Abstract
At least 53 distinct isoforms of Plasmodium falciparum chloroquine resistance transporter (PfCRT) protein are expressed in strains or isolates of P. falciparum malarial parasites from around the globe. These parasites exhibit a range of sensitivities to chloroquine (CQ) and other drugs. Mutant PfCRT is believed to confer cytostatic CQ resistance (CQRCS) by transporting CQ away from its DV target (free heme released upon hemoglobin digestion). One theory is that variable CQ transport catalyzed by these different PfCRT isoforms is responsible for the range of CQ sensitivities now found for P. falciparum. Alternatively, additional mutations in drug-selected parasites, or additional functions of PfCRT, might complement PfCRT-mediated CQ transport in conferring the range of observed resistance phenotypes. To distinguish between these possibilities, we recently optimized a convenient method for measuring PfCRT-mediated CQ transport, involving heterologous expression in Saccharomyces cerevisiae. Here, we use this method to quantify drug transport activity for 45 of 53 of the naturally occurring PfCRT isoforms. Data show that variable levels of CQR likely depend upon either additional PfCRT functions or additional genetic events, including perhaps changes that influence DV membrane potential. The data also suggest that the common K76T PfCRT mutation that is often used to distinguish a P. falciparum CQR phenotype is not, in and of itself, a fully reliable indicator of CQR status.
Graphical abstract

Mutations in the Plasmodium falciparum chloroquine (CQ) resistance transporter (PfCRT) are believed to be the primary determinant of P. falciparum malaria chloroquine resistance (CQR) as measured by a shift in CQ IC50.1–3 However, higher-level resistance to cytocidal (parasite kill) effects of CQ is only partially mediated by PfCRT.3,4 PfCRT is a polytopic, integral membrane protein that under physiologic conditions likely functions to transport important osmolytes across the digestive vacuole (DV) membrane.5,6 Mutant, CQR-associated isoforms of PfCRT are believed to confer CQR by mediating an increased level of transport of charged CQ out of the DV down its electrochemical gradient and by decreasing the number of CQ–heme target interactions via perturbations in DV volume and/or pH.1–3,5,7
P. falciparum CQR evolved independently in at least six distinct geographic locations and is continuing both to evolve and to spread. There are now known to be at least 53 distinct isoforms of PfCRT, which are distinguished by the number and pattern of amino acid substitutions relative to wild-type (e.g., “HB3” or “3D7”) PfCRT. Although no one simple explanation has yet been identified, these different mutational patterns are segregated on the basis of parasite geographic origin and are thought to have arisen in part by selection with variable drug pressure due to different antimalarial drug-use histories around the globe.2,8 Of the 53 isoforms, 11 are found in culture-adapted strains for which highly reproducible CQ IC50 data quantifying CQ cytostatic potency are available.9 However, the remaining isoforms are found in field isolates whose drug susceptibilities are typically determined via more qualitative single-pass assays, or not determined at all.
Several recent field studies have found that the suspension of CQ treatment within a region and the subsequent absence of continued CQ selective pressure allow parasites expressing wild-type PfCRT to repopulate malaria endemic regions, possibly because of a parasite fitness cost associated with the expression of some CQR-associated mutant PfCRTs.10,11 However, other parasite populations in some regions have shown little or no change in the ratio of mutant to wild-type PfCRT expression years after CQ withdrawal.8,12,13 This suggests that certain mutant PfCRT isoforms are found in CQR isolates that are more “fit” than others, as has been suggested for some isoforms of South American origin.8 Alternatively, parasite populations in some regions may have acquired additional genetic mutations that compensate for fitness costs associated with expressing a given mutant PfCRT.14,15 A third possibility is that certain mutant isoforms of PfCRT persist because they confer cross-resistance to other antimalarials that are still in use in that region,8,16 and a fourth is that some mutant PfCRT isoforms are actually revertants that do not confer CQR phenotypes at all but actually confer reacquired “fit” CQS phenotypes.17
Distinction between these models is critical and relies in part on the ability to rapidly quantify PfCRT function. Unfortunately, very few (if any) quantitative data regarding the function of most PfCRT isoforms are available. This is because it is often difficult (and expensive) to establish P. falciparum isolates as stably growing laboratory strains, and to then isolate the contribution of PfCRT function to altered drug susceptibility versus contributions from additional mutations that may be present. Recently, we optimized a fast, inexpensive method to rapidly screen CQ transport mediated by PfCRT.9,18,19 This method, based on tight inducible heterologous expression in growing Saccharomyces cerevisiae, separates PfCRT function from other P. falciparum factors that can influence drug accumulation, allows us to easily distinguish differences in CQ transport mediated by CQ sensitive (CQS) versus resistant (CQR) PfCRT isoforms, and permits distinction between even subtly different CQR isoforms.9 Initial work with 11 PfCRT isoforms that are expressed in laboratory-cultured P. falciparum strains suggested that PfCRT mutations are necessary but not sufficient for the full shift in CQ IC50 seen for these strains.9 To further aid our understanding of the relative contribution of PfCRT isoforms to geographically disposed CQR phenotypes, in this paper we report on 45 of the currently known naturally occurring PfCRT isoforms.
MATERIALS AND METHODS
Materials
Yeast DOB were obtained in powder form from MP Biomedicals (Solon, OH). Cell culture plastics were from BD Falcon. Glucose, galactose, and raffinose were obtained from Sigma (St. Louis, MO). Glass beads for yeast cell lysis were from B. Braun Biotech (Allentown, PA). Anti-V5-HRP antibody was from Invitrogen (Carlsbad, CA). Mutagenesis reagents were obtained from Agilent (Santa Clara, CA). All other chemicals were reagent grade or better and were purchased from Sigma and used without additional purification.
Yeast Strains and Methods
CH1305 (MATa ade2 ade3 ura3-52 leu2 lys2-801) was kindly provided by J. F. Cannon.20 Solid and liquid media were prepared as described by Sherman et al.21 and included synthetic complete (SC) medium lacking one or more specified amino acids, as well as rich medium (YPD). Induction of CRT protein expression, standard yeast growth methods, yeast transfections, and other routine methods were as described previously.9,18
Plasmids
The pYES2 backbone containing PfHB3vh, PfDd2vh, and Pf7G8vh was constructed previously18 and used as template DNA in subsequent rounds of multi-site-directed mutagenesis with oligonucleotides detailed in Table 1 via the Agilent QUICKChange method to create the various isoforms of PfCRT (see Table 2). All constructs were confirmed by direct DNA sequencing of the full pfcrt gene.
Table 1.
