Abstract
Purpose
Orally administered anticancer medications are among the fastest growing components of cancer care. These medications are expensive, and cost-sharing requirements for patients can be a barrier to their use. For Medicare beneficiaries, the Affordable Care Act will close the Part D coverage gap (doughnut hole), which will reduce cost sharing from 100% in 2010 to 25% in 2020 for drug spending above $2,960 until the beneficiary reaches $4,700 in out-of-pocket spending. How much these changes will reduce out-of-pocket costs is unclear.
Methods
We used the Medicare July 2014 Prescription Drug Plan Formulary, Pharmacy Network, and Pricing Information Files from the Centers for Medicare & Medicaid Services for 1,114 stand-alone and 2,230 Medicare Advantage prescription drug formularies, which represent all formularies in 2014. We identified orally administered anticancer medications and summarized drug costs, cost-sharing designs used by available plans, and the estimated out-of-pocket costs for beneficiaries without low-income subsidies who take a single drug before and after the doughnut hole closes.
Results
Little variation existed in formulary design across plans and products. The average price per month for included products was $10,060 (range, $5,123 to $16,093). In 2010, median beneficiary annual out-of-pocket costs for a typical treatment duration ranged from $6,456 (interquartile range, $6,433 to $6,482) for dabrafenib to $12,160 (interquartile range, $12,102 to $12,262) for sunitinib. With the assumption that prices remain stable, after the doughnut hole closes, beneficiaries will spend approximately $2,550 less.
Conclusion
Out-of-pocket costs for Medicare beneficiaries taking orally administered anticancer medications are high and will remain so after the doughnut hole closes. Efforts are needed to improve affordability of high-cost cancer drugs for beneficiaries who need them.
INTRODUCTION
Orally administered anticancer medications are among the fastest growing category of prescription drugs.1,2 Spending on anticancer medications and costs to patients and insurers has been the focus of intense debate in recent years.2-8 Historically, many cancer therapies were physician administered, but medical innovations have increased the number of orally administered anticancer therapies. These innovations allow patients to receive treatments at home rather than in the clinic, which increases convenience for patients who need long-term therapy.9,10 This shift away from physician-administered medication has also shifted insurers’ reimbursement of these treatments from patients’ medical benefits to their outpatient pharmacy benefits, which has important implications for patients’ total out-of-pocket costs.11,12
Cancer is disproportionately a disease of older adults; thus, Medicare Part D is a key payer for orally administered anticancer medications. Traditional Medicare is composed of three parts: A, B, and D, which correspond to hospital, outpatient, and prescription drug coverage, respectively. Medicare Parts A and B have been available as part of the public insurance plan since 1966, whereas Medicare Part D is offered through private health insurance and has only been available since 2006. Given the increasing availability of oral cancer medications, a large and growing proportion of anticancer medications are now reimbursed through Medicare Part D.
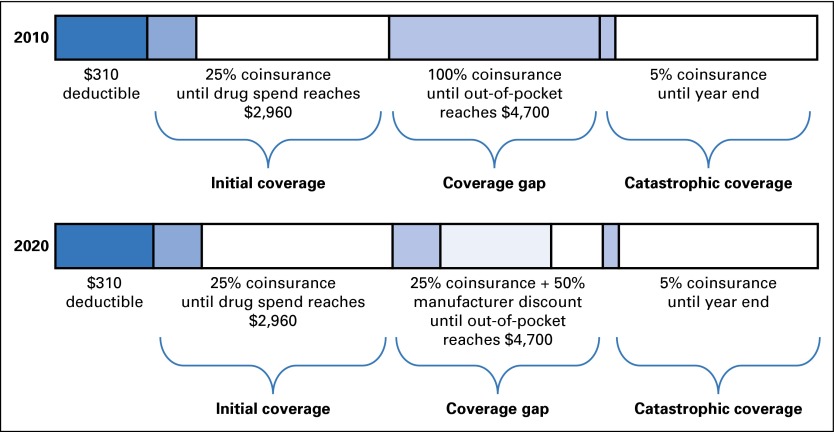
Although the Affordable Care Act (ACA) includes many provisions to improve affordability of health care services, most of the changes focus on the privately insured market. One key change introduced in the ACA for Medicare beneficiaries is the closing of the Part D coverage gap (ie, the doughnut hole), which has been praised as a way to reduce out-of-pocket spending for Medicare enrollees. In 2010, before implementation of the ACA, the standard Medicare Part D benefit included a deductible of $310, 25% coinsurance up to an initial coverage limit of $2,830, 100% coinsurance for drugs purchased during the doughnut hole (for drug spending > $2,830 until a patient reached $4,550 in out-of-pocket spending), and 5% coinsurance in the catastrophic phase.13 This benefit cycle resets at the beginning of each calendar year. With the ACA, beginning in 2011, the Part D benefit design changes annually to decrease out-of-pocket costs to patients during the doughnut hole from 100% to 25% coinsurance by 2020. This reduction in patient cost sharing in the doughnut hole is largely due to manufacturers’ 50% discount on drug prices during the doughnut hole. Contributions made by the manufacturer during this benefit phase are counted toward the patient’s out-of-pocket spending, which reduces the amount of time the patient spends in the doughnut hole (Fig 1).14
Fig 1.
