in the CTCAE (version 4). Codes were used as necessary to resolve ambiguities in descriptions.
We defined a DLT as any grade 3 or higher nonhematologic toxicity or any grade 4 or higher hematologic toxicity, in each case at least possibly related to treatment. For this purpose, decreased white blood cells, platelets, neutrophils, or lymphocytes, other blood and lymphatic system disorders, anemia, febrile neutropenia, coagulation, and hemorrhage were treated as hematologic toxicities. To track the treatment-related consequences of DLTs, we also evaluated dose reductions or treatment discontinuations because of toxicity. Finally, we also evaluated protocol-reported DLTs, even though the definitions of DLT are heterogeneous among the 14 trials. In some trials, a toxicity had to be probably or definitely related to treatment to be considered a DLT,13 whereas in others, a toxicity could also possibly be related to treatment to be considered a DLT.22 In addition, some studies considered few hematologic toxicities to be DLTs,11 whereas others considered all grade 4 hematologic toxicities to be DLTs.18 This is to be expected, given that trials for patients with hematologic malignancies and solid tumors were included.
To examine the impact of the DLT assessment time frame on the incidence of DLTs, the proportion of patients receiving bortezomib who had at least one DLT was calculated on the basis of toxicities from cycle 1 only, cycles 1 and 2, and across all cycles, on an aggregate basis across all 14 trials. We also computed, for each patient, the cycle in which the patient first experienced a treatment-related toxicity of the highest grade across all cycles.
For the trials that used the currently approved dosing schedule (days 1, 4, 8, and 11 of each 21-day cycle), we computed cumulative incidence curves for the first DLT, dose reduction, or treatment discontinuation because of toxicity and a protocol-reported DLT, as a function of the cycle of treatment, treating discontinuation because of progression or death as a competing risk and treating other events as censoring events. Cumulative incidence curves were computed for four groups of patients with similar initial bortezomib doses (0.5 to 0.75 mg/m2, 1.0 mg/m2, 1.3 mg/m2, and 1.5 to 1.7 mg/m2). The approved schedule was used to ensure comparability among the dose groups. Two hundred fifty-four patients in eight different trials were treated with this dosing schedule. The etm package in R was used to compute cumulative incidence functions and to compute pointwise CIs for the cumulative incidence functions using the Greenwood-type estimator of the variance.24 We estimated the progression-free survival function using the Kaplan-Meier estimator, again stratifying by initial bortezomib dose. Cox proportional hazards models were used to examine the effect of dose on toxicity after adjusting for baseline patient characteristics.
RESULTS
Toxicity data for the 14 trials were available for 13,008 toxicity events. For 12,352 of these toxicity events, CTCAE codes from various versions of the CTCAE were available; for all the events, descriptions were available. On an aggregate basis, 61% of patients had a treatment-related toxicity of their highest grade across all cycles in cycle 1 (range, 13% to 100% across studies), and 87% of patients had a treatment-related toxicity of their highest grade across all cycles in the first two cycles (range, 75% to 100% across studies).
On an aggregate basis, 101 patients (21%) had a DLT in the first cycle, 156 patients (32%) had a DLT in the first or second cycles, and 188 patients (39%) had a DLT across all cycles. Twelve patients (2%) had a dose reduction or treatment discontinuation because of toxicity in the first cycle, 52 patients (11%) in the second cycle, and 97 patients (20%) across all cycles. Fifty-five patients (11%) had a protocol-reported DLT in the first cycle, 67 (14%) in the second cycle, and 74 (15%) in any cycle.
The incidence of both DLTs and dose reductions or treatment discontinuations because of toxicity is high after the first cycle: 46% of all patients’ first DLTs were observed after the first cycle, and 88% of all dose reductions or treatment discontinuations because of toxicity were observed after the first cycle. The lack of a substantial incidence of protocol-reported DLTs after the first cycle may have been a result, in large part, of the protocol-defined DLT assessment period normally including only the first cycle,13-15 so that protocol-reported DLTs were often not recorded after the first cycle. There is evidence for this in inconsistencies between the recording of discontinuations because of toxicity and protocol-reported DLTs. For example, of the 26 patients who were recorded as discontinuing treatment because of toxicity in the first cycle, 10 (38%) had a protocol-reported DLT in the first cycle, eight had grade 1 or 2 treatment-related toxicities that resulted in treatment discontinuation because of intolerability, two had no treatment-related toxicities but had grade 3 or 4 non–treatment-related toxicities that led to discontinuation, and six had grade 3 or 4 treatment-related toxicities that were not reported as DLTs (three hematologic, three nonhematologic). There is additional evidence to suggest the lack of recording of protocol-reported DLTs after the first cycle on the basis of the higher degree of overlap between DLTs (as defined here) and protocol-reported DLTs in the first cycle as compared with after the first cycle. Of the 195 DLTs in the first cycle (some patients had more than one), 43 (22%) were also protocol-reported DLTs, whereas of the 208 DLTs after the first cycle, only 23 (11%) were also protocol-reported DLTs.