Oligonucleotides Used in This Study
| name | sequence (5′-3′) |
|---|---|
| Q10K | CGCATCTAAGAAGAACAACAAGAAGAACTCCTCTAAGAACG |
| D24Y | CGAACGTTACAGAGAATTGTACAACCTGGTTCAAGAGGG |
| S39P | GATTGGGTGGAGGTCCCTGTTTGGGCAAGTG |
| S72C | GTCCATCATCTACCTGTCAGTTTGCGTGATGAACAC |
| M74I | CATCTACCTGTCAGTTTGCGTGATAAACAAGATCTTCG |
| N75E | CCTGTCAGTTTGCGTGATGGAGAAGATCTTCGCTAAGAGAA |
| E75D | CCTGTCAGTTTGCGTGATCGATACCATCTTCGC |
| K76T | CAGTTTGCGTGATGAACACGATCTTCGCTAAGAGAAC |
| T76K | GTCAGTTTGCGTGATCGAAAAGATCTTCGCGAAGAGAACCT |
| T76A | GCGTGATAGAGGCGATCTTCGCTAAGAGAAC |
| CK72,76ST | CCATCATCTACCTGTCAGTTAGCGTGATGAACACGATCTTCGC |
| NK75–76ET | ACCTGTCAGTTTGCGTGATGGAGACGATCTTCGCTAAGAGAACCT |
| MNK74–76IET | CATCTACCTGTCAGTTTGCGTGATAGAGACGATCTTCGCTAAGAGAACCTTGAA |
| H97Q | CGTGACTAGTGAAACCCAGAACTTCATCTGCATGATC |
| A144T | CTTGCAGCGTCATCTTGACCTTCATCGGTCTTACC |
| AL144,148FI | CTTGCAGCGTCATCTTGTTCTTCATCGGTATTACCAGAACCACAGGT |
| L148I | CTTGGCCTTCATCGGTATTACCAGAACCACAGG |
| I148L | GGCCTTCATCGGTCTTACCAGAACCACAGG |
| L160Y | CAGGTAACATTCAGTCCTTCGTCTATCAACTATCAATTCCAATCAACATG |
| L160P | GGTAACATTCAGTCCTTCGTCCCGCAACTATCAATTCC |
| I166V | CAACTATCAATTCCAGTCAACATGTTCTTCTGCTTCC |
| S163R | TCAGTCCTTCGTCTTGCAACTAAGAATTCCAATCAACATGTTCTTC |
| I194T | CAGTAATCATCGTAGTCACAACCGCATTGGTGGAAATG |
| I194S | CAGTAATCATCGTAGTCACATCCGCATTGGTGGAAATG |
| E198K | CACAATCGCATTGGTGAAAATGAAGCTGAGCTTCG |
| A220S | CATCTACCTGTCAGTTTGCGTGATAGAGACGATCTTCGCTAAGAGAACCTTGAA |
| S220A | CCTAGTCCTGATATCCGCTCTGATCCCTGTCTG |
| F251Δ | GATTGAACGCTATGGTTAGCTTCCAACTGTTCACTTCATGC |
| H273N | CCATTCCTGAAGCAATTAAACTTGCCATACAACGAAATC |
| Q271E | CACACTACCATTCCTGAAGGAGTTACACTTGCCATACAACG |
| N277D | CACTTGCCATACGACGAAATCTGGACCAAC |
| N326S | GCCTTGTTCTCATTCTTCAGCATCTGTGATAACCTGATC |
| N326D | CGCCTTGTTCTCATTCTTCGACATCTGTGATAACCTGAT |
| I327Δ | CGCCTTGTTCTCATTCTTCTCCTGTGATAACCTGATCACCAGC |
| T333S | ATCTGTGATAACCTGATCAGCAGCTACATCATCGATAAG |
| S333T | CTGTGATAACCTGATCACCAGCTACATCATCGAC |
| T333A | CTGTGATAACCTGATCGCCAGCTACATCATCAAC |
| S334N | GATAACCTGATCACCAACTACATCATCGACAAG |
| I356T | CATCCAGGGTCCCGCAACCGCTATTGCCT |
| T356I | ATCCAGGGGCCCGCAATCGCTATTGCC |
| R371I | CTTAGCAGGTGATGTCGTAATAGAACCACGTTTGTTG |
| I371R | CTTAGCAGGTGATGTCGTAAGAGAACCACGTTTGTTG |
Table 2.
Amino Acid Sequences of 53 Known Naturally Occurring PfCRT Isoformsa
| Origin | Clone/ Isolate | 10 | 24 | 39 | 72 | 74 | 75 | 76 | 97 | 123 | 144 | 148 | 160 | 163 | 166 | 194 | 198 | 205 | 220 | 251 | 271 | 273 | 277 | 326 | 527 | 333 | 334 | 350 | 356 | 371 | Isolations | IC50 (nM) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Honduras | HB3 | Q | D | S | C | M | N | K | H | H | A | L | L | S | 1 | I | E | T | A | F | Q | H | N | X | I | T | S | C | I | R | 12.3,33.9 | |
| Thailand | Dd2 | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | E | H | N | S | I | T | S | C | T | I | 48, 404 | |
| Sudan | 106/1 | 0 | D | S | C | I | E | K | H | H | A | L | L | S | I | I | E | T | S | F | E | H | N | S | I | T | S | C | I | I | 15,37.8 | |
| Ecuador | Ecu1110 | Q | D | S | C | M | N | T | H | H | A | L | L | S | I | I | E | T | S | F | Q | H | N | D | I | T | S | C | L | R | 90.0, 156 | |
| Solomon | PNG4 | Q | D | S | S | M | N | T | H | H | A | L | L | S | I | I | E | T | A | F | Q | H | N | D | I | T | S | C | L | R | 251 | |
| Brazil | 7G8 | Q | D | S | S | M | N | T | H | H | A | L | L | S | I | I | E | T | S | F | Q | H | N | D | I | T | S | C | L | R | 34, 220 | |
| Colombia | Jav | Q | D | S | C | M | E | T | Q | H | A | L | L | S | I | I | E | T | S | F | Q | H | N | N | I | T | S | C | I | T | 137,305 | |
| Ghana | GB4 | Q | D | S | C | 1 | E | T | H | H | A | L | L | S | I | I | E | T | S | F | E | H | N | N | I | T | S | C | I | I | 89.8, 144 | |
| Thailand | FCB | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | E | H | X | S | I | T | S | C | I | I | 135,492 | |
| Thailand | TM6 | Q | D | S | C | I | E | T | H | R | A | L | L | S | I | I | E | A | S | F | E | H | N | S | I | T | S | C | I | I | 111, 167 | |
| Thailand | TM93 | Q | D | S | C | I | E | T | L | A | L | L | S | I | I | E | T | S | F | E | H | N | S | I | T | S | C | T | I | 1200 | ||
| Cambodia | 734 | Q | D | S | C | I | D | T | H | H | F | I | L | S | I | T | E | T | S | F | E | H | N | N | I | S | S | C | I | R | 39 | 33.2-169.4 |
| Cambodia | 738 | Q | D | S | C | I | D | T | H | H | A | I | L | S | I | T | E | T | S | F | E | H | N | N | I | S | S | C | I | R | I | 157 |
| Cambodia | 783 | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | E | H | N | N | I | T | S | C | T | I | 1 | 134 |
| China | B | Q | D | S | C | I | D | T | H | Y | L | L | S | I | I | E | T | A | F | E | H | N | N | I | T | S | C | I | R | 7 | X.D. | |
| China | C | Q | D | S | C | I | D | T | H | Y | L | L | S | I | I | E | T | A | F | E | H | N | N | I | T | S | C | I | I | 1 | N.D. | |
| China | D | Q | D | S | C | I | E | T | H | Y | L | L | S | I | I | E | T | A | F | E | H | N | N | I | T | S | C | I | R | 1 | N.D. | |
| China | E | Q | D | S | C | I | E | T | H | A | L | L | S | I | I | E | T | S | F | E | H | N | N | I | T | S | C | I | R | I | X.D. | |
| Thailand | BC7 | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | E | H | N | S | - | T | S | C | T | I | 1 | 31-78 |
| Thailand | KS28 | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | - | E | H | N | S | I | T | S | C | T | I | 1 | 74.0 |
| Thailand | BC22 | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | K | T | S | F | E | H | N | S | I | T | S | C | T | I | 4 | 31-78 |
| Thailand | J9 | Q | D | p | C | I | E | A | H | H | A | L | L | S | I | I | E | T | S | F | F | H | D | S | I | T | S | C | T | I | 1 | 123 |
| Colombia | TA6182 | Q | D | S | C | M | E | T | Q | H | A | L | L | S | I | I | E | T | S | F | Q | H | N | S | I | T | S | C | I | I | 2 | N.D. |
| Colombia | TU741 | Q | D | S | C | M | N | T | H | H | A | L | L | S | I | I | E | T | S | F | Q | H | N | D | I | T | N | C | L | R | 1 | N.D. |
| Vietnam | IsoI | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | E | H | N | N | I | S | S | C | I | I | 1 | N.D. |
| Vietnam | IsoII | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | E | H | N | N | I | S | S | C | T | I | 1 | N.D. |
| Vietnam | IsoIII | Q | D | S | C | I | D | T | H | H | A | L | L | S | I | T | E | T | S | F | E | H | N | N | I | S | S | C | I | R | 1 | N.D. |
| Vietnam | IsoIV | Q | D | S | C | I | D | T | H | H | A | L | L | I | I | T | E | T | S | F | E | H | N | N | I | T | S | C | I | R | 1 | N.D. |
| Vietnam | IsoV | Q | D | S | C | I | D | T | H | H | F | I | L | S | 1 | S | E | T | S | F | E | H | N | N | I | S | S | C | I | R | 1 | N.D. |
| I. Papau | 2300 | Q | D | S | C | I | K | T | H | H | A | L | L | S | I | I | E | T | S | F | E | H | N | S | I | T | S | C | I | I | 1 | N.D. |
