Abstract
Many enzymes in pathways such as glycolysis associate reversibly with cellular substructures. The spatiotemporal behavior of a "limit-cycle" oscillation model is studied under the condition that the "ambiquitous" oscillophor, phosphofructokinase, is partitioned between "bulk-phase" and "bound" forms in a heterogeneous system. Computer simulation demonstrates the occurrence of sustained, wave-like spatiotemporal patterns of chemical concentration in the bulk medium. Kinetic dissimilarity among the localized populations of bound enzyme leads to a "polarity" effect in the wave phenomenon. It is suggested that a key physiological role of the limit-cycle regime is to engender a rapid, site-to-site, signal-transmission modality in large eukaryotic (e.g., mammalian) cells.
Full text
PDF
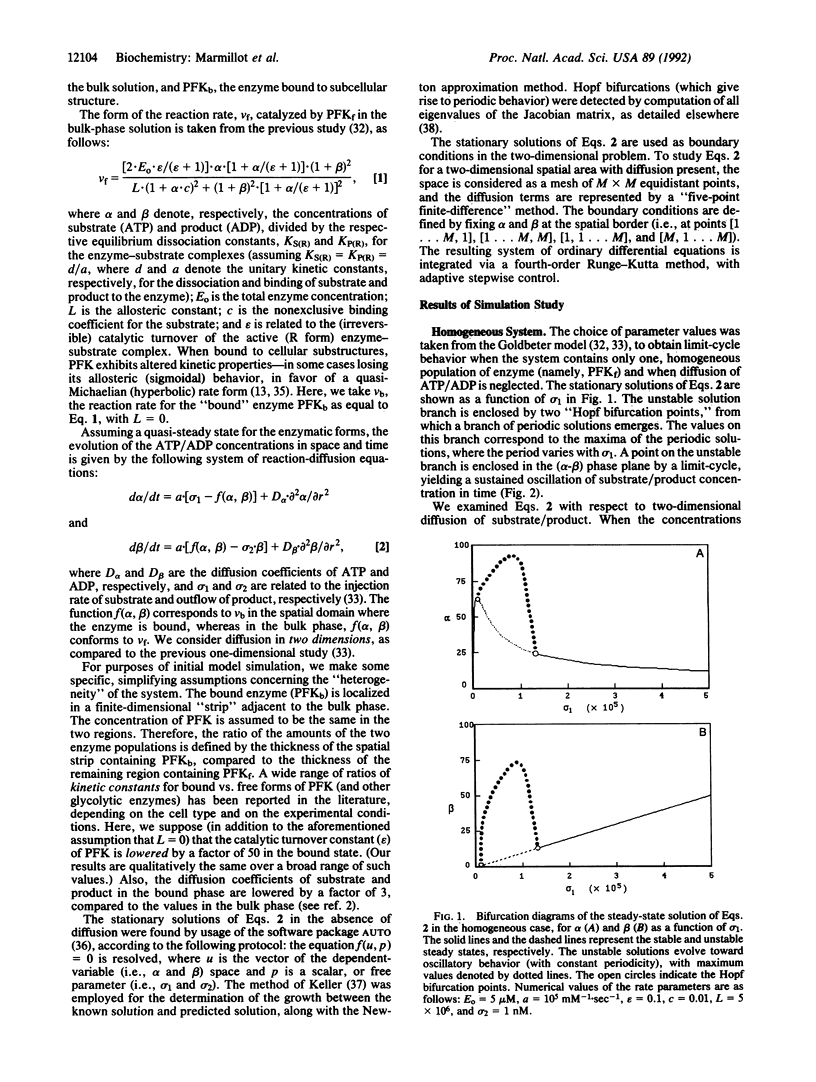
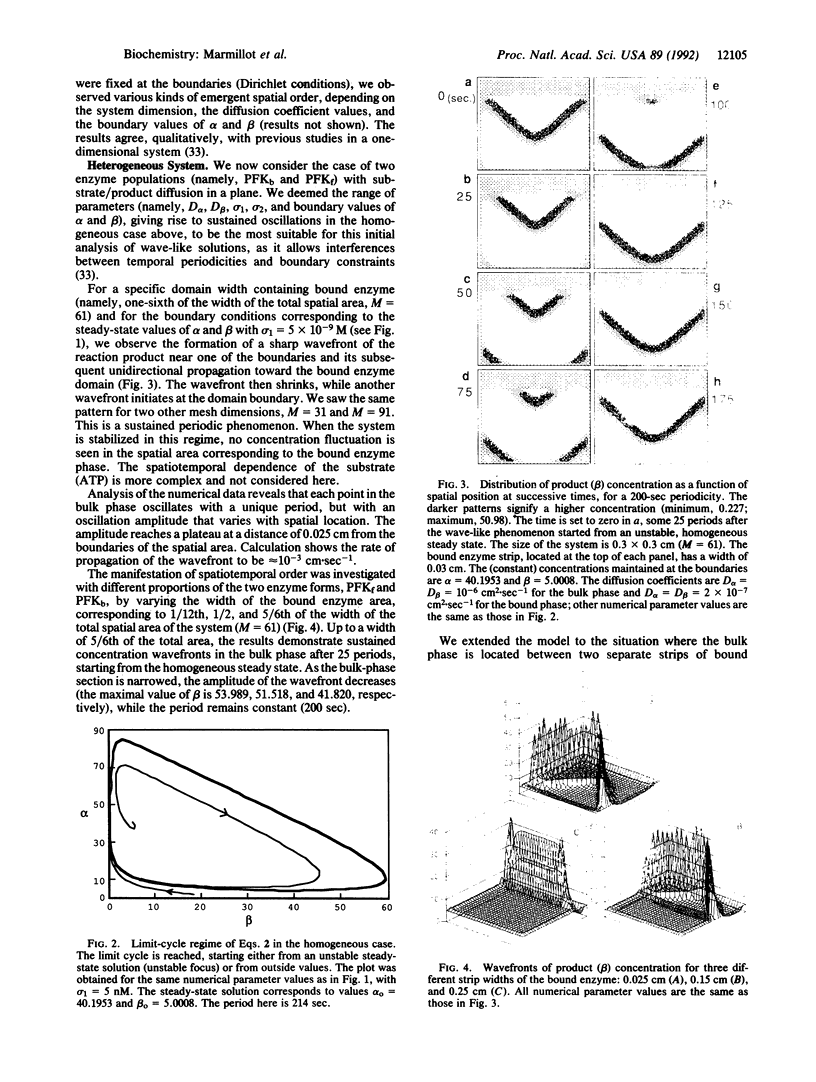
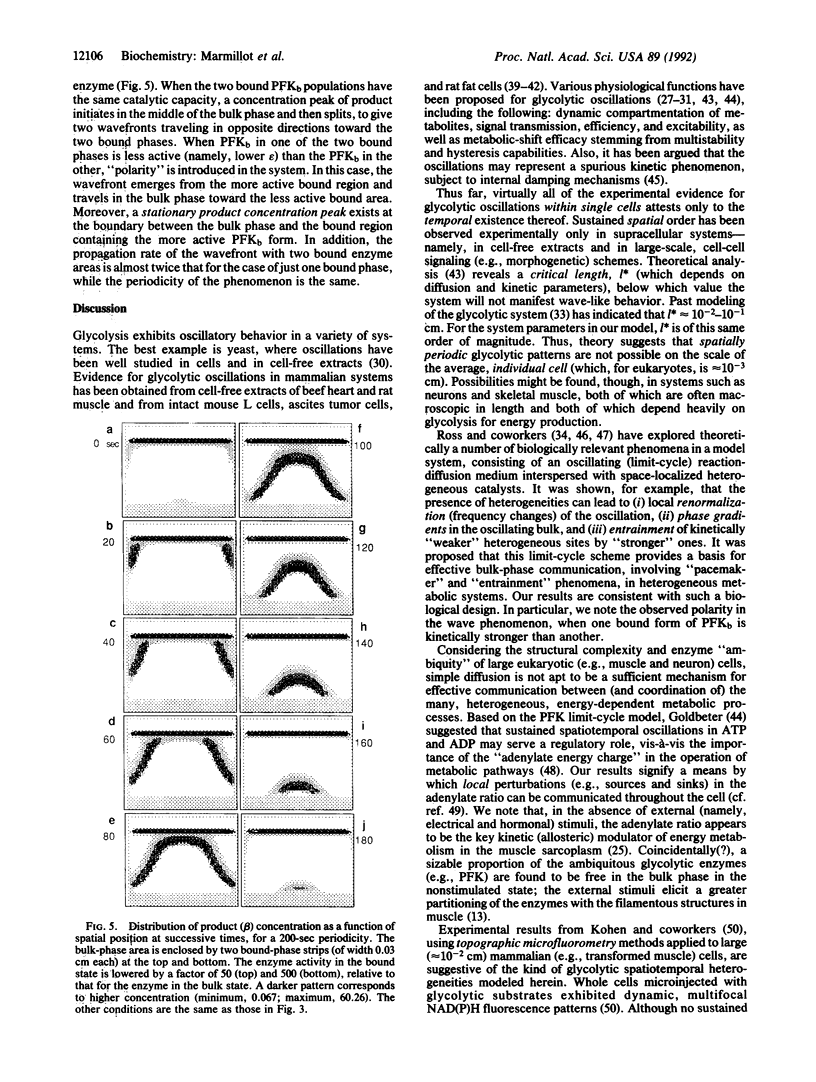

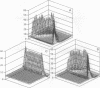
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aon M. A., Cortassa S., Westerhoff H. V., Berden J. A., Van Spronsen E., Van Dam K. Dynamic regulation of yeast glycolytic oscillations by mitochondrial functions. J Cell Sci. 1991 Jun;99(Pt 2):325–334. doi: 10.1242/jcs.99.2.325. [DOI] [PubMed] [Google Scholar]
- Arnold H., Henning R., Pette D. Quantitative comparison of the binding of various glycolytic enzymes to F-actin and the interaction of aldolase with G-actin. Eur J Biochem. 1971 Sep 13;22(1):121–126. doi: 10.1111/j.1432-1033.1971.tb01522.x. [DOI] [PubMed] [Google Scholar]