The 10 most common DLTs in the first cycle, in the second cycle, and after the second cycle are listed in Table 3. Consistent with previous findings,25 peripheral sensory neuropathy is an important toxicity for bortezomib, especially after the second cycle. Other toxicities that are noteworthy in all cycles of treatment are fatigue, diarrhea, and decreased neutrophil count.
Table 3.
Ten Most Reported DLTs* in Cycles 1 and 2 and After Cycle 2 Among Patients Receiving One, Two, or More Than Two Cycles of Treatment
| Rank | Cycle 1 | No. of Patients (n = 481) | Cycle 2 | No. of Patients (n = 341) | After Cycle 2 | No. of Patients (n = 186) |
|---|---|---|---|---|---|---|
| 1 | Fatigue | 23 | Fatigue | 25 | Diarrhea | 13 |
| 2 | Neutrophil count decreased | 16 | Diarrhea | 10 | Fatigue | 13 |
| 3 | Diarrhea | 14 | Neutrophil count decreased | 10 | Neutrophil count decreased | 7 |
| 4 | Platelet count decreased | 12 | WBC count decreased | 6 | Peripheral sensory neuropathy | 6 |
| 5 | WBC count decreased | 9 | Hypotension | 5 | WBC count decreased | 4 |
| 6 | Hypotension | 8 | Nausea | 5 | Hyperglycemia | 3 |
| 7 | Peripheral sensory neuropathy | 7 | Dyspnea | 4 | Vomiting | 3 |
| 8 | Hyperglycemia | 6 | Peripheral sensory neuropathy | 4 | Abdominal pain | 2 |
| 9 | Dehydration | 5 | Rash maculopapular | 4 | Constipation | 2 |
| 10 | Dyspnea | 5 | Syncope | 4 | Dizziness | 2 |
Abbreviation: DLT, dose-limiting toxicity.
Defined as any grade 3 or higher nonhematologic toxicity or grade 4 or higher hematologic toxicity, in each case at least possibly related to treatment.
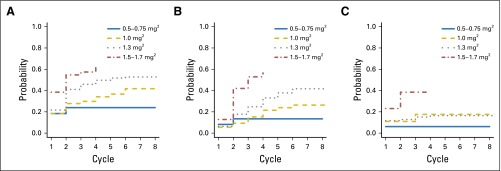
Figure 1 illustrates cumulative incidence functions for first DLT (Fig 1A), first dose reduction or treatment discontinuation because of toxicity (Fig 1B), and first protocol-reported DLT (Fig 1C), stratified by dose category. The cumulative incidence of experiencing a first DLT, dose reduction, or discontinuation because of toxicity or a protocol-reported DLT is higher for the higher dose levels in all cycles, as expected. The cumulative incidence of protocol-reported DLT does not change substantially after the first few cycles, because few additional protocol-reported DLT events are recorded after the first cycle.
Fig 1.
Cumulative incidence curves of (A) dose-limiting toxicity (any grade 3 or higher nonhematologic toxicity or any grade 4 or higher hematologic toxicity, in each case at least possibly related to treatment), (B) dose reduction or treatment discontinuation because of toxicity, and (C) protocol-reported dose-limiting toxicity up to cycle 8, stratified by dose category.
Point estimates and CIs for the cumulative incidence of DLTs and dose reductions or treatment discontinuations because of toxicity at one, two, and five cycles are displayed in Table 4. For the approved bortezomib dose of 1.3 mg/m2, the cumulative incidence of DLT at five cycles is 0.52 (95% CI, 0.43 to 0.61), and the cumulative incidence of dose reduction or treatment discontinuation because of toxicity at five cycles is 0.38 (95% CI, 0.29 to 0.48). For the bortezomib dose of 1.0 mg/m2, the cumulative incidence of DLT at five cycles is 0.37 (95% CI, 0.25 to 0.51), and the cumulative incidence of dose reduction or treatment discontinuation because of toxicity at five cycles is 0.24 (95% CI, 0.14 to 0.38). The hazard for all three toxicity outcomes increased with dose even after adjusting for age, sex, performance status, and body mass index.
