Abstract
IN BRIEF Congenital lipodystrophy is a rare genetic disorder characterized by a near-complete absence of fat cells, hypoleptinemia leading to a voracious appetite, and marked insulin resistance. This article focuses on the known cardiovascular manifestations of patients with congenital lipodystrophy, including cardiomyopathy, cardiac arrhythmias, and accelerated atherosclerosis arising from a markedly deranged metabolic milieu. Future research that targets leptin deficiency (metreleptin) and apoC3 mRNA (antisense oligonucleotide) could open a window for potential pharmacological treatment of this challenging disorder.
Congenital generalized lipodystrophy (CGL), also called Berardinelli-Seip congenital lipodystrophy, is a rare genetic disorder characterized by loss of adipose tissue and marked insulin resistance. A hallmark of this disorder is a low leptin level, leading to a voracious appetite in affected individuals. There appear to be ∼300–500 cases of CGL worldwide (1). Mutations in a variety of genes can lead to the various subtypes of congenital lipodystrophy (1). At the cellular level, dysfunctional adipocytes are unable to store triglycerides, leading to high chylomicron levels. The disorder is characterized by protean clinical manifestations, including diabetes, pancreatitis, fatty liver, and endothelial cell dysfunction (2).
Berardinelli (3) first described CGL in a 2.5-year-old boy in Brazil in 1954. Subsequently, in 1959, Seip (4) described three more cases in Norway. CGL has an autosomal recessive pattern of inheritance. It has multisystemic manifestations, including muscular hypertrophy, hypertrophic cardiomyopathy, hepatomegaly, steatohepatitis, acanthosis nigricans, hypertriglyceridemia, and diabetes (5). Affected patients develop a hypertrophic “lipotoxic” cardiomyopathy, with both diastolic and systolic dysfunction, and have a predilection for cardiac dysrhythmias and early sudden cardiac death (6). In this article, we summarize the literature on this rare genetic disorder, focusing on the translational biology of the disease, its cardiac manifestations, and novel treatment considerations.
Genetics and Translational Biology of CGL
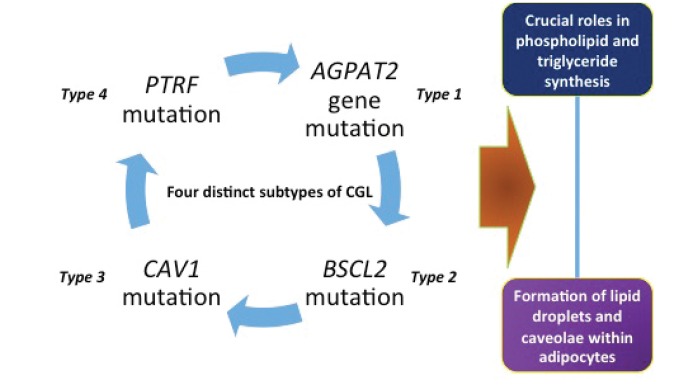
CGL has four subclinical phenotypes (CGL1, -2, -3, and -4) secondary to mutations in the acylglycerol-3-phosphate-O-acyltransferase (AGPAT2) gene, the gamma-3–linked gene located at chromosome 11q13 (BSCL2), caveolin-1 (CAV1), and RNA polymerase 1 and transcript release factor (PTRF), respectively (7–12) (Figure 1). Agarwal et al. (9) reported mutations in many genes, one of which is the AGPAT2 gene found on chromosome 9 in humans. This gene has been found to code the protein located in the endoplasmic reticulum, affecting phospholipid biosynthesis. Severeal other mutations associated with CGL have been described in the literature (1).
FIGURE 1.
Subtypes of congenital lipodystrophy. Adapted from Ref. 1.