- Arnold H., Pette D. Binding of aldolase and triosephosphate dehydrogenase to F-actin and modification of catalytic properties of aldolase. Eur J Biochem. 1970 Aug;15(2):360–366. doi: 10.1111/j.1432-1033.1970.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Arnold H., Pette D. Binding of glycolytic enzymes to structure proteins of the muscle. Eur J Biochem. 1968 Nov;6(2):163–171. doi: 10.1111/j.1432-1033.1968.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Dagher S. M., Hultin H. O. Association of glyceraldehyde-3-phosphate dehydrogenase with the particulate fraction of chicken skeletal muscle. Eur J Biochem. 1975 Jun 16;55(1):185–192. doi: 10.1111/j.1432-1033.1975.tb02150.x. [DOI] [PubMed] [Google Scholar]
- Frenkel R. DPNH oscillations in glycolyzing cell free extracts from beef heart. Biochem Biophys Res Commun. 1965 Dec 9;21(5):497–502. doi: 10.1016/0006-291x(65)90411-0. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Caplan S. R. Oscillatory enzymes. Annu Rev Biophys Bioeng. 1976;5:449–476. doi: 10.1146/annurev.bb.05.060176.002313. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Lefever R. Dissipative structures for an allosteric model. Application to glycolytic oscillations. Biophys J. 1972 Oct;12(10):1302–1315. doi: 10.1016/S0006-3495(72)86164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A. Modulation of the adenylate energy charge by sustained metabolic oscillations. FEBS Lett. 1974 Aug 1;43(3):327–330. doi: 10.1016/0014-5793(74)80672-1. [DOI] [PubMed] [Google Scholar]