| F. Guiana | H209 | Q | D | S | S | M | N | T | H | H | A | L | L | S | I | I | E | T | S | F | Q | H | N | D | I | T | S | R | L | R | 1 | 34.8 |
| SEA | Pfl64 | Q | D | S | C | I | E | T | H | H | A | L | L | R | I | I | E | T | S | F | H | H | N | S | I | T | S | C | I | I | 1 | 21 |
| Tanzania | GadI | Q | Y | S | C | M | N | K | H | H | A | L | L | S | I | I | E | T | A | F | Q | H | N | N | I | T | S | C | I | R | 1 | N.D. |
| Tanzania | GadII | K | Y | S | C | M | N | K | H | H | A | L | L | S | I | I | E | T | A | F | Q | H | N | N | I | T | S | C | I | R | 1 | N.D. |
| Tanzania | GadIII | Q | D | S | C | M | N | K | H | H | A | L | L | S | I | I | E | T | A | F | Q | H | N | N | I | T | S | C | I | I | I | N.D. |
| Tanzania | GadIV | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | N | H | N | N | I | T | S | C | I | R | 1 | N.D. |
| Tanzania | GadV | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | N | H | N | N | I | T | S | C | I | I | 1 | N.D. |
| Tanzania | GadVI | Q | D | S | C | I | E | T | H | H | A | L | L | S | I | I | E | T | S | F | N | H | N | S | I | T | S | C | I | 1 | I | N.D. |
| Phillipines | PH1 | Q | D | S | C | M | N | T | H | H | T | L | Y | S | I | I | E | T | A | F | Q | H | N | D | I | T | S | C | I | R | 33 | 30.4 |
| Phillipines | PH2 | Q | D | S | S | M | N | T | H | H | I | L | Y | S | I | I | E | T | A | F | Q | H | N | D | I | T | S | C | I | R | 15 | N.D. |
| Malaysia | KT099 | Q | D | S | C | M | N | K | H | H | A | L | L | S | I | I | E | I | A | F | D | H | N | D | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KK038 | Q | D | S | S | M | N | T | H | H | A | L | L | S | I | I | E | T | A | F | D | H | N | N | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KK004 | Q | D | S | C | M | N | K | H | H | A | L | L | S | I | I | E | T | S | F | Q | H | N | D | I | A | S | C | L | R | 1 | N.D. |
| Malaysia | KK005 | Q | D | S | S | M | N | T | H | H | A | L | L | S | I | I | E | T | S | F | Q | H | N | I | I | A | S | C | L | R | 14 | N.D. |
| Malaysia | KT088 | Q | D | S | S | M | N | T | H | H | A | L | L | S | I | I | E | T | A | F | Q | N | N | N | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KT096 | Q | D | S | S | M | N | T | H | H | A | L | L | S | I | I | E | T | A | F | Q | N | N | D | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KT070 | Q | D | S | C | M | N | K | H | H | A | L | L | S | I | I | E | T | A | F | Q | N | N | N | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KT052 | Q | D | S | C | M | N | K | H | H | T | L | Y | S | V | I | E | T | A | F | Q | H | N | N | I | T | S | C | I | R | 3 | N.D. |
| Malaysia | KT097 | Q | D | S | C | M | N | K | H | H | I | L | Y | S | V | I | E | T | A | F | Q | N | N | N | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KT094 | Q | D | S | S | M | N | T | H | H | H | L | P | S | I | I | E | T | A | F | Q | N | N | N | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KT072 | Q | D | S | S | M | N | T | H | H | T | L | Y | S | V | I | E | T | A | F | Q | H | N | N | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KT055 | Q | D | S | S | M | N | T | H | H | T | L | Y | S | V | I | E | T | A | F | Q | N | N | N | I | T | S | C | I | R | 1 | N.D. |
| Malaysia | KT066 | Q | D | S | S | M | N | T | H | H | T | L | Y | S | V | I | E | T | A | F | D | H | N | D | I | T | S | C | I | R | 1 | N.D. |
Isoforms found in isolates that have been established as strains are colored black, while those that have not been adapted to stable laboratory culture are colored red. Residues mutated relative to the wild type are highlighted in green. Empty cells denote residues for which sequence data are not available, and a dash denotes a deletion mutation. Where multiple CQ IC50 values were found in the literature, the high and low values are reported (low, high). If a range of values for isolates bearing a single PfCRT isoform was reported in a single study, the range is indicated (low-high).
Preparation of Yeast Membranes
Yeast membranes were isolated using a glass bead lysis method with some modifications.22 Overnight cultures of yeast cells grown in inducing SGR-ura medium were harvested at 2000g for 5 min at 25 °C. Cells were washed three times in harvest buffer [100 mM glucose, 50 mM imidazole, and 5 mM DTT (pH 7.5)] and lysed by vortexing in the presence of glass beads (1.00–1.05 mm diameter glass beads). Suspensions {0.5 g wet weight of cells, 500 μL of breaking buffer [250 mM sucrose, 100 mM glucose, 50 mM imidazole, and 1.0 mM MgCl2 (pH 7.50)], equal volume of glass beads} were homogenized via vortexing (maximum speed) for 30 s followed by 30 s on ice, a process that was repeated 20 times. Suspensions were pipetted away from the glass beads and centrifuged at 700g for 5 min followed by centrifugation for additional 5 min at 1300g to remove unlysed cells and cellular debris. The supernatant was collected and spun again at 100000g to collect the crude membrane (CM) fragment. An amido black assay was conducted to determine the protein content of each CM sample, and membranes were stored at −80 °C prior to determination of PfCRT content via Western blotting.
Western Blotting
Western blots were performed with anti-V5-HRP as described previously.9
Quantitative Growth Rate Analysis
Assays were performed under CRT-inducing and noninducing conditions as previously described.9,18 Growth under each condition was measured in triplicate with the starting cell density set to an OD600 of 0.1 via back dilution of the strain grown under noninducing conditions (SD medium lacking uracil); 96-well plates were wrapped in parafilm and placed in a Tecan (Durham, NC) GENious plate reader, and growth curve data were collected using the following parameters: measurement wavelength, 595 nm; number of flashes, 3; number of kinetic cycles, 125; kinetic interval, 30 min; valid temperature range, 28–32 °C; orbital shake duration, 30 s. PfCRT-induced, CQ-dependent growth delays under standard conditions (pH 6.75 and 16 mM CQ) as well as under conditions of PQ-induced recovery of CQ growth inhibition (pH 6.75, 16 mM CQ, and 0.7 mM PQ) were calculated as described previously9 via determination of the difference in time to reach the maximal growth rate in PfCRT noninducing versus inducing medium. Growth delays in the presence of elevated Δψ values were calculated by direct comparison to growth of the empty vector control under identical inducing conditions. PQ growth assays were conducted at pH 6.75 in inducing medium containing 1.8 mM PQ. PfCRT-induced, PQ-dependent growth delays were determined via comparison to control cells grown under identical inducing conditions.