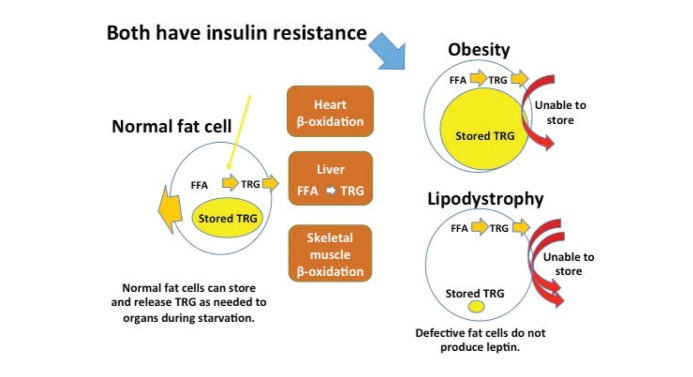
At the cellular level, the dysfunctional adipocytes in CGL are unable to store triglycerides. Figure 2 illustrates differences between an adipocyte of an obese individual and one of a person with CGL. It is noteworthy that, in obesity, the enlarged adipocyte is no longer able to store triglycerides and, in CGL, the abnormal adipocyte is unable to store triglycerides. Both conditions lead to increased circulating levels of free fatty acids (FFAs). It is well known from numerous studies that high levels of FFAs increase cardiovascular risk (13). Cardiovascular interest in CGL comes, in part, from the marked increase in circulating FFAs found in this condition.
FIGURE 2.
An adipocyte in an obese individual is contrasted with a dysfunctional adipocyte in congenital lipodystrophy. It is noteworthy that in obesity, the enlarged adipocyte is no longer able to store triglycerides, and in CGL, the abnormal adipocyte is unable to store triglycerides. Both conditions lead to increased circulating FFAs. TRG, triglycerides.
Cardiac Manifestations of CGL
The cardiac manifestations of CGL were described as early as 1959 (4). A variety of cardiac issues have been described, ranging from dilated cardiomyopathy to hypertrophic cardiomyopathy and severe biventricular heart failure. In addition, patients have been noted to have both brady- and tachyarrhythmias, particularly long QT syndrome and sudden cardiac death. Following is a summary of the literature on CGL as it pertains to the heart; ∼200 cases of CGL (or Berardinelli-Seip syndrome) have been reported (14,15).
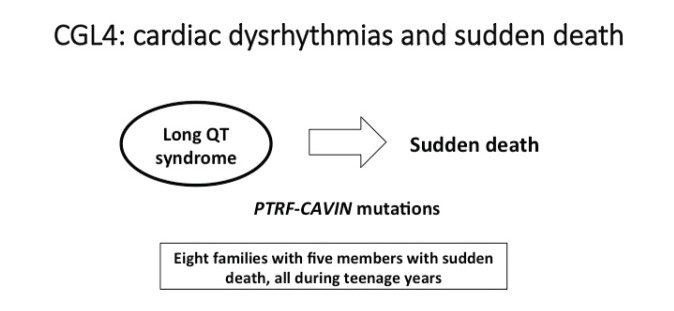
Rajab et al. (16) described the cardiac manifestations of CGL4. Patients with CGL4 have a wide variety of clinical manifestations, including cardiac dysrhythmias, joint involvement, skeletal and smooth muscle hypertrophy, and myopathies. Individuals with CGL4 are described to have mutations in the PTRF-CAVIN gene, which in turn interferes with the functioning of CAV1 and CAV3, manifesting as lipodystrophy or myopathy, respectively (17). At the cardiac physiology level, these mutations adversely affect the functioning of various ion channels in the heart, including the nodal pacemaker channel hyperpolarization-activated cyclic nucleotide-gated potassium channel 4 (HCN4), voltage-gated Na+ channel (Nav1.5, SCN5A), and the L-type Ca2+ channels (CACNA1C), leading to various dysrhythmias. Mutations in HCN4 can cause sinus node dysfunction/sick sinus syndrome, whereas mutations in SCN5A, CAV3, and CACNA1C are linked to sudden cardiac death, ventricular tachycardia, and long QT syndromes (LQT3, -8, and -9) (17) (Figure 3).
FIGURE 3.