Table 4.
Cumulative Incidence of DLT* and Dose Reduction or Treatment Discontinuation Because of Toxicity for Four Different Bortezomib Dose Ranges at One, Two, and Five Cycles
| Dose Range | ||||
|---|---|---|---|---|
| 0.5-0.75 mg/m2 | 1.0 mg/m2 | 1.3 mg/m2 | 1.5-1.7 mg/m2 | |
| DLT, cycle | ||||
| 1 | 0.18 (0.10 to 0.32) | 0.18 (0.10 to 0.31) | 0.22 (0.15 to 0.30) | 0.38 (0.25 to 0.55) |
| 2 | 0.24 (0.14 to 0.39) | 0.28 (0.18 to 0.42) | 0.41 (0.32 to 0.51) | 0.55 (0.40 to 0.71) |
| 5 | 0.24 (0.14 to 0.39) | 0.37 (0.25 to 0.51) | 0.52 (0.43 to 0.61) | 0.63 (0.48 to 0.78) |
| Dose reduction or treatment discontinuation because of toxicity, cycle | ||||
| 1 | 0.08 (0.03 to 0.20) | 0.05 (0.02 to 0.16) | 0.06 (0.03 to 0.13) | 0.13 (0.06 to 0.28) |
| 2 | 0.13 (0.06 to 0.28) | 0.09 (0.04 to 0.21) | 0.18 (0.12 to 0.26) | 0.42 (0.28 to 0.60) |
| 5 | 0.13 (0.06 to 0.28) | 0.24 (0.14 to 0.38) | 0.38 (0.29 to 0.48) | 0.57 (0.41 to 0.73) |
NOTE. Data are presented as cumulative incidence (95% CI).
Abbreviation: DLT, dose-limiting toxicity.
Any grade 3 or higher nonhematologic toxicity or any grade 4 or higher hematologic toxicity, in each case at least possibly related to treatment.
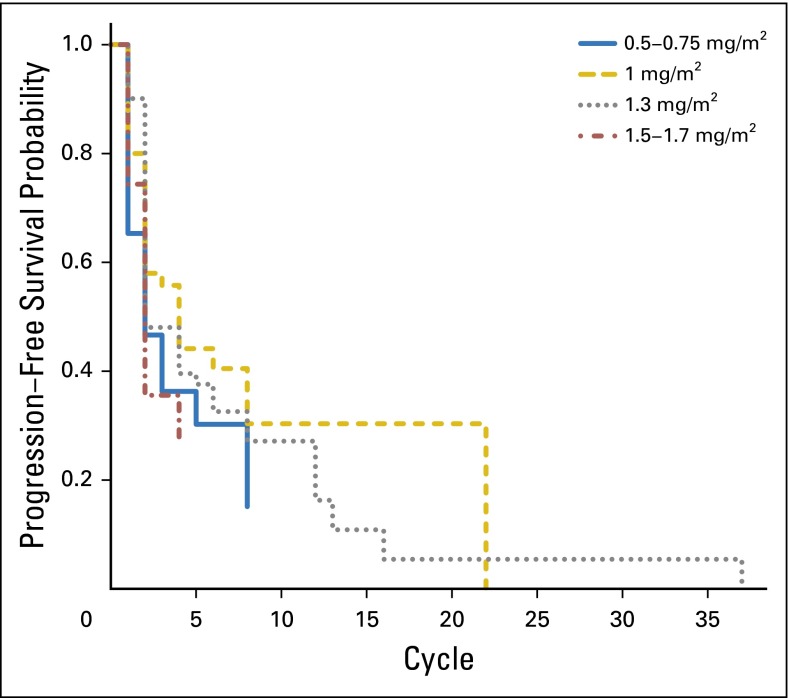
Figure 2 illustrates Kaplan-Meier estimates of progression-free survival, stratified by initial bortezomib dose. Although the survival curve for the bortezomib dose of 1.0 mg/m2 is generally the highest, the survival curves for the four doses are not significantly different from one another (P = .071 by the log-rank test).
Fig 2.
Kaplan-Meier estimates of progression-free survival functions, stratified by dose category.