RESULTS
Previously, we optimized a fast, inexpensive assay for quantifying CQ transport mediated by PfCRT. The approach relies on tight galactose induction of PfCRT in S. cerevisiae yeast, plasma membrane localization of PfCRT, and CQ inhibition of yeast growth. Native PfCRT within the malarial parasite DV membrane transports charged CQ from inside the DV (positive membrane potential, low pH) to the parasite cytosol (negative potential, higher pH) (Figure 1A). For yeast, the majority of expressed PfCRT is found within the plasma membrane18 and PfCRT cytosolic domains remain disposed to the cytosol, with intra-DV domains disposed outside the cell (Figure 1B). Similar magnitude ΔΨ and ΔpH values relative to the DV membrane are found across the yeast plasma membrane of growing yeast (Figure 1B), with negative potential and higher pH again cytosolic. Thus, the orientation of thermodynamic driving forces for transport versus PfCRT membrane topology is preserved. Because CQ2+ transport by PfCRT is stimulated by ΔΨ and ΔpH oriented as in the DV,7,9,23 in yeast, PfCRT mediates fast downhill transport of CQ2+ from outside the cell (positive membrane potential, low pH) toward the yeast cytosol (negative potential, high pH) (Figure 1B; see also ref 9). The inward transport of toxic CQ by PfCRT expressed in the yeast leads to decreased rates of yeast growth relative to control.18 Importantly, previously we showed that these decreased rates of growth were linearly correlated with an increased rate of [3H]CQ transport; thus, quantitative analysis of yeast growth rates can be used to calculate the rate of [3H]CQ transport.9 A higher ΔΨ and/or ΔpH of the correct orientation (Figure 1C) is known to stimulate drug transport by CQR (but not CQS)-associated isoforms of PfCRT that are either expressed in yeast9 or purified and reconstituted into proteoliposomes.7
Figure 1.
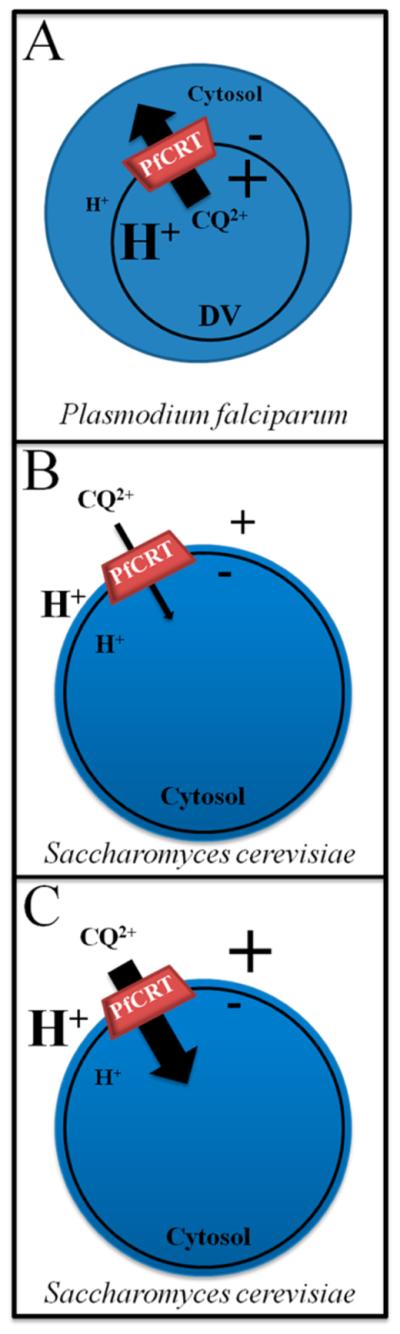
Cartoon schematic depicting PfCRT localization and topology and direction of PfCRT-mediated CQ transport in (A) P. falciparum and (B and C) S. cerevisiae. (A) Native PfCRT within the malaria parasite DV membrane transports charged CQ from inside the DV (positive membrane potential, low pH) to the parasite cytosol (negative potential, high pH), and cytosolic domains of PfCRT are disposed to the cytosol. (B) Codon-optimized PfCRT expressed at the yeast plasma membrane transports charged CQ from the external growth medium (positive potential, low pH) to the yeast cytosol (negative potential, high pH). (C) Yeast ΔΨ can be clamped to a magnitude similar to that across the DV membrane. The rate of PfCRT-mediated transport of CQ2+ is increased at higher ΔΨ (see refs 7 and 9).
We have created “yeast-optimized” versions of PfCRT genes encoding all 53 known, naturally occurring PfCRTs (Table 2; see ref 24 for the optimized wild-type PfCRT sequence used as the template for mutagenesis). The geographic origin of isolates harboring these PfCRTs and all available CQ susceptibility data for the corresponding isolates culled from the literature are summarized in Table 2. All isoforms are expressed to nearly equal levels in S. cerevisiae yeast upon galactose induction, as shown for 33 isoforms in Figure 2 (note that ref 9 quantifies another 12 and ref 19 eight more).9,19 As previously described for a limited selection of 13 PfCRT isoforms,9 screening these isoforms for their ability to transport CQ revealed a wide range of activity, as also now seen for the collection of 45 (Figure 3). In all screens that we perform, Dd2 PfCRT (CQR conferring) and HB3 PfCRT (CQS) are included as internal controls.9,18,19 Although conflicting interpretation can be found in the literature (see ref 1 for a summary), in our hands, both purified, reconstituted wild-type PfCRT (e.g., HB3 PfCRT) and HB3 PfCRT expressed in yeast transport CQ, albeit less well than CQR isoforms of PfCRT.7,9,18 This result was also recently confirmed independently via analysis of a purified and reconstituted wild-type PfCRT–YbeL2 chimera.25 In this paper, we thus scale mutant PfCRT isoform CQ transport relative to that catalyzed by wild-type HB3 PfCRT under identical conditions (Figure 3).
Figure 2.

Western blot analysis of 33 PfCRT isoforms expressed in S. cerevisiae. Each lane contains 7 μg of total protein. The α-V5 blot shows similar levels of protein expression are found for each PfCRT isoform. HB3 PfCRT (“wild type”) is included in each blot as an internal control. EV indicates the “empty vector” negative control.
Figure 3.
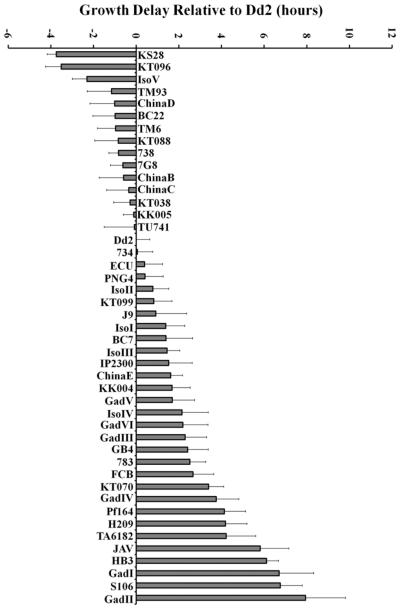
Growth delays (left axis) and calculated CQ uptake (right axis) mediated by 45 of 53 naturally occurring PfCRT isoforms, as well as an artificial A144Y Dd2 mutant PfCRT (far left, two asterisks), expressed in yeast (see refs 9 and 18). Data are expressed relative to HB3 PfCRT, which confers a delay of 13.9 h and [3H]CQ transport of 0.48 pmol (106 cells)−1 min−1.9 Error bars represent the SEM after analysis of at least three independent yeast clones harboring each PfCRT isoform, each clone analyzed in triplicate (nine determinations in total). Isoforms for which growth delay and CQ transport were previously reported9 are marked with one asterisk.
Isoforms harboring some, but not all, of the eight mutations seen in CQR-associated Dd2 PfCRT relative to CQS-associated (“wild type”) HB3 PfCRT [e.g., isoforms 783, ChinaE, and GadV (see Table 2)] transport CQ at levels between those of HB3 (low) and Dd2 (high) (Figure 3). The same holds true for isoforms TA6182 and TU741,26 which have amino acid substitution patterns similar to those of PfCRTs found in CQR strains Jav and Ecu, respectively (Table 2). Two additional isoforms, BC22 and Pf164, are distinguished by single-amino acid substitutions that deliver additional positive charge to an otherwise “Dd2-like” PfCRT (Table 2). We find that the E198K substitution in BC22 reduces transport ~40% relative to that of Dd2 PfCRT (Figure 3), while the S163R mutation in Pf164 PfCRT did not significantly alter transport relative to that of Dd2 PfCRT (Figure 3).