CGL4 (PTRF mutation) has been associated with cardiac arrhythmias. Rajab et al. (16) characterized patients with fatal cardiac arrhythmias and long QT syndrome and reported patients with sudden death in the teenage years. The treatment of these patients is best evaluated by an electrophysiologist because of the complexity related to polymorphic ventricular tachycardia seen in CGL4 patients. CGL2 patients also are at increased risk, and avoidance of exercise might be considered, but this remains unclear. Napolitano and Priori (33) reported on the diagnosis and treatment of patients with catecholaminergic polymorphic ventricular tachycardia, which has been seen in lipodystrophy.
Rajab et al. (16) described 11 cases of CGL, of which 6 experienced sudden cardiac death at an early age. They reported the case of a 14-year-old boy with CGL who was found to have had multiple episodes of palpitations and syncope secondary to both ventricular and supraventricular tachycardia, as well as sinus bradycardia. Electrocardiogram (ECG) revealed a prolonged corrected QT of ∼ 450–480 ms, for indicating long QT syndrome. Long QT syndrome is a congenital disorder characterized by ECG evidence of a prolonged QT interval, which may lead to ventricular tachyarrhythmias, syncope, cardiac arrest, or sudden cardiac death. Another patient was noted to have syncope, with a cardiac loop recorder revealing supraventricular and ventricular tachycardia and frequent ventricular extrasystoles; eventually, at the age of 13 years, this patient sustained cardiac arrest secondary to ventricular fibrillation that was refractory to defibrillation. Another five patients reported by Rajab et al. sustained sudden cardiac death in their teens. Postmortem examinations of these children were precluded because they lived in rural areas of Oman.
Shastry et al. (18) described five patients with CGL4, associated with mutations in the PTRF gene. Two of these patients showed catecholaminergic polymorphic ventricular tachycardia on exercise treadmill testing, three had normal left ventricular (LV) size and function on transthoracic echocardiography, and one had hypertension.
Rahman et al. (19) described CGL in two Pakistani children. From the cardiac standpoint, both children demonstrated echocardiographic evidence of LV hypertrophy, although LV function was found to be normal. One child had a small patent foramen ovale, and the other had a small secundum atrial septal defect. Electrocardiography of the affected children showed a right bundle branch pattern.
Bjornstad et al. (6) reported the cardiac manifestations of CGL in seven patients. There were four deaths in their case series, in patients ranging in age from 24 to 37 years, with a mean age of death of 32 years. Biventricular hypertrophy and dysfunction with pulmonary hypertension was also described. Echocardiographically, the thickened myocardium showed evidence of vacuolization or intramyocardial channels. Moreover, on histopathological assessment of endomyocardial biopsies, diffuse interstitial deposition of collagen was noted in addition to marked subendocardial fibrosis. There was no disarray in terms of myocyte arrangement; however, the myocytes were hypertrophic, with increases in the diameters of the nuclei and fibers.
Rheuban et al. (20) described the cardiac manifestations of total generalized lipodystrophy in four patients. These patients were between the ages of 16 and 23 years, and echocardiography revealed symmetric hypertrophic cardiomyopathy and preserved systolic function. Autopsy of one patient showed hypertrophy of the myocardial fibers.
Klar et al. (21) described a case of CGL in a 13-year-old girl who was found on echocardiography to have asymmetric septal hypertrophy without evidence of outflow tract obstruction. Marked myocardial hypertrophy in a 6-month-old child with insulin resistance and CGL was described by Geffner et al. (22). Viegas et al. (23) described the cardiovascular manifestations of a woman with total generalized lipodystrophy who presented with decompensated heart failure. Transthoracic echocardiography revealed severe concentric LV hypertrophy with hyperdynamic LV function and advanced diastolic dysfunction with a restrictive mitral inflow pattern. Cardiac MRI confirmed LV hypertrophy. The patient underwent diuresis and was started on calcium channel blockers, with marked clinical improvement.
Cardiac Imaging in CGL
Lupsa et al. (24) described the echocardiographic features of 25 patients with CGL; 18 had an increased LV mass, 4 had LV dysfunction, 1 had a patent ductus arteriosus, 1 had concentric remodeling, and 1 had moderate LV dysfunction and dilation. Nelson et al. (25) used MRI and localized photon spectroscopy to demonstrate that patients with CGL had a myocardial triglyceride content that was threefold higher than in control subjects (0.6–0.2 vs. 0.2–0.1%, P = 0.004). In addition, patients with CGL were found to have increased LV mass index, LV concentricity, and LV hypertrophy independent of blood pressure. Interestingly, this group described MRI evidence of pericardial fat in CGL patients that was in contrast to previous autopsy studies showing an absence of pericardial fat (26).