DISCUSSION
Our analysis of toxicity data from 14 bortezomib dose-finding trials suggests that undercounting of DLTs occurs when the DLT assessment period is limited to cycle 1. This undercounting of toxicity may have led to an approved bortezomib dose that resulted in a higher than expected overall toxicity rate. On the basis of our analysis, with the approved dose of 1.3 mg/m2, the estimated cumulative incidence over five cycles of grade 3 or higher nonhematologic and grade 4 or higher hematologic toxicity is > 50%, and the estimated cumulative incidence of dose reduction or treatment discontinuation because of toxicity is nearly 40%. These rates exceed the conventional ceiling DLT rate of 20% to 33%.26 The estimated cumulative incidence functions suggest that a dose of 1.0 mg/m2 may be more appropriate, because with the lower dose, the cumulative incidence of toxicity is reduced, whereas the risk of disease progression seems unchanged. The lack of difference in disease progression should be interpreted with caution, given that the population participating in phase I trials may not accurately reflect changes in the rates of disease progression that may be associated with the lower doses.
As demonstrated here, using methods that incorporate only toxicities in the first one or two cycles may result in recommended doses that are, in hindsight, too high, when excessive late-onset toxicities are present. Thus, future dose-finding studies of MTAs and immunotherapies, which have a different toxicity profile from that of conventional cytotoxic chemotherapies, may benefit from assessing toxicities across the entire course of treatment and incorporating this longer toxicity profile into dose escalation decisions and the estimation of the MTD. Even if trials use the 3+3 design and dose escalation decisions are based on the first cycle, the estimation of the MTD can incorporate toxicities across all cycles in a longitudinal or time-to-event approach. Moreover, methods such as the time-to-event continual reassessment method, which takes into account toxicities arising over the entire course of treatment, could provide a better estimate of tolerable MTA doses for long-term treatment.27 The time-to-event continual reassessment method in combination with targeted agents has been recommended by the NCI Radiation Therapy Oncology Group for use in phase I trials of radiotherapy to improve the design of these trials with late-onset toxicities.28 To use these methods, protocol-defined DLTs will need to incorporate toxicities beyond the first one or two cycles of treatment and perhaps will need to be extended through all cycles of treatment. To make full use of these designs, patients will have to be observed for longer than one or two cycles to evaluate the impact of late-onset and cumulative toxicities. Given the advanced stage of disease of patients enrolled in phase I studies, with more than one half of these trials having a median follow-up of two cycles or fewer, consideration should also be given to alternative study designs that recommend several doses for phase II, or randomized phase II, studies with different dosing regimens, to evaluate the safety and efficacy of a drug with a longer follow-up and in a population that is more similar to the target population. This is particularly important given that, in the context of MTAs and immunotherapies, higher doses may not necessarily imply higher efficacy. Furthermore, in this study, two of the DLTs (fatigue and peripheral neuropathy) have subjective components. Consideration should be given to the need to assess the aggregate effects of mild toxicities over extended periods of time, as well as to the inclusion of selected patient-reported subjective adverse effects, because clinical investigators may underreport these low-grade adverse effects.29,30
To remedy the shortcoming that protocol-defined DLTs were captured generally only in the first cycle, we used a definition of DLT that mirrors traditional definitions but does not take into account certain limitations of the DLT definition. For example, in some of the trials included here, grade 4 vomiting and diarrhea were not considered DLTs if they were well controlled with medication. Other trials excluded certain hematologic conditions from the DLT definition.12,14 This may account for some of the discrepancy between the incidence of DLTs as defined here and protocol-reported DLTs. To remedy this defect, we also used dose reductions or treatment discontinuations because of toxicity, which tracks two of the consequence of a DLT, but which ignores another of the possible consequences of a DLT, a delayed or skipped dose. Thus, the three measures we used, although supported by the literature,8 may lead to overestimation (which is likely for our definition of DLT) or underestimation (which is likely for dose reductions or treatment discontinuations because of toxicity) when compared with the actual rate of DLTs. However, the relevant findings here were consistent for both our definition of DLT and for dose reductions or treatment discontinuations because of toxicity, because a substantial portion of both events occurred after the first cycle.
This analysis has other potential limitations. There are confounding factors, such as single agent versus combination therapy, that have not been accounted for in our analysis because of the small number of trials. Moreover, because this study focused on a single MTA, its conclusions may not be generalizable to other MTAs. However, this and similar analyses are useful for suggesting dose revisions to already approved drugs that are poorly tolerated in the clinical setting.
Our case example using bortezomib suggests that as new anticancer therapies are developed, it is essential to consider alternative designs to ensure that tolerable doses are identified early for evaluation in future efficacy trials. It also suggests that dose reductions are not common occurrences in clinical settings. If recommended doses for MTAs from phase I studies are not well tolerated over time, and if approved doses have to be reduced in a large percentage of patients, we are left with a limited understanding of the decrement in efficacy caused by dose reduction.