Interestingly, five recently isolated PfCRTs show increased CQ transport relative to that of HB3 PfCRT even though they harbor only a partial complement of the full spectrum of amino acid substitutions found for Dd2 PfCRT.27 For example, “Gad IV” retains 74I, 75E, 76T, and 220S substitutions found in Dd2 PfCRT but is missing 271E, 326S, 356T, and 371I mutations (Table 2). Until now, it has been thought that mutant PfCRT cannot mediate increased CQ transport relative to that of the wild type without at least six of eight and four of five of the mutations found in “archtypical” CQR-associated mutants such as Dd2 and 7G8, respectively, with the K76T mutation being particularly important.28–30 Drug susceptibility has been measured only for P. falciparum harboring one of these unusual isoforms (783 PfCRT), and this strain was predicted to be CQR in one study,17 consistent with increased CQ transport by 783 PfCRT, relative to the wild type that we now quantify (Figure 3). The resistance conferring ability of the other unusual isoforms [China E (five of eight), GadIV (four of eight), GadV (five of eight), and GadVI (six of eight)] has been unknown until now. We find that all of these isoforms transport CQ better than wild-type PfCRT (Figure 3); thus, our data predict that P. falciparum parasites expressing China E, GadIV, GadV, and GadVI PfCRT are indeed CQR but will likely show levels of resistance that are lower than that exhibited by strain Dd2 (see Discussion).
Previously, we found two PfCRT isoforms that transported CQ less well than wild-type HB3 PfCRT.9 These were Sudan 106/1, which does not carry the key K76T mutation typically associated with CQR, and IP2300, which is K76T (as found in CQR strains) but carries a proximal N75K mutation (asterisk, left-hand side of Figure 3). It is thought introduction of positive charge at position 75 substitutes for loss of K at position 76 to create a CQS PfCRT isoform. We now identify 12 additional isoforms that also transport less well than HB3 (Figure 3). Surprisingly, eight of them harbor the key K76T mutation that is usually characteristic of CQR-conferring PfCRT, with another (J9) harboring K76A (Figure 3 and Table 2). The ability of mutant PfCRT to confer a cytostatic CQR phenotype relies on increased CQ transport relative to that of wild-type HB3 PfCRT,1–3 so CQ transport that is lower than that catalyzed by HB3 suggests expression of the PfCRT isoform may not confer a CQR phenotype. On the basis of these data, like others,17,31,32 we suggest that the K76T PfCRT mutation, the traditional marker for a CQR phenotype, may not in and of itself be fully reliable for predicting CQR status. We suggest that some newly evolving isoforms carrying K76T that do not catalyze increased CQ transport relative to that of HB3 represent “revertants” selected by a decreased use of CQ in the field. Indeed, these nine unusual isoforms are expressed in a widely dispersed collection of isolates from China, Thailand, Papua, Cambodia, and Tanzania (Table 2), regions that have all decreased use of CQ in recent years.
However, importantly, our previous studies showed that CQ transport by HB3 PfCRT displays little dependence on membrane potential, whereas CQR-conferring isoforms such as Dd2 show significantly heightened drug transport when the membrane potential is elevated.7,9 To test how CQ transport by the nine unusual isoforms might vary with an increasing membrane potential, using previously perfected protocols,9 we assayed yeast harboring these isoforms under conditions where the yeast membrane potential is elevated (Figures 4 and 5). We found that four of nine that show transport lower than that of HB3 PfCRT [J9, KS28, 734, and IsoV, all from SEA (cf. Table 2)] indeed catalyzed heightened CQ transport relative to that of wild-type HB3 PfCRT when the yeast plasma membrane potential is maximal (Figures 4 and 5). This is shown in detail for IsoV at a range of ΔΨ and external pH values [Figure 4, white triangles (IsoV) vs gray diamonds (HB3)] and summarized for the others in Figure 5 [compare gray (high ΔΨ) vs black (low ΔΨ) bars]. On the basis of these data, we suggest that additional mutations that promote an elevated DV membrane potential in isolates harboring these PfCRTs are perhaps required to confer CQR status to the isolate. However, the other five of nine PfCRTs harboring K76T mutations (China B–D, BC7, and Cam738 PfCRT) do not catalyze CQ transport any more efficiently than HB3 even at high ΔΨ (Figure 5), suggesting that they do not confer CQR phenotypes (see Discussion). The behavior of the 738 mutant PfCRT is unique versus those of all other 53 isoforms we have investigated and will be explored in detail elsewhere (S. Gabryszewski, P. Callaghan, P. D. Roepe, and D. A. Fidock, manuscript in preparation). Notably, three of these five “HB3-like but K76T” (“HL76T”) isoforms harbor an interesting mutation, A144Y, which is not found in other PfCRT isoforms (Table 2). To further investigate the importance of this unusual mutation, we created a Dd2 mutant harboring mutation A144Y. The introduction of the A144Y mutation into the Dd2 PfCRT background indeed produced a mutant with transport activity similar to that of HL76T isoforms (Figure 3, far left, two asterisks).
Figure 4.

ΔΨ dependence of PfCRT isoform CQ transport for a representative set of PfCRT isoforms. Growth delay was measured relative to empty vector in the presence of 5 mM CQ and at the indicated pH values for yeast expressing HB3 PfCRT (gray diamonds), Dd2 PfCRT (black squares), and IsoV PfCRT (white triangles). Only under conditions under which ΔΨ is clamped to maximal values (high external pH) does isoform IsoV transport CQ better than HB3, which is characteristic of other CQR PfCRTs.
Figure 5.
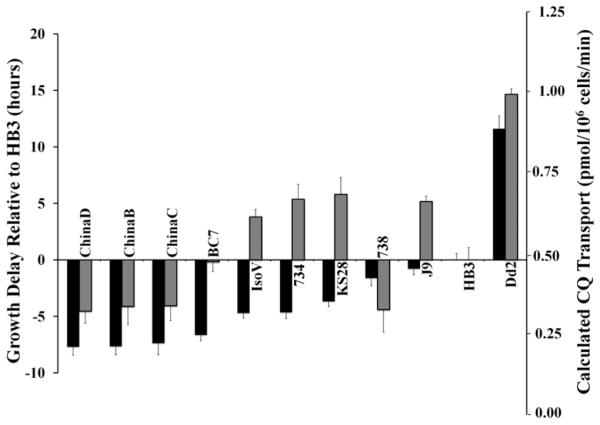
CQ transport under standard conditions (pH 6.75, 16 mM CQ, black bars) vs elevated ΔΨ conditions (pH 7.45, 5 mM CQ, gray bars). For elevated ΔΨ conditions, the growth delay is expressed as the difference between yeast expressing the indicated PfCRT isoform and yeast expressing the empty vector.
Multiple reports suggest that PfCRT may interact with the 8-amino quinoline antimalarial drug primaquine (PQ),33–35 and recent data of Wendler et al. suggest a reciprocal relationship between CQR and PQ susceptibility.33 Bray et al. showed that PQ potentiates CQ versus some CQR strains,34 and recent genomewide association studies demonstrate increased sensitivity to PQ in some CQR isolates.33 However, it is not clear whether this reciprocal relationship is due to PQ blocking CQ transport by PfCRT or perhaps to PQ transport by PfCRT in some of these strains.34,36
Panels A and B of Figure 6 show that under appropriate conditions PQ potently inhibits CQ transport by PfCRT, which is similar to previous observations using intact iRBC harboring trophozoite stage P. falciparum parasites.34 Figure 6 shows that yeast expressing Dd2 PfCRT (gray line) grow noticeably slower in the presence of CQ than those expressing no PfCRT (black line) or expressing wild-type PfCRT (dotted line) in the presence of the same concentration of CQ, because of the increased inward transport of CQ catalyzed by Dd2 PfCRT.9,18 In the presence of PQ and the same concentration of CQ (Figure 6B), yeast expressing Dd2 PfCRT (gray line, Figure 6B) now grow noticeably faster, which is similar to the case for yeast expressing HB3 PfCRT (dashed line, Figure 6B), suggesting that heightened CQ transport by Dd2 PfCRT is blocked by PQ. Figure 6C shows results in the presence of PQ alone, which suggests in addition that PfCRT mediates PQ transport, but with HB3 PfCRT (dashed line, Figure 6C) catalyzing increased transport relative to that of Dd2 PfCRT (gray line). These data suggest inhibition of Dd2 PfCRT CQ transport by PQ is due to isoform-specific competition between the two drugs for transport, which is consistent with the recently observed reciprocal relationship between CQR status and PQ susceptibility for African P. falciparum isolates.33
Figure 6.