Medicare Part D benefit design and beneficiary cost-sharing requirements in 2010 and 2020. The standard 2014 Medicare Part D benefit included a deductible of $310, 25% coinsurance up to an initial coverage limit of $2,960, coinsurance for drugs purchased during the doughnut hole (100% in 2010, 25% in 2020) for drug spending greater than $2,960 until a patient reached $4,700 in out-of-pocket spending, and 5% coinsurance in the catastrophic phase. Although initial coverage limits and out-of-pocket maximum costs were slightly lower in 2010, we used 2014 limits for all analyses for consistency (by changing only the percentage of coinsurance during the coverage gap). This benefit cycle resets at the beginning of each calendar year. As part of the Affordable Care Act, beginning in 2011, the Part D benefit design changes annually to decrease out-of-pocket costs to patients during the doughnut hole. This reduction in patient cost sharing in the doughnut hole is largely due to manufacturers’ 50% discount on drug prices during the doughnut hole. Contributions made by the manufacturer during this benefit phase are counted toward patient out-of-pocket spending, which reduces the amount of time that a patient spends in the doughnut hole. Patient deductibles are shown with dark blue shading, patient coinsurance with medium blue shading, manufacturer discounts with light blue shading (in 2020 only).
We examined the formularies of Medicare Part D plans to better understand coverage for oral anticancer medications. First, we explored whether variation exists in the benefits offered for these medications. Second, we estimated out-of-pocket expenditures for Medicare beneficiaries with Part D coverage who require oral anticancer therapy and who do not receive low-income subsidies. Finally, we assessed the extent to which the ACA closing of the doughnut hole will reduce costs for oral chemotherapy users enrolled in Medicare Part D.
METHODS
We used the July 15, 2014, quarterly release of the Prescription Drug Plan Formulary, Pharmacy Network, and Pricing Information Files from the Centers for Medicare & Medicaid Services (CMS formulary files) to identify Part D coverage for orally administered anticancer medications. We followed the methodology proposed by Howard et al15 to include products first approved by the US Food and Drug Administration in 1995 or later and that are administered with the primary intent of improving overall or progression-free survival. From this subset of products, we focused on products covered by Medicare Part D in 2014 (Table 1). We excluded endocrine therapy from consideration (tamoxifen and aromatase inhibitors) because these drugs are inexpensive relative to the selected products and primarily available as generic products at this time.
Table 1.
Monthly Formulary Price for Orally Administered Chemotherapy Offered by Medicare Part D Formularies in 2014 and Median Duration of Drug Use
| Drug | Median Monthly Price ($) | 25th Percentile of Monthly Price ($) | 75th Percentile of Monthly Price ($) | Median Duration of Use (months) |
|---|---|---|---|---|
| Abiraterone | 7,009 | 6,956 | 7,089 | 8 |
| Afatinib | 6,279 | 6,211 | 6,319 | 11 |
| Axitinib | 10,057 | 9,920 | 10,183 | 7 |
| Cabozantinib | 10,888 | 10,770 | 10,960 | 7 |
| Crizotinib | 11,908 | 11,816 | 12,016 | 8 |
| Dabrafenib | 8,359 | 8,269 | 8,414 | 5 |
| Dasatinib | 9,516 | 9,413 | 9,633 | 12 |
| Enzalutamide | 8,128 | 8,046 | 8,132 | 9 |
| Erlotinib | 6,353 | 6,313 | 6,417 | 11 |
| Everolimus | 9,976 | 9,877 | 10,034 | 5 |
| Imatinib | 7,832 | 7,764 | 7,898 | 12 |
| Lapatinib | 5,123 | 5,073 | 5,176 | 9 |
| Lenalidomide | 13,683 | 13,537 | 13,779 | 4 |
| Nilotinib | 9,543 | 9,480 | 9,655 | 12 |
| Pazopanib | 7,924 | 7,843 | 7,904 | 8 |
| Pomalidomide | 16,093 | 15,923 | 16,202 | 8 |
| Regorafenib | 15,206 | 15,020 | 15,213 | 3 |
| Sorafenib | 10,811 | 10,728 | 10,937 | 6 |
| Sunitinib | 13,003 | 12,919 | 13,170 | 12 |
| Trametinib | 9,564 | 9,466 | 9,632 | 5 |
| Vandetanib | 11,421 | 11,304 | 11,502 | 12 |
| Vemurafenib | 11,241 | 11,071 | 11,355 | 5 |
| Vorinostat | 11,469 | 11,312 | 11,609 | 4 |
NOTE. Prices are from the Prescription Drug Plan Formulary, Pharmacy Network, and Pricing Information Files available through the Centers for Medicare & Medicaid Services and represent the plan level average monthly reimbursement (amount paid from all sources [patient, plan, and Medicare]) for formulary Part D drugs for a 30-day supply of the most commonly covered dose of each drug selected. Median costs were calculated across all plans, and interquartile ranges are indicated. Median duration of use was obtained from data provided in Howard et al.15 For products not included in Howard et al, we used the drug product label to identify median duration of therapy (Data Supplement).