Cardiac Autopsy Findings in CGL
Autopsy studies from Bjornstad’s CGL group (6) revealed “moderately symmetrically enlarged heart with normal cusps.” The histopathological examination showed “mild thickening of the smaller intramural coronary arteries with intimal fibrosis, as well as a moderate subendocardial collagen deposition. Larger intramural arteries were normal, and the epicardial branches revealed only sparse focal intimal fibrosis. The myocytes were slightly hypertrophic and normally arranged. The content of perivascular and subepicardial fat was decreased.” One case in particular was described as having concentric hypertrophy with the posterior wall and the interventricular septum, both measuring up to 16 mm, whereas another case had asymmetrical septal hypertrophy.
Haque et al. (27) described the autopsy findings of two patients with familial partial lipodystrophy, Dunnigan variety. The first case was of a white female who died at the age of 66 years. The heart was enlarged (weight 670 g vs. normal weight of 250–300 g in adult women). The authors reported finding old myocardial scars, but no new infarct was apparent. Moderate to severe atherosclerosis of the coronary arteries, proximal aorta, and carotid arteries was noted.
Accelerated Atherosclerosis in CGL
Atherosclerotic and vascular disease in patients with CGL may stem from a number of metabolic derangements. Marked elevations in triglyceride and insulin levels with low HDL cholesterol levels have been well documented to be associated with increased atherosclerosis, and people with CGL fit this metabolic profile (28). In addition, fatty liver, which is frequently associated with lipodystrophy, is also known to be associated with an increased risk for cardiovascular disease (CVD) (29).
The National Institutes of Health (NIH) and FHA101 trials were open-label, single-arm, U.S. Food and Drug Administration regulatory trials that focused on the long-term safety and efficacy of metreleptin for the treatment of patients with CGL (30,31). Goldens (31) described the burden of atherosclerotic and CVD in this small cohort of CGL patients: 72 patients with CGL in the NIH trials and 28 patients with CGL in the FHA101 trial. In the NIH trials, two patients (2.8%) had a medical history of coronary artery disease (CAD), and four patients (5.6%) had cardiomyopathy (two with hypertrophic cardiomyopathy, one with cardiomyopathy, and one with decreased ejection fraction). In the FHA101 trial, seven (28%) of 25 patients had CVD (four with CAD, one with aortic atherosclerosis, one with myocardial infarction, and one with ischemic cardiomyopathy). Needless to say, older patients with CGL may accrue a higher risk for atherosclerotic cardiovascular events over time. The FHA101 trial had a high proportion of adults with CGL in contrast to the NIH trials, in which more than half of the patients were <18 years of age, which likely explains the higher proportion of CVD in the FHA101 cohort. However, further studies and longer-term follow-up of individuals with CGL are needed to better understand these patients’ predilection for atherosclerotic vascular disease.