Representative growth curves for yeast expressing the empty vector (—), HB3 PfCRT (−−−), and Dd2 PfCRT (gray line) in the presence of (A) 16 mM CQ (chemical structure inset), (B) 16 mM CQ and 700 μ M PQ, and (C) 1.8 mM PQ (chemical structure inset).
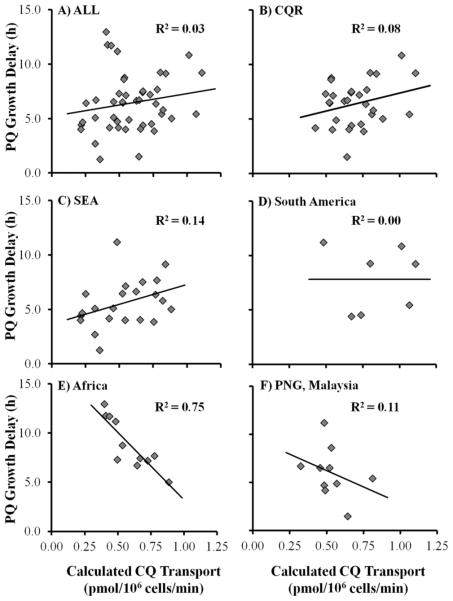
Figure 7 summarizes the magnitude of PQ-induced growth delay (proportional to PQ transport)9 for yeast expressing 45 naturally occurring PfCRT isoforms. We note that some CQS-associated isoforms [e.g., HB3, S106/1, GadI, and GadII (cf. Figure 3)] tend to transport PQ better than do CQR-associated PfCRT isoforms. However, when we plot CQ transport for all PfCRT isoforms versus PQ growth delay (proportional to PQ transport), we obtain a scatter plot with no discernible correlation (Figure 8A). When all CQR-conferring isoforms from all geographic regions are inspected (Figure 8B), there is again no correlation. We similarly do not find any correlation for CQR-conferring PfCRT isoforms from several geographic regions (Figure 8C,D,F). However, if we consider only isoforms found in Africa [wild type (HB3), S106/1, Dd2 (identical to S102/1 from Sudan), FCB (identical to RB8 from South Africa), GB4, and GadI–VI], a strong inverse correlation is found (R2 = 0.75) (Figure 8E). This is the most relevant subset of isoforms for comparison to the work of Wendler et al. as their study was limited to African isolates, and sequence information published in that paper indicated that the CQR isolates harbored Dd2-like mutations.33 Perhaps this strong correlation for African PfCRT isoforms is due to the relatively sparse use of PQ on that continent, relative to the use in other geographic regions.37
Figure 7.
Growth delays [the difference growth rate for PfCRT-expressing vs non-PfCRT-expressing yeast in the presence of 1.8 mM PQ under PfCRT-inducing conditions (see Materials and Methods)] expressed relative to Dd2 PfCRT yeast (5.1 h delay). Error bars represent the SEM after analysis of at least three independent yeast clones harboring each PfCRT isoform, each clone analyzed in triplicate (nine determinations in total).
Figure 8.
Plot of calculated CQ accumulation vs PQ growth delay for the following subgroups of PfCRT isoforms: (A) all 45 PfCRT isoforms analyzed in this study, (B) CQR isoforms [as defined by CQ transport greater than that of canonical CQS isoform HB3 (Figure 3)], and isoforms found in strains and isolates from (C) Southeast Asia, (D) South America, (E) Africa, PNG, and (F) Malaysia. Data for wild-type (HB3) PfCRT were included on all plots as wild-type isoforms are found in all geographic regions. Isoforms that have been found in parasites from more than one region (e.g., the FCB isoform has been found in FCB from Thailand as well as in RB8 from South Africa) were included on all applicable plots.
Two recent studies have identified PfCRT isoforms that may be derived from “hybrid” haplotypes resulting from intragenic recombination between CQR and CQS parasites within a population.27,38 Some of these isoforms, such as GadIII and KT099, harbor only a single CQR-associated mutation in an otherwise wild-type background (Table 2). While each of these isoforms was found to transport CQ at levels similar to that of HB3, their PQ transport was found to be substantially mitigated relative to HB3, especially in the case of KT099 (Figure 7, middle). KT099 harbors mutation N326D only. Interestingly, other common isoforms harboring N326D (e.g., 7G8, Ecu, and PNG4) were also found to be poor transporters of PQ, providing additional evidence that this mutation tends to reduce PfCRT PQ transport. Similarly, GadIII possessed only one mutation relative to HB3 (R371I). This isoform was also found to transport PQ with an efficiency between that of HB3 and Dd2 (Figure 7).
Previously, we compared PfCRT isoform-mediated CQ transport and cognate parasite strain CQ IC50 and found no correlation between these parameters.9 Here, we have expanded this analysis to include all PfCRT isoforms for which parasite CQ susceptibility data were available (Figure 9). For isoforms found in strains whose drug susceptibility has been assayed by multiple laboratories, we have culled data from the literature to calculate an average resistance factor (RF), defined as the CQ IC50 for the resistant isolate divided by the CQ IC50 for the CQS control used in the same study. Consistent with our previous results, we once again do not observe any correlation between PfCRT-mediated CQ transport and cognate parasite CQ IC50, further indicating that CQ transport by mutant PfCRT is necessary but not sufficient for conferring the full shift in CQ IC50 found in parasite strains and isolates.9 Figure 9 predicts that either additional functions of PfCRT (functions other than CQ transport) or additional mutations in other drug resistance proteins add with mutant PfCRT CQ transport to confer variable levels of CQR now found around the globe.
Figure 9.

Scatter plot of PfCRT isoform-mediated growth delay vs “resistance factor” for unique PfCRTs found in strains (gray) and isolates (white) for which CQ IC50 data are available. Resistance factor (RF) was determined by dividing the CQ IC50 of the strain or isolate of interest by the CQ IC50 of the CQS control in the same study. Horizontal error bars represent the range of CQ IC50 data found in the literature.
DISCUSSION
This study is the first to assess function for dozens of naturally occurring PfCRT isoforms. To the best of our knowledge, 42 unique PfCRT isoforms are expressed only in isolates [meaning the parasites harboring these PfCRTs cannot yet be maintained in continuous culture (Table 2)]. Of these 42, 38 have been found in fewer than 10 isolates. We designate these “rare” and suggest that methods described here provide a fast, inexpensive way to assay rare PfCRT contributions to parasite phenotypes. Our previous investigation of CQ transport mediated by 13 PfCRT isoforms expressed in laboratory strains of P. falciparum revealed a very strong linear correlation between PfCRT-mediated [3H]CQ uptake and CQ-dependent yeast growth inhibition.9 That is, the magnitude of PfCRT-dependent, CQ-induced growth delay for these yeast is a direct measure of the efficiency of CQ transport by PfCRT.9 Also in this work, no correlation between PfCRT-mediated CQ transport and CQ susceptibility of the cognate P. falciparum strain expressing that PfCRT isoform was found.9 This provided clear evidence that PfCRT drug transport alone is not necessarily the only cause of shifts in malarial parasite CQ IC50 values. Other genome mutations or other resistance-conferring function(s) of PfCRT must also influence parasite CQ susceptibility, a conclusion also consistent with data from other studies.5,17,39,40 The results presented here (Figures 3 and 9) provide additional evidence that strongly supports this model. In contrast, another recent study reports good correlation between CQ transport for six PfCRT isoforms and the CQ IC50 found for the cognate strains expressing those isoforms.41 Our data for these isoforms show some degree of correlation; however, the correlation does not hold when the data are included within larger data sets (Figure 9).