We identified 1,114 stand-alone Part D Plan/Product formularies (PDPs) and 2,230 Medicare Advantage Part D Plan/Product formularies (MA-PDs) in the CMS formulary files. Because antineoplastic medications are a protected class within Medicare Part D, nearly all products studied were covered on available formularies. For each covered therapy, we evaluated the formulary structure used by stand-alone PDPs and MA-PDs to describe variation in benefit design by plan. Next, we estimated beneficiary total out-of-pocket costs for each drug by using the median duration of therapy expected for each treatment. Median duration of therapy was based on previously published methodology and data that used drug product labels, published randomized trial results, and cost-effectiveness studies (Data Supplement).15 We rounded duration of therapy to the next full month when a partial month was indicated and capped use of a product at 12 months given that the benefit design reset each calendar year.
We obtained the price for a 30-day supply of each included product at a standard dose directly from the CMS formulary files. These prices represent the amount reimbursed for the medication (patient, plan, Medicare contributions) and were used as the input for the out-of-pocket price calculation for each Part D formulary (Data Supplement). Next, we predicted the median and interquartile range of out-of-pocket costs for a Medicare Part D enrollee who takes a single specialty drug for a full course of treatment (of median duration, capped at 12 months) by using the Part D initial coverage limit and the out-of-pocket limits in 2015. To assess the effect of closing the doughnut hole on out-of-pocket costs, we estimated out-of-pocket costs for 2010 and 2020 (before and after the closing of the doughnut hole). Specifically, we calculated annual out-of-pocket costs first by calculating the deductible (if any) plus the coinsurance/copayments for the initial coverage period up to $2,960 (initial coverage limit). Next, we calculated the out-of-pocket costs in the doughnut hole (100% in 2010, 25% in 2020) up to $4,700 (out-of-pocket limit before catastrophic coverage, which includes the 50% manufacturer discount during the coverage gap in 2020). Finally, we calculated the costs in the catastrophic period through the end of the benefit year. Because annual out-of-pocket costs were similar for patients in PDPs and MA-PDs, we present results for PDPs only unless otherwise indicated.
Sensitivity Analyses
Increases in drug prices have occurred rapidly over the past decade, although newer policies to increase biologic competition and the introduction of generics could result in price reductions for some therapies. Because product prices may change over time, we also estimated patient out-of-pocket prices and potential cost savings by assuming that anticancer medication prices increased and decreased by 50%, respectively, between 2014 (the prices used in the 2010 estimate) and 2020. Furthermore, to account for patients who used more or less therapy than the median duration tested in the primary analysis, we replicated analyses by using a 3- and 12-month duration to mimic minimum and maximum durations of therapy during a benefit year. Finally, to ensure the robustness of the drug price data, we re-estimated comparisons of out-of-pocket spending in 2010 and 2020 by using published cost estimates, which were relatively lower than those identified in the 2014 formulary files.16 Results and the detailed methodology are included in the Data Supplement. All results were substantively similar to the primary analysis presented here.
RESULTS
As anticipated, orally administered anticancer therapies were available on a majority of formularies across both MA-PDs and stand-alone PDPs. The median total cost for a 30-day supply of included products (patient and plan contributions) across all plans was $10,060 (range, $5,123 for lapatinib to $16,093 for pomalidomide; Table 1). Stand-alone PDP formularies typically use one of two formulary designs for coverage of oral anticancer medications as follows: $310 deductible and 25% coinsurance in the initial coverage period or no deductible and 33% coinsurance in the initial coverage period. Both designs used the standard 45% coinsurance in the coverage gap in 2014 and 5% catastrophic coverage. MA-PDs most often used the latter design (Table 2).
Table 2.