Conclusion
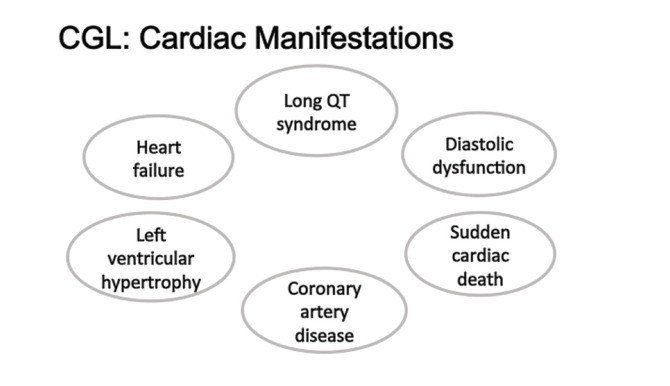
CGL is a rare genetic disorder with unusual cardiac manifestations (Figure 4). Cardiac involvement may include electrophysiological abnormalities resulting from long QT syndrome and a predisposition to tachyarrhythmias, including catecholaminergic polymorphic ventricular tachycardia and sudden cardiac death. Patients with CGL may develop heart failure and advanced diastolic dysfunction from marked LV hypertrophy that may be either symmetrical or asymmetrical. Histologically, the absence of rearrangement of myocardial fibers in CGL differentiates this condition from hypertrophic cardiomyopathy. In addition, because of increased circulating FFAs and low HDL cholesterol levels, such patients may develop accelerated atherosclerosis. Patients with this rare genetic disorder require cardiac care focused on the diagnosis and treatment of heart failure, appropriate screening and management of rhythm disorders, and aggressive treatment of dyslipidemia (Table 1). Since leptin deficiency is a key abnormality in lipodystrophy, leptin replacement has been found to ameliorate insulin resistance, hyperglycemia, hypertriglyceridemia, and hepatic steatosis (32). Future directions for treatment could include novel pharmacological therapies that target leptin deficiency (metreleptin) and hypertriglyceridemia via inhibition of apoC3 mRNA (antisense oligonucleotide) (32–34).
FIGURE 4.
The cardiac manifestations of CGL are wide-ranging. Involvement of the cardiovascular system is very apparent, ranging from sudden death to likely increased atherosclerotic risk.
TABLE 1.
Treatment Considerations in Lipodystrophy
| • Diagnosis can lead to major anxiety for patients and their families. • Cosmetic surgery to address fat loss can be considered with caution. • Dietary considerations: high-carbohydrate, low-fat diet ○ Improves marked chylomicronemia but may increase VLDL cholesterol ■ Fibric acid agents and peroxisome proliferator–activated receptor agents may be helpful but are untested ■ Fish oil supplementation should be considered • There are special cardiovascular concerns with CGL4 because of arrhythmia risk: ○ Avoid strenguous exercise ○ Possible role for β-blockers ○ Electrophysiology consultation should be given strong consideration • Newest consideration: metreleptin ○ Leads to improved metabolic parameters ○ Glucose, triglyercides, fatty organ disease |
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Patni N, Garg A. Congenital generalized lipodystrophies: new insights into metabolic dysfunction. Nat Rev Endocrinol 2015;11:522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol 2011;7:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berardinelli W. An undiagnosed endocrinometabolic syndrome: report of 2 cases. J Clin Endocrinol Metab 1954;14:193–204 [DOI] [PubMed] [Google Scholar]
- 4.Seip M. Lipodystrophy and gigantism with associated endocrine manifestations: a new diencephalic syndrome? Acta Paediatr 1959;48:555–574 [PubMed] [Google Scholar]
- 5.Fu M, Kazlauskaite R, Baracho Mde F, et al. . Mutations in Gng3lg and AGPAT2 in Berardinelli-Seip congenital lipodystrophy and Brunzell syndrome: phenotype variability suggests important modifier effects. J Clin Endocrinol Metab 2004;89:2916–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjornstad PG, Foerster A, Ihlen H. Cardiac findings in generalized lipodystrophy. Acta Paediatr 1996;413(Suppl.):39–43 [DOI] [PubMed] [Google Scholar]
- 7.Van Maldergem L, Magre J, Khallouf TE, et al. . Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet 2002;39:722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simha V, Garg A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J Clin Endocrinol Metab 2003;88:5433–5437 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal AK, Arioglu E, De Almeida S, et al. . AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 2002;31:21–23 [DOI] [PubMed] [Google Scholar]
- 10.Magre J, Delepine M, Khallouf E, et al. . Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 2001;28:365–370 [DOI] [PubMed] [Google Scholar]
- 11.Kim CA, Delepine M, Boutet E, et al. . Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab 2008;93:1129–1134 [DOI] [PubMed] [Google Scholar]