Previously, we also provided evidence of a strong ΔΨ dependency for CQ transport by CQR-associated (but not CQS-associated) PfCRT isoforms,7,9,18 suggesting that charged CQ is the favored PfCRT substrate. Also, the quadratic shape of a plot of ΔΨ versus transport rate constant for purified PfCRT suggested that the diprotonated drug (not monoprotonated) is favored.7 Data in this paper further highlight this ΔΨ dependence and show that transport measurements taken at lower ΔΨ values do not necessarily reveal the complete CQ transport potential for some PfCRT isoforms. As previously discussed,1 this concept is important when comparing data obtained via oocyte expression models36,41 versus these yeast models or versus proteoliposome preparations.7,9,18,25 For yeast and proteoliposomes, ΔΨ can be manipulated to much higher values whereas ΔΨ is quite low for the oocyte plasma membrane.1 Yeast and proteoliposome data therefore yield substantially higher drug transport turnover for PfCRT and further clarify the role that these isoforms may play in CQR phenomena.
This concept is also important for assessing which PfCRT isoforms are capable of conferring CQR phenotypes in the absence of CQ IC50 data for cognate P. falciparum isolates harboring those PfCRTs. We note that some PfCRT isoforms showing CQ transport that is less efficient than that mediated by CQS-associated HB3 PfCRT are perhaps still capable of mediating CQR if the DV membrane potential is increased (Figures 4 and 5). Some isoforms designated “CQS-like” on the basis of their CQ transport behavior at low membrane potentials (e.g., Cam734, IsoV, J9, and KS28) are actually “CQR-like” when their transport is measured in the presence of higher membrane ΔΨ values.
Of all the naturally occurring isoforms of PfCRT, only five have been found to harbor a mutation that places an aromatic residue at position 144 (Cam734, IsoV, China B, China C, and China D). Strikingly, all five of these transport CQ less well than HB3 under standard assay conditions, yet Cam734 is the only one of the five for which host parasite drug susceptibility has been measured. In a field study conducted by Durrand et al.,17 eight isolates expressing Cam734 PfCRT were catalogued. The CQ IC50 values for these isolates ranged from 33.2 nM (CQS) to 169.2 nM (moderate CQR). Further analysis of Cam734 PfCRT in our yeast model revealed that under conditions where membrane potential is sufficiently elevated, Cam734 transports CQ at levels between those found for HB3 and Dd2. This behavior could have important implications in terms of secondary genetic changes required to confer CQR within a background harboring a Cam734 PfCRT isoform. Our data suggest that some isolates studied by Durrand et al.17 might have low DV ΔΨ values (conferring low CQ IC50 values), whereas others might have higher ΔΨ values (higher CQ IC50 values).
Multiple isoforms show CQ transport no greater than that of CQS-associated HB3 PfCRT. One isoform that is clearly CQS-associated, BC7, is identical to Dd2 except that it harbors a single-amino acid deletion. The deletion of amino acid I327 in BC7 produced the fourth lowest CQ transport phenotype of all naturally occurring PfCRT isoforms we have yet analyzed. One possible explanation for this is the likely importance of residue 326 in mediating CQ transport. With the exception of residue 76, residue 326 is the only other PfCRT residue that is mutated in CQR parasites from all geographic regions, including those of Southeast Asian (N326S), South American (N326S/D), and Fillipino (N326D) origin (Jav and GB4 PfCRT are notable exceptions to this trend).
We assign PfCRTs with HB3-like CQ transport that do not show ΔΨ dependence (China B, China C, China D, BC7, GadI, GadII, GadIII, and Cam738) to two new categories of CQS-conferring PfCRT isoforms. One (GadI, GadII, and GadIII) was recently identified by Sutherland and colleagues and entails single-amino acid substitutions relative to HB3 (Q10K, D24Y, and R371I).27 The second (China B–D, BC7, and Cam738) includes PfCRTs that otherwise appear to be CQR-conferring. All five of these isoforms harbor the K76T mutation, which until now has been considered “diagnostic” for P. falciparum CQR status.42 The existence of PfCRT isoforms harboring K76T that are nonetheless unable to catalyze increased CQ transport relative to that of CQS PfCRT, at both high and low ΔΨ values, suggests that previously CQR-conferring PfCRTs may be reverting back to CQS-associated isoforms upon sequential loss or gain of other key PfCRT mutations. We are unable to fully ascertain the complete drug selection history for the region of China from which China B–D isolates originate; however, we note that isolates BC7 and Cam738 originate from Thailand and Cambodia, where CQ use has largely been discontinued. Also consistent with this hypothesis, we note that BC7 is identical to Dd2 PfCRT (CQR-conferring, originating from SEA), except for a single-amino acid deletion at position 327. We suggest that additional PfCRT isoforms with sequences nearly identical to that of Dd2 (up to and including the K76T mutation) but with single-amino acid deletions or substitutions will be found from regions that have discontinued CQ use, and that these may represent CQS-conferring PfCRT revertants. This observation has obvious important implications for targeted local use of quinoline antimalarial drugs, perhaps even limited reintroduction of CQ as a partner in artemisinin combination therapy.
Consistent with this idea, although the full PfCRT sequence was not determined, we note two recent reports identified several P. falciparum isolates carrying K76T PfCRT isoforms that were nonetheless measured to be CQS via CQ susceptibility assays.31,32 Several field studies have cited the gradual resurgence of CQS parasites carrying 76K PfCRT as evidence of a fitness cost associated with expression of CQR-conferring PfCRT isoforms of Asian origin.6,11 However, other studies have found populations of parasites harboring K76T PfCRT that are stable even decades after the withdrawal of CQ.8,12 There are several possible explanations for these apparent contradictions. Deep genome sequencing of CQR versus CQS parasite strains and isolates has identified various genes with mutations or altered expression that are associated with the presence of mutant PfCRT14,15 that might abrogate mutant PfCRT fitness costs. It is also possible that additional mutations within PfCRT itself may reduce the fitness burden associated with some isoforms. For example, Cam734 harbors four mutations (A144F, L148I, I194T, and T333S) that are not found in canonical SEA or South American PfCRT isoforms.
In the study describing identification of BC22 PfCRT,43 four isolates expressing BC22 PfCRT were found. Single-pass quantification of the drug susceptibility of these isolates revealed a noticeable reduction in CQ IC50. This finding led the authors to suggest that the E198K mutation in BC22 PfCRT may partially counteract the K76T mutation by reintroducing a positive charge into the “PfCRT channel”. Our finding that BC22 mediates reduced CQ transport relative to that of Dd2 PfCRT is consistent with this hypothesis. A similar argument has previously been applied to isoforms bearing the S163R mutation, such as Pf164 PfCRT. This mutation was first found in CQR laboratory clones that were further selected with halofantrine and amantadine.44 The additional drug selection yielded parasite clones (named K1AM and K1HF) that were resistant to the selecting compounds but were resensitized to CQ. Sequencing pfcrt in these lab-derived strains identified the S163R mutation, leading the authors to identify PfCRTs in the clones as second-site revertants. However, a recent field study reported elevated recrudescence after single- or double-dose treatment with CQ or AQ in parasites harboring the S163R mutation in PfCRT (97% of recrudescent parasites were 163R vs only 36% of the parasite pretreatment group).45 Our finding that Pf164 PfCRT transports CQ at high levels coupled with the results of this field study suggests that genetic mutations other than S163R in PfCRT may have played an important role in resensitizing lab strains K1AM and K1HF to CQ.
Several recent field studies in SEA have identified isolates expressing PfCRT isoforms with the mutational IDT pattern at residues 74–76.12,13,17,46–48 In the studies in which CQ susceptibility of the isolates was assayed, a correlation between the IDT pattern and reduced CQ IC50 [relative to isolates harboring the canonical SEA haplotype (IET)] was observed.17,46 Data in the work presented here show a general decrease in CQ transport mediated by PfCRTs with the 74–76 IDT pattern of mutations, consistent with these previous observations.