Common Formulary Structures Used by Stand-Alone Part D and Medicare Advantage Part D Plan/Product Formularies for Orally Administered Cancer Medications in 2014
| Benefit Structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| $310 Deductible, 25% Coinsurance Initial, 45% Coinsurance Gap, 5% Coinsurance Catastrophic (%) | $0 Deductible, 33% Coinsurance Initial, 45% Coinsurance Gap, 5% Coinsurance Catastrophic (%) | $0 Deductible, 33% Coinsurance Initial, 33% Coinsurance Gap, 5% Coinsurance Catastrophic (%) | $0 Deductible, 25% Coinsurance Initial, 45% Coinsurance Gap, 5% Coinsurance Catastrophic (%) | Cumulative Coverage of Selected Designs (%) | ||||||
| Drug | PDP | MA-PD | PDP | MA-PD | PDP | MA-PD | PDP | MA-PD | PDP | MA-PD |
| Abiraterone | 45.6 | 12.6 | 43.4 | 48.3 | 3.3 | 15.1 | 0.4 | 5.3 | 92.7 | 81.3 |
| Afatinib | 45.7 | 13.0 | 43.3 | 45.5 | 3.3 | 17.6 | 1.2 | 6.5 | 93.5 | 82.6 |
| Axitinib | 45.7 | 12.7 | 43.2 | 45.9 | 3.3 | 16.3 | 1.0 | 7.0 | 93.2 | 81.9 |
| Cabozantinib | 45.7 | 13.0 | 43.3 | 45.4 | 3.3 | 17.6 | 1.2 | 6.6 | 93.5 | 82.6 |
| Crizotinib | 45.7 | 12.8 | 43.2 | 47.1 | 3.3 | 16.3 | 1.0 | 5.8 | 93.2 | 82.0 |
| Dabrafenib | 45.7 | 12.8 | 43.2 | 47.1 | 3.3 | 16.3 | 1.0 | 5.8 | 93.2 | 82.0 |
| Dasatinib | 45.3 | 12.5 | 44.8 | 44.2 | 3.2 | 21.8 | 0.4 | 6.0 | 93.7 | 84.5 |
| Enzalutamide | 45.6 | 12.5 | 43.4 | 48.6 | 3.3 | 15.1 | 0.4 | 4.8 | 92.7 | 81.0 |
| Erlotinib | 45.7 | 13.1 | 43.3 | 45.3 | 3.3 | 17.6 | 1.2 | 6.8 | 93.5 | 82.8 |
| Everolimus | 45.3 | 12.5 | 45.0 | 42.6 | 3.0 | 22.1 | 0.7 | 6.6 | 94.0 | 83.8 |
| Imatinib | 45.7 | 12.8 | 43.2 | 45.9 | 3.3 | 16.3 | 1.0 | 7.2 | 93.2 | 82.2 |
| Lapatinib | 45.7 | 12.6 | 43.4 | 48.4 | 3.3 | 15.1 | 0.5 | 5.3 | 92.9 | 81.4 |
| Lenalidomide | 45.3 | 12.7 | 44.8 | 44.3 | 3.2 | 21.9 | 0.4 | 5.7 | 93.7 | 84.6 |
| Nilotinib | 45.7 | 12.8 | 43.2 | 47.0 | 3.3 | 16.3 | 1.0 | 6.0 | 93.2 | 82.1 |
| Pazopanib | 45.6 | 12.6 | 43.4 | 47.6 | 3.3 | 15.1 | 0.4 | 5.9 | 92.7 | 81.2 |
| Pomalidomide | 45.4 | 12.8 | 44.0 | 45.3 | 3.3 | 19.2 | 0.7 | 6.5 | 93.4 | 83.8 |
| Regorafenib | 45.6 | 12.5 | 43.3 | 48.5 | 3.3 | 15.1 | 0.4 | 4.9 | 92.6 | 81.0 |
| Sorafenib | 45.6 | 12.6 | 43.4 | 47.6 | 3.3 | 15.1 | 0.8 | 5.8 | 93.1 | 81.1 |
| Sunitinib | 45.7 | 13.1 | 43.3 | 44.5 | 3.3 | 17.6 | 1.2 | 7.4 | 93.5 | 82.6 |
| Trametinib | 45.7 | 12.7 | 43.2 | 46.9 | 3.3 | 16.3 | 1.0 | 6.0 | 93.2 | 81.9 |
| Vandetanib | 45.7 | 13.0 | 43.2 | 47.8 | 3.3 | 16.7 | 1.0 | 4.2 | 93.2 | 81.7 |
| Vemurafenib | 45.6 | 12.6 | 43.4 | 48.4 | 3.3 | 15.1 | 0.4 | 5.0 | 92.7 | 81.1 |
| Vorinostat | 45.6 | 12.5 | 43.4 | 48.2 | 3.3 | 15.1 | 0.4 | 5.3 | 92.7 | 81.1 |
NOTE. Proportions reflect the percentage of plan/products that cover the relevant drug that uses the listed formulary structure. Analysis is based on the July 15, 2014, quarterly release of the Prescription Drug Plan Formulary, Pharmacy Network, and Pricing Information Files from the Centers for Medicare & Medicaid Services. Abbreviations: MA-PD, Medicare Advantage Part D Plan/Product formulary; PDP, Part D Plan/Product formulary.