- Goldbeter A. Patterns of spatiotemporal organization in an allosteric enzyme model. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3255–3259. doi: 10.1073/pnas.70.11.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervagault J. F., Duban M. C., Kernevez J. P., Thomas D. Multiple steady states and oscillatory behavior of a compartmentalized phosphofructokinase system. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5455–5459. doi: 10.1073/pnas.80.18.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T., Richards C. S., Uyeda K. The interaction of phosphofructokinase with erythrocyte membranes. J Biol Chem. 1979 Oct 10;254(19):9542–9550. [PubMed] [Google Scholar]
- Ibsen K. H., Schiller K. W. Control of glycolysis and respiration in substrate-depleted Ehrlich ascites tumor cells. Arch Biochem Biophys. 1971 Mar;143(1):187–203. doi: 10.1016/0003-9861(71)90199-8. [DOI] [PubMed] [Google Scholar]
- Karadsheh N. S., Uyeda K. Changes in allosteric properties of phosphofructokinase bound to erythrocyte membranes. J Biol Chem. 1977 Nov 10;252(21):7418–7420. [PubMed] [Google Scholar]
- Kemp R. G., Foe L. G. Allosteric regulatory properties of muscle phosphofructokinase. Mol Cell Biochem. 1983;57(2):147–154. doi: 10.1007/BF00849191. [DOI] [PubMed] [Google Scholar]
- Knull H. R. Association of glycolytic enzymes with particulate fractions from nerve endings. Biochim Biophys Acta. 1978 Jan 12;522(1):1–9. doi: 10.1016/0005-2744(78)90316-9. [DOI] [PubMed] [Google Scholar]
- Lipkin E. W., Teller D. C., de Haën C. Dynamic aspects of insulin action: synchronization of oscillatory glycolysis in isolated perifused rat fat cells by insulin and hydrogen peroxide. Biochemistry. 1983 Feb 15;22(4):792–799. doi: 10.1021/bi00273a013. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Lanni F., Taylor D. L. The submicroscopic properties of cytoplasm as a determinant of cellular function. Annu Rev Biophys Biophys Chem. 1988;17:369–396. doi: 10.1146/annurev.bb.17.060188.002101. [DOI] [PubMed] [Google Scholar]