Footnotes
Supported in part by Grant No. MRSG-13-146-01-CPHPS from the American Cancer Society and by Grant No. UL1 TR000040 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Shing M. Lee, Ying Kuen Ken Cheung, Dawn L. Hershman, Percy Ivy, Lori Minasian
Financial support: Shing M. Lee
Collection and assembly of data: Diana Vulih, Percy Ivy
Data analysis and interpretation: Shing M. Lee, Daniel Backenroth, Ying Kuen Ken Cheung, Dawn L. Hershman, Barry Anderson, Percy Ivy, Lori Minasian
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Case Example of Dose Optimization Using Data From Bortezomib Dose-Finding Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Shing M. Lee
No relationship to disclose
Daniel Backenroth
No relationship to disclose
Ying Kuen Ken Cheung
Consulting or Advisory Role: The Remedy Pharm
Dawn L. Hershman
No relationship to disclose
Diana Vulih
Employment: Theradex Systems
Barry Anderson
Employment: Theradex Systems
Percy Ivy
No relationship to disclose
Lori Minasian
No relationship to disclose
REFERENCES
- 1.National Cancer Institute CTEP . Common Terminology Criteria for Adverse Events. Bethesda, MD: National Cancer Institute CTEP; 2009. (CTCAE) [Google Scholar]
- 2.Postel-Vinay S, Gomez-Roca C, Molife LR, et al. Phase I trials of molecularly targeted agents: Should we pay more attention to late toxicities? J Clin Oncol. 2011;29:1728–1735. doi: 10.1200/JCO.2010.31.9236. [DOI] [PubMed] [Google Scholar]
- 3.Niraula S, Seruga B, Ocana A, et al. The price we pay for progress: A meta-analysis of harms of newly approved anticancer drugs. J Clin Oncol. 2012;30:3012–3019. doi: 10.1200/JCO.2011.40.3824. [DOI] [PubMed] [Google Scholar]
- 4.Niraula S, Amir E, Vera-Badillo F, et al. Risk of incremental toxicities and associated costs of new anticancer drugs: A meta-analysis. J Clin Oncol. 2014;32:3634–3642. doi: 10.1200/JCO.2014.55.8437. [DOI] [PubMed] [Google Scholar]
- 5.Soria JC. Phase 1 trials of molecular targeted therapies: Are we evaluating toxicities properly? Eur J Cancer. 2011;47:1443–1445. doi: 10.1016/j.ejca.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Minasian L, Rosen O, Auclair D, et al. Optimizing dosing of oncology drugs. Clin Pharmacol Ther. 2014;96:572–579. doi: 10.1038/clpt.2014.153. [DOI] [PubMed] [Google Scholar]
- 7.Le Tourneau C, Razak AR, Gan HK, et al. Heterogeneity in the definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents: A review of the literature. Eur J Cancer. 2011;47:1468–1475. doi: 10.1016/j.ejca.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Postel-Vinay S, Collette L, Paoletti X, et al. Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents: Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group for Early Trials of Targeted Therapies, an European Organisation for research and treatment of cancer-led study. Eur J Cancer. 2014;50:2040–2049. doi: 10.1016/j.ejca.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Paoletti X, Le Tourneau C, Verweij J, et al. Defining dose-limiting toxicity for phase 1 trials of molecularly targeted agents: Results of a DLT-TARGETT international survey. Eur J Cancer. 2014;50:2050–2056. doi: 10.1016/j.ejca.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal S, Cole S, Yang D, et al. 2004. Phase I study of PS-341 (bortezomib) with 5-fluorouracil/leucovorin (5-FU/LV) in advanced solid tumors: A California Cancer Consortium study. J Clin Oncol (Meeting Abstracts) 22:2057, 2004. [Google Scholar]
- 11.Cortes J, Thomas D, Koller C, et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10:3371–3376. doi: 10.1158/1078-0432.CCR-03-0508. [Erratum: Clin Cancer Res 10:7787, 2004] [DOI] [PubMed] [Google Scholar]