Plan Coverage and Estimated Out-of-Pocket Expenses
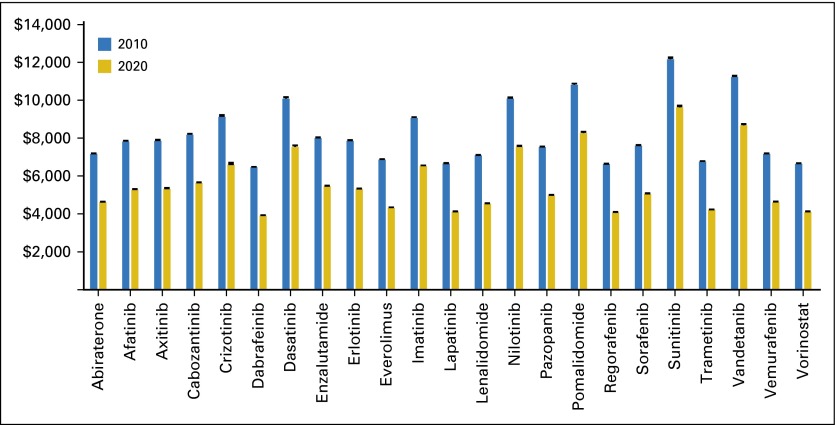
Median out-of-pocket costs for Medicare Part D enrollees in 2010 for a median duration course ranged from $6,456 for dabrafenib to $12,160 for sunitinib with the assumption that patients paid the full drug costs while in the doughnut hole before the ACA (Fig 2). Out-of-pocket costs varied little, with the range between the upper and lower quartiles across all drugs and formularies equal to $82 on average. After the doughnut hole is closed in 2020, we estimate that median out-of-pocket costs would still be high, with an average of $5,663 across all products (a savings of approximately $2,550 per year).
Fig 2.
Median total out-of-pocket costs for a course of oral anticancer therapies for Part D beneficiaries by drug before (2010) and after (2020) the doughnut hole closes, with the assumption of a median duration treatment course. Analysis is based on the July 15, 2014, quarterly release of the Prescription Drug Plan Formulary, Pharmacy Network, and Pricing Information Files from the Centers for Medicare & Medicaid Services. Input price is from the formulary files and represents the average reimbursed amount across each plan for the product listed for 1 month of therapy at the mean recommended dose. Median duration of use was obtained from data provided in Howard et al.15 For products not included in Howard et al, we used the drug product label to identify median duration of therapy (Data Supplement).
In the base case analysis, we assumed that drug prices will not change between 2010 and 2020. To evaluate the impact of drug price increases or decreases, we varied the drug price by ± 50% for the 2020 estimate. We found that if prices declined by 50%, average out-of-pocket costs would be $3,738, which would save beneficiaries an average of $4,468 per year in 2020 compared with 2010 costs (Table 3). However, if drug prices increased by 50%, average out-of-pocket costs would be $7,584, which would save beneficiaries only $621 over the 2010 prices (Table 3).
Table 3.