- Masters C. J. Interactions between soluble enzymes and subcellular structure. CRC Crit Rev Biochem. 1981;11(2):105–143. doi: 10.3109/10409238109108700. [DOI] [PubMed] [Google Scholar]
- Porter K. R. The cytomatrix: a short history of its study. J Cell Biol. 1984 Jul;99(1 Pt 2):3s–12s. doi: 10.1083/jcb.99.1.3s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P. H., Ross J. Concentration oscillations and efficiency: glycolysis. Science. 1981 Feb 13;211(4483):715–717. doi: 10.1126/science.6450447. [DOI] [PubMed] [Google Scholar]
- Sigel P., Pette D. Intracellular localization of glycogenolytic and glycolytic enzymes in white and red rabbit skeletal muscle: a gel film method for coupled enzyme reactions in histochemistry. J Histochem Cytochem. 1969 Apr;17(4):225–237. doi: 10.1177/17.4.225. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Tornheim K., Lowenstein J. M. The purine nucleotide cycle. Control of phosphofructokinase and glycolytic oscillations in muscle extracts. J Biol Chem. 1975 Aug 25;250(16):6304–6314. [PubMed] [Google Scholar]
- Walder J. A., Chatterjee R., Steck T. L., Low P. S., Musso G. F., Kaiser E. T., Rogers P. H., Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984 Aug 25;259(16):10238–10246. [PubMed] [Google Scholar]
- Welch G. R. On the role of organized multienzyme systems in cellular metabolism: a general synthesis. Prog Biophys Mol Biol. 1977;32(2):103–191. [PubMed] [Google Scholar]