- 12.Walker AR, Klisovic R, Johnston JS, et al. Pharmacokinetics and dose escalation of the heat shock protein inhibitor 17-allyamino-17-demethoxygeldanamycin in combination with bortezomib in relapsed or refractory acute myeloid leukemia. Leuk Lymphoma. 2013;54:1996–2002. doi: 10.3109/10428194.2012.760733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LoRusso PM, Venkatakrishnan K, Ramanathan RK, et al. Pharmacokinetics and safety of bortezomib in patients with advanced malignancies and varying degrees of liver dysfunction: Phase I NCI Organ Dysfunction Working Group Study NCI-6432. Clin Cancer Res. 2012;18:2954–2963. doi: 10.1158/1078-0432.CCR-11-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leal TB, Remick SC, Takimoto CH, et al. Dose-escalating and pharmacological study of bortezomib in adult cancer patients with impaired renal function: A National Cancer Institute Organ Dysfunction Working Group Study. Cancer Chemother Pharmacol. 2011;68:1439–1447. doi: 10.1007/s00280-011-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dy GK, Thomas JP, Wilding G, et al. A phase I and pharmacologic trial of two schedules of the proteasome inhibitor, PS-341 (bortezomib, velcade), in patients with advanced cancer. Clin Cancer Res. 2005;11:3410–3416. doi: 10.1158/1078-0432.CCR-04-2068. [DOI] [PubMed] [Google Scholar]
- 16.Messersmith WA, Baker SD, Lassiter L, et al. Phase I trial of bortezomib in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res. 2006;12:1270–1275. doi: 10.1158/1078-0432.CCR-05-1942. [DOI] [PubMed] [Google Scholar]
- 17.LoConte NK, Thomas JP, Alberti D, et al. A phase I pharmacodynamic trial of bortezomib in combination with doxorubicin in patients with advanced cancer. Cancer Chemother Pharmacol. 2008;63:109–115. doi: 10.1007/s00280-008-0719-5. [DOI] [PubMed] [Google Scholar]
- 18.Aghajanian C, Dizon DS, Sabbatini P, et al. Phase I trial of bortezomib and carboplatin in recurrent ovarian or primary peritoneal cancer. J Clin Oncol. 2005;23:5943–5949. doi: 10.1200/JCO.2005.16.006. [DOI] [PubMed] [Google Scholar]
- 19.Ma C, Mandrekar SJ, Alberts SR, et al. A phase I and pharmacologic study of sequences of the proteasome inhibitor, bortezomib (PS-341, Velcade), in combination with paclitaxel and carboplatin in patients with advanced malignancies. Cancer Chemother Pharmacol. 2007;59:207–215. doi: 10.1007/s00280-006-0259-9. [DOI] [PubMed] [Google Scholar]
- 20.Ramaswamy B, Bekaii-Saab T, Schaaf LJ, et al. A dose-finding and pharmacodynamic study of bortezomib in combination with weekly paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2010;66:151–158. doi: 10.1007/s00280-009-1145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton AL, Eder JP, Pavlick AC, et al. Proteasome inhibition with bortezomib (PS-341): A phase I study with pharmacodynamic end points using a day 1 and day 4 schedule in a 14-day cycle. J Clin Oncol. 2005;23:6107–6116. doi: 10.1200/JCO.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 22.Davies AM, Ruel C, Lara PN, et al. The proteasome inhibitor bortezomib in combination with gemcitabine and carboplatin in advanced non-small cell lung cancer: A California Cancer Consortium Phase I study. J Thorac Oncol. 2008;3:68–74. doi: 10.1097/JTO.0b013e31815e8b88. [DOI] [PubMed] [Google Scholar]
- 23.Barr PM, Fu P, Lazarus HM, et al. Phase I trial of fludarabine, bortezomib and rituximab for relapsed and refractory indolent and mantle cell non-Hodgkin lymphoma. Br J Haematol. 2009;147:89–96. doi: 10.1111/j.1365-2141.2009.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allignol A, Schumacher M, Beyersmann J. Empirical transition matrix of multi-state models: The etm package. J Stat Softw. 2011;38:1–15. [Google Scholar]
- 25.Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma : A comprehensive review of the literature. Blood. 2008;112:1593–1599. doi: 10.1182/blood-2008-04-149385. [DOI] [PubMed] [Google Scholar]
- 26.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101:708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence YR, Vikram B, Dignam JJ, et al. NCI-RTOG translational program strategic guidelines for the early-stage development of radiosensitizers. J Natl Cancer Inst. 2013;105:11–24. doi: 10.1093/jnci/djs472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33:910–915. doi: 10.1200/JCO.2014.57.9334. [DOI] [PubMed] [Google Scholar]
- 30.Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire-based study. Lancet Oncol. 2006;7:903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]