Median Total Patient OOP Cost Savings for a Course of Oral Anticancer Therapies for Closing the Doughnut Hole Under Varying Assumptions With Regard to Drug Pricing
| 2010 |
Base Case (No Change in Price; $) | 50% Price Increase ($) | 50% Price Decrease ($) | ||||
|---|---|---|---|---|---|---|---|
| Drug | Estimated OOP 2010 | Estimated OOP 2020 | Savings From 2010 | Estimated OOP 2020 | Savings From 2010 | Estimated OOP 2020 | Savings From 2010 |
| Abiraterone | 7,156 | 4,621 | 2,535 | 6,023 | 1,133 | 3,220 | 3,936 |
| Afatinib | 7,817 | 5,242 | 2,575 | 6,968 | 849 | 3,532 | 4,285 |
| Axitinib | 7,877 | 5,345 | 2,532 | 7,108 | 769 | 3,582 | 4,295 |
| Cabozantinib | 8,174 | 5,597 | 2,577 | 7,508 | 666 | 3,709 | 4,465 |
| Crizotinib | 9,120 | 6,552 | 2,568 | 8,917 | 203 | 4,187 | 4,933 |
| Dabrafenib | 6,454 | 3,889 | 2,565 | 4,923 | 1,531 | 2,855 | 3,599 |
| Dasatinib | 10,075 | 7,531 | 2,544 | 10,386 | (311) | 4,676 | 5,399 |
| Enzalutamide | 8,002 | 5,480 | 2,522 | 7,320 | 682 | 3,646 | 4,356 |
| Erlotinib | 7,853 | 5,309 | 2,544 | 7,054 | 799 | 3,563 | 4,290 |
| Everolimus | 6,836 | 4,291 | 2,545 | 5,525 | 1,311 | 3,056 | 3,780 |
| Imatinib | 9,062 | 6,508 | 2,554 | 8,855 | 207 | 4,162 | 4,900 |
| Lapatinib | 6,660 | 4,121 | 2,539 | 5,280 | 1,380 | 2,970 | 3,690 |
| Lenalidomide | 7,084 | 4,561 | 2,523 | 5,932 | 1,152 | 3,189 | 3,895 |
| Nilotinib | 10,083 | 7,545 | 2,538 | 10,410 | (327) | 4,680 | 5,403 |
| Pazopanib | 7,518 | 4,981 | 2,537 | 6,566 | 952 | 3,400 | 4,118 |
| Pomalidomide | 10,800 | 8,223 | 2,577 | 11,451 | (651) | 5,006 | 5,794 |
| Regorafenib | 6,630 | 4,099 | 2,531 | 5,242 | 1,388 | 2,959 | 3,671 |
| Sorafenib | 7,598 | 5,063 | 2,535 | 6,696 | 902 | 3,440 | 4,158 |
| Sunitinib | 12,163 | 9,623 | 2,540 | 13,528 | (1,365) | 5,721 | 6,442 |
| Trametinib | 6,756 | 4,188 | 2,568 | 5,371 | 1,385 | 3,005 | 3,751 |
| Vandetanib | 11,218 | 8,650 | 2,568 | 12,092 | (874) | 5,230 | 5,988 |
| Vemurafenib | 7,149 | 4,623 | 2,526 | 6,029 | 1,120 | 3,221 | 3,928 |
| Vorinostat | 6,650 | 4,112 | 2,538 | 5,259 | 1,391 | 2,965 | 3,685 |
NOTE. Analysis is based on the July 15, 2014, quarterly release of the Prescription Drug Plan Formulary, Pharmacy Network, and Pricing Information Files from the Centers for Medicare & Medicaid Services. Input price is from the formulary files and represents the average reimbursed amount across each plan for the product listed for 1 month of therapy at the mean recommended dose. Savings in the base case scenario are attributed to the 50% manufacturer discount during the doughnut hole. These contributions from the manufacturer are included in the patient out-of-pocket limit of $4,700. After reaching this limit, beneficiaries pay 5% of the drug price until the beginning of the next calendar year.
Abbreviation: OOP, out of pocket.
DISCUSSION
Medicare beneficiaries who do not receive low-income subsidies and who need to use cancer medications long term will spend approximately $4,000 to $10,000 out of pocket, depending on the therapy prescribed, even after the doughnut hole is closed. In 2012, the average Medicare beneficiary’s household budget was estimated at $33,993.17 By taking into consideration the mean annual expenditures of $11,673 for housing, $5,189 for food, and $5,087 for transportation, let alone other health care costs, few can afford the high costs of specialty cancer therapies.
In 2014, 37 million beneficiaries were enrolled in a Medicare drug plan. Although 30% of Part D enrollees receive low-income subsidies through dual eligibility for Medicaid or through other needs-based qualifications (eg, annual income of < $17,235 for an individual or < $23,265 for a couple), those who do not qualify for subsidies have few options for affordable care.18 In fact, Medigap policies and out-of-pocket limits on Medicare Advantage plans meant to limit cost sharing for Medicare beneficiaries exclude Part D spending, which further exposes beneficiaries to high costs.18-20
High out-of-pocket expenditures negatively affect patient adherence to oral cancer therapies.21-23 Even in the setting of relatively low out-of-pocket costs, such as endocrine therapy for breast cancer, large declines have been seen in adherence as patients move into the doughnut hole and face larger cost sharing, with subsequent rebounds in adherence as out-of-pocket prices reduce.22 Even in the catastrophic phase of the Medicare Part D benefit, enrollees are expected to contribute 5% toward the price of their medications. Even this generous level of cost sharing results in high costs to patients. The average price across orally administered cancer therapies covered by Medicare in 2014 was more than $10,000 per month of therapy, with 5% cost sharing resulting in $500 out of pocket per month for the beneficiary.