- 12.Hayashi YK. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest 2009;119:2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilz S, Marz W. Free fatty acids as a cardiovascular risk factor. Clin Chem Lab Med 2008;46:429–434 [DOI] [PubMed] [Google Scholar]
- 14.Agarwal AK, Garg A. Genetic disorders of adipose tissue development, differentiation, and death. Ann Rev Genomics Hum Genet 2006;7:175–199 [DOI] [PubMed] [Google Scholar]
- 15.Machado PV, Daxbacher EL, Obadia DL, Cunha EF, Alves Mde F, Mann D. Do you know this syndrome? Berardinelli-Seip syndrome. An Bras Dermatol 2013;88:1011–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajab A, Straub V, McCann LJ, et al. . Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet 2010;6:e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol 2008;98:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shastry S, Delgado MR, Dirik E, Turkmen M, Agarwal AK, Garg A. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet A 2010;152A:2245–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman OU, Khawar N, Khan MA, et al. . Deletion mutation in BSCL2 gene underlies congenital generalized lipodystrophy in a Pakistani family. Diagn Pathol 2013;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rheuban KS, Blizzard RM, Parker MA, Carter T, Wilson T, Gutgesell HP. Hypertrophic cardiomyopathy in total lipodystrophy. J Pediatr 1986;109:301–302 [DOI] [PubMed] [Google Scholar]
- 21.Klar A, Brand A, Hurvitz H, Gross-Kieselstein E, Branski D. Cardiomyopathy in lipodystrophy and the specificity spillover hypothesis. Isr J Med Sci 1993;29:50–52 [PubMed] [Google Scholar]
- 22.Geffner ME, Golde DW. Selective insulin action on skin, ovary, and heart in insulin-resistant states. Diabetes Care 1988;11:500–505 [DOI] [PubMed] [Google Scholar]
- 23.Viegas RF, Diniz RV, Viegas TM, Lira EB, Almeida DR. Cardiac involvement in total generalized lipodystrophy (Berardinelli-Seip syndrome). Arq Bras Cardiol 2000;75:243–248 [DOI] [PubMed] [Google Scholar]
- 24.Lupsa BC, Sachdev V, Lungu AO, Rosing DR, Gorden P. Cardiomyopathy in congenital and acquired generalized lipodystrophy: a clinical assessment. Medicine (Baltimore) 2010;89:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson MD, Victor RG, Szczepaniak EW, Simha V, Garg A, Szczepaniak LS. Cardiac steatosis and left ventricular hypertrophy in patients with generalized lipodystrophy as determined by magnetic resonance spectroscopy and imaging. Am J Cardiol 2013;112:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandalia M, Garg A, Vuitch F, Nizzi F. Postmortem findings in congenital generalized lipodystrophy. J Clin Endocrinol Metab 1995;80:3077–3081 [DOI] [PubMed] [Google Scholar]
- 27.Haque WA, Vuitch F, Garg A. Post-mortem findings in familial partial lipodystrophy, Dunnigan variety. Diabet Med 2002;19:1022–1025 [DOI] [PubMed] [Google Scholar]
- 28.Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract 2010;16:324–333 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez A, Mastronardi CA, Paz-Filho GJ. New advances in the treatment of generalized lipodystrophy: role of metreleptin. Ther Clin Risk Manag 2015;11:1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colman C. Myalept (metreleptin). Presentation at a U.S. Food and Drug Administration Advisory Committee Meeting, 11 December 2013. Available online from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM379648.pdf. Accessed 3 May 2016
- 31.Goldens J. Metreleptin clinical efficacy and safety review. Presentation at a U.S. Food and Drug Administration Advisory Committee Meeting, 11 December 2013. Available online from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM379648.pdf. Accessed 3 May 2016
- 32.Oral EA, Simha V, Ruiz E, et al. . Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346:570–578 [DOI] [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov Safety, tolerability, and pharmacokinetic study of ISIS ApoC-III Rx in hypertriglyceridemia. Available from http://clinicaltrials.gov/ct2/show/NCT01529424. Accessed 22 March 2016
- 34.Meehan CA, Cochran E, Kassai A, Brown RJ, Gorden P. Metreleptin for injection to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy. Expert Rev Clin Pharmacol 2016;9:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]