One recently published study of total and out-of-pocket spending on specialty medications among Medicare Advantage plan members suggested that large decreases in out-of-pocket spending follow the initial phases of the doughnut hole closure.11 The authors observed a 26% reduction in out-of-pocket spending between 2010 and 2011 across all drugs that met the $600 per specialty tier threshold. The study also highlighted the benefits of closing the doughnut hole on average but did not address the limitations of the policy for very-high-priced medications, which include those that are the focus of the current report. Beneficiaries who remain in the doughnut hole for the longest periods will receive the most benefits of the policy change. A single fill for oral chemotherapy prescriptions places the beneficiary in the doughnut hole, and most patients exit the doughnut hole at 1.6 fills, which leaves little time to gain benefits from this phase of coverage.
The current study has several important limitations. First, we estimated use of only a single drug for each beneficiary in the calculations. Given the age of the population, many beneficiaries take additional medications aside from cancer therapies. These other medications move the beneficiary through the doughnut hole faster, with the same out-of-pocket maximums required. Thus, comparisons between the 2020 projected costs and the 2010 costs would not be changed with the inclusion of other medications. Second, our estimates of 2020 out-of-pocket costs are based on assumptions that the benefit design is only changed through a reduction of patient cost sharing from 100% to 25% and inclusion of the 50% manufacturer discount toward the out-of-pocket maximum during the coverage gap. However, prices paid to manufacturers for oral chemotherapies have increased significantly over time. At the same time, newer policies related to increasing biologic competition and the introduction of generics could result in price reductions for some therapies, although the extent of cost savings through this form of competition currently is not clear.24,25 To address these conflicting scenarios, in sensitivity analyses, we varied the drug input price by increasing and decreasing drug prices by 50%. These price changes had little impact on the relative effect of the doughnut hole closing on patient out-of-pocket spending, but they did have an impact on the accumulated annual cost to patients, as would be expected. Finally, beneficiaries who use patient assistance programs or those eligible for low-income subsidies or Medicaid were not considered because their out-of-pocket costs differ from those of patients without these protections.
Out-of-pocket costs for orally administered cancer medications for Medicare Part D beneficiaries without low-income subsidies are very high and will remain so after the doughnut hole is closed. We found almost no variation in out-of-pocket costs to patients across all available formularies, which suggests that patients who need these products can do little to reduce their out-of-pocket costs through better plan selection. With consideration of the rising prices for specialty drugs over the past decade,26 any projected cost savings from closing the doughnut hole likely would be lost for these drugs. Efforts should be made to improve affordability of specialty drugs for Medicare beneficiaries given that medication nonadherence and a forgoing of basic needs are associated with high out-of-pocket medication costs.21,23,27,28 In particular, some key protections offered to privately insured individuals through the ACA are unavailable to Medicare-insured beneficiaries, specifically those on traditional Medicare plans. These include the annual out-of-pocket maximum that includes both medical and pharmacy spending ($6,600 for an individual in 2015). The current findings demonstrate the extraordinary financial burden of orally administered cancer medications for Medicare Part D enrollees and the need for policies that improve access to high-value therapies. Examples of such policies are a substantial lowering of drug prices or a shift in formulary designs from coinsurance to copayments.
Supplementary Material
Acknowledgments
We thank Jeff Souza for assistance with data extraction and analysis.
Footnotes
Supported by Research Scholar Grant RSGI-14-030-01-CPHPS from the American Cancer Society. S.B.D. is supported by the National Institutes of Health Building Interdisciplinary Research Careers in Women’s Health K12 Program and the North Carolina Translational and Clinical Sciences Institute (UL1TR001111). N.L.K. is supported by K24CA181510 from the National Cancer Institute.
No funder or sponsor was involved in any phase of manuscript development.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Stacie B. Dusetzina
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Mind the Gap: Why Closing the Doughnut Hole Is Insufficient for Increasing Medicare Beneficiary Access to Oral Chemotherapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Stacie B. Dusetzina
No relationship to disclose
Nancy L. Keating
No relationship to disclose
REFERENCES
- 1.Lotvin AM, Shrank WH, Singh SC, et al. Specialty medications: Traditional and novel tools can address rising spending on these costly drugs. Health Aff (Millwood) 2014;33:1736–1744. doi: 10.1377/hlthaff.2014.0511. [DOI] [PubMed] [Google Scholar]
- 2.Conti RM, Fein AJ, Bhatta SS. National trends in spending on and use of oral oncologics, first quarter 2006 through third quarter 2011. Health Aff (Millwood) 2014;33:1721–1727. doi: 10.1377/hlthaff.2014.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weil AR. The promise of specialty pharmaceuticals. Health Aff (Millwood) 2014;33:1710. doi: 10.1377/hlthaff.2014.1091. [DOI] [PubMed] [Google Scholar]
- 4.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian H, Steensma D, Rius Sanjuan J, et al. High cancer drug prices in the United States: Reasons and proposed solutions. J Oncol Pract. 2014;10:e208–e211. doi: 10.1200/JOP.2013.001351. [DOI] [PubMed] [Google Scholar]
- 6.Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology Statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch BR, Balu S, Schulman KA. The impact of specialty pharmaceuticals as drivers of health care costs. Health Aff (Millwood) 2014;33:1714–1720. doi: 10.1377/hlthaff.2014.0558. [DOI] [PubMed] [Google Scholar]
- 8. Bach PB, Saltz LB, Wittes R: In cancer care, cost matters. New York Times, October 15, 2012:A5.
- 9.Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–115. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 10.Borner M, Scheithauer W, Twelves C, et al. Answering patients’ needs: Oral alternatives to intravenous therapy. Oncologist. 2001;6(suppl 4):12–16. doi: 10.1634/theoncologist.6-suppl_4-12. 6:12-16, 2001 (suppl 4) [DOI] [PubMed] [Google Scholar]
- 11.Trish E, Joyce G, Goldman DP. Specialty drug spending trends among Medicare and Medicare Advantage enrollees, 2007-11. Health Aff (Millwood) 2014;33:2018–2024. doi: 10.1377/hlthaff.2014.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Government Accountability Office . Medicare Part D: Spending, Beneficiary Cost Sharing, and Cost-Containment Efforts for High-Cost Drugs Eligible for a Specialty Tier. Washington, DC, Government Accountability Office: 2010. [Google Scholar]
- 13. Centers for Medicare & Medicaid Services: Medicare & You 2010. Baltimore, MD, Department of Health and Human Services, 2010. [Google Scholar]
- 14. Centers for Medicare & Medicaid Services: Understanding true out-of-pocket (TrOOP) costs. https://www.cms.gov/Outreach-and-Education/Outreach/Partnerships/downloads/11223-P.pdf.
- 15.Howard DH, Bach PB, Berndt ER, et al. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29:139–162. doi: 10.1257/jep.29.1.139. [DOI] [PubMed] [Google Scholar]
- 16.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 17. Cubanski J, Swoope C, Damico A, et al: Health Care on a Budget: The Financial Burden of Health Spending by Medicare Households. Oakland, CA, The Henry J. Kaiser Family Foundation, 2014. [Google Scholar]
- 18. Hoadley J, Summer L, Hargrave E, et al: Medicare Part D in its ninth year: The 2014 marketplace and key trends, 2006-2014. Section 4: The low-income subsidy program. http://kff.org/report-section/medicare-part-d-in-its-ninth-year-section-4-the-low-income-subsidy-program.
- 19. Jacobson G, Damico A, Neuman T, et al: Medicare Advantage 2015 Data Spotlight: Overview of Plan Changes. Oakland, CA: The Henry J. Kaiser Family Foundation, 2014. [Google Scholar]
- 20. Centers for Medicare & Medicaid Services: Choosing a Medigap Policy: A Guide to Health Insurance for People With Medicare. Baltimore, MD, US Department of Health and Human Services, 2015.
- 21.Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32:306–311. doi: 10.1200/JCO.2013.52.9123. [DOI] [PubMed] [Google Scholar]
- 22.Neuner JM, Kamaraju S, Charlson JA, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv130. djv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaisaeng N, Harpe SE, Carroll NV. Out-of-pocket costs and oral cancer medication discontinuation in the elderly. J Manag Care Spec Pharm. 2014;20:669–675. doi: 10.18553/jmcp.2014.20.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarpatwari A, Avorn J, Kesselheim AS. Progress and hurdles for follow-on biologics. N Engl J Med. 2015;372:2380–2382. doi: 10.1056/NEJMp1504672. [DOI] [PubMed] [Google Scholar]
- 25.Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: Statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood) 2015;34:294–301. doi: 10.1377/hlthaff.2014.0482. [DOI] [PubMed] [Google Scholar]
- 26.Shih YC, Smieliauskas F, Geynisman DM, et al. Trends in the cost and use of targeted cancer therapies for the privately insured nonelderly: 2001 to 2011. J Clin Oncol. 2015;33:2190–2196. doi: 10.1200/JCO.2014.58.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naci H, Soumerai SB, Ross-Degnan D, et al. Medication affordability gains following Medicare Part D are eroding among elderly with multiple chronic conditions. Health Aff (Millwood) 2014;33:1435–1443. doi: 10.1377/hlthaff.2013.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.