In conclusion, we suggest that partial PfCRT sequence data are insufficient for assigning CQR status to P. falciparum isolates. Our data show that PfCRT isoforms containing the key K76T mutation often used to assign CQR status actually show a wide range of activities, including activities that are consistent only with PfCRT isoforms found in CQS strains and isolates. Ever since the discovery that PfCRT mutations serve as the primary determinant of CQR,28 most researchers have envisioned a model in which CQ IC50 values are elevated in proportion to the increase in CQ transport conferred by mutations in PfCRT. Because archtypical mutant PfCRT isoforms found in CQR isolates and strains have always included the K76T mutation, monitoring global CQR has relied on testing for the presence of this mutation. However, recent findings, including those reported here, point to a model of PfCRT-mediated CQR that is more complex. We propose that some newer PfCRT isoforms, and those that continue to evolve under changing drug-use policies, may contain K76T mutations yet be unable to confer CQR. We also suggest that CQR P. falciparum isolates expressing PfCRT isoforms that show unusually ΔΨ-dependent CQ transport like those we identify here likely carry additional genetic mutations that modify DV ΔΨ.
Acknowledgments
Funding
Supported by National Institutes of Health Grants AI056312 and AI090832 to P.D.R.
ABBREVIATIONS
- CQ
chloroquine
- CQR(S)
chloroquine resistant (sensitive)
- CS
cytostatic
- DV
digestive vacuole
- PfCRT
P. falciparum chloroquine resistance transporter
- PM
plasma membrane
- PNG
Papau New Guinea
- PQ
primaquine
- SA
South America
- SEA
Southeast Asia
- SEM
standard error of the mean
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Roepe PD. PfCRT-mediated drug transport in malarial parasites. Biochemistry. 2011;50:163–171. doi: 10.1021/bi101638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ecker A, Lehane AM, Clain J, Fidock DA. PfCRT and its Role in Antimalarial Drug Resistance. Trends Parasitol. 2012;28:504–514. doi: 10.1016/j.pt.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Roepe PD. To kill or not to kill, that is the question: cytocidal antimalarial drug resistance. Trends Parasitol. 2014;30:130–135. doi: 10.1016/j.pt.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Gaviria D, Paguio M, Turnbull L, Asako T, Siriwardana A, Ghosh D, Ferdig M, Sinai A, Roepe PD. Dysregulation of Autophagy is Associated with Cytocidal Chloroquine Resistane in Plasmodium falciparum Malaria. PLoS One. 2013;8:e79059. doi: 10.1371/journal.pone.0079059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Gligorijevic B, Bennett T, McAllister R, Urbach JS, Roepe PD. Spinning Disk Confocal Microscopy of Live, Intraerythrocytic Malarial Parasites. 2. Altered Vacuolar Volume Regulation in Drug Resistant Malaria. Biochemistry. 2006;45:12411–12423. doi: 10.1021/bi0610348. [DOI] [PubMed] [Google Scholar]
- (6).Lewis IA, Wacker M, Olszewski KL, Cobbold SA, Baska KS, Tan A, Ferdig MT, Llinas M. Metabolic QTL analysis links chloroquine resistance in Plasmodium falciparum to impaired hemoglobin catabolism. PLoS Genet. 2014;10:e1004085. doi: 10.1371/journal.pgen.1004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Paguio MF, Cabrera M, Roepe PD. Chloroquine transport in P. falciparum. 2. Analysis of PfCRT-mediated drug transport using proteoliposomes and a fluorescent chloroquine probe. Biochemistry. 2009;48:9482–9491. doi: 10.1021/bi901035j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sa J, Twu O, Hayton K, Reyes S, Fay M, Ringwald P, Wellems T. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Baro NK, Callaghan PS, Roepe PD. Function of Resistance Conferring Plasmodium falciparum Chloroquine Resistance Transporter Isoforms. Biochemistry. 2013;52:4242–4249. doi: 10.1021/bi400557x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Chen N, Gao Q, Wang S, Wang G, Gatton M, Cheng Q. No genetic bottleneck in Plasmodium falciparum wild-type pfcrt alleles reemerging in Hainan Island, China, following high-level chloroquine resistance. Antimicrob. Agents Chemother. 2008;52:345–347. doi: 10.1128/AAC.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Laufer MK, Takala-Harrison S, Dzinjalamala FK, Stine O, Taylor T, Plowe CV. Return of chloroquine-susceptible falciparum malaria in Malawi was a reexpansion of diverse susceptible parasites. J. Infect. Dis. 2010;202:801–808. doi: 10.1086/655659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Yang Z, Zhang Z, Sun X, Wan W, Cui L, Zhang X, Zhong D, Yan G, Cui L. Molecular analysis of chloroquine resistance in Plasmodium falciparum in Yunnan Province, China. Trop. Med. Int. Health. 2007;12:1051–1060. doi: 10.1111/j.1365-3156.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- (13).Isozumi R, Uemura H, Dao L, Van Hanh T, Giang N, Vien H, Phuc B, Van Tuan N, Nakazawa S. Longitudinal Survey of Plasmodium falciparum Infection in Vietnam: Characteristics of Antimalarial Resistance and Their Associated Factors. J. Clin. Microbiol. 2010;48:70–77. doi: 10.1128/JCM.01449-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Jiang H, Patel J, Yi M, Mu J, Ding J, Stephens R, Cooper R, Ferdig M, Su X.-z. Genome-wide compensatory changes accompanying drug-selected mutations in the Plasmodium falciparum crt gene. PLoS One. 2008;3:e2484. doi: 10.1371/journal.pone.0002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Jovel I, Ferreira P, Veiga M, Malmberg M, Martensson A, Kaneko A, Zakeri S, Murillo C, Nosten F, Bjorkman A, Ursing J. Single nucleotide polymorphisms and Plasmodium falciparum V type H+ pyrophosphatase gene (pfvp2) and their association with pfcrt and pfmdr1 polymorphisms. Infect., Genet. Evol. 2014;24:111–115. doi: 10.1016/j.meegid.2014.03.004. [DOI] [PubMed] [Google Scholar]
- (16).Summers RL, Nash MN, Martin RE. Know your enemy: Understanding the role of PfCRT in drug resistance could lead to new antimalarial tactics. Cell. Mol. Life Sci. 2012;69:1967–1995. doi: 10.1007/s00018-011-0906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Durrand V, Berry A, Sem R, Glaziou P, Beaudou J, Fandeur T. Variations in the sequence and expression of the Plasmodium falciparum chloroquine resistance transporter (Pfcrt) and their relationship to chloroquine resistance. Mol. Biochem. Parasitol. 2004;136:273–285. doi: 10.1016/j.molbiopara.2004.03.016. [DOI] [PubMed] [Google Scholar]
- (18).Baro NK, Pooput C, Roepe PD. Analysis of chloroquine resistance transporter (CRT) isoforms and orthologues in S. cerevisiae yeast. Biochemistry. 2011;50:6701–6710. doi: 10.1021/bi200922g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Callaghan PS, Siriwardana A, Hassett MR, Roepe PD. Plasmodium falciparum chloroquine resistance transporter (PfCRT) isoforms PH1 and PH2 perturb malarial parasite digestive vacuole physiology. 2015 doi: 10.1186/s12936-016-1238-1. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Nigavekar SS, Cannon JF. Characterization of genes that are synthetically lethal with ade3 or leu2 in Saccharomyces cerevisiae. Yeast. 2002;19:115–122. doi: 10.1002/yea.807. [DOI] [PubMed] [Google Scholar]
- (21).Sherman F, Baim SB, Hampsey DM, Gooodhue CT, Friedman LR, Stiles JI. In: Translational Control. Matthews MB, editor. Cold Spring Harbor Laboratory Press; Plainview, NY: 1986. [Google Scholar]
- (22).Delhez J, Dufour JP, Thines D, Goffeau A. Comparison of the properties of plasma membrane-bound and mitochondria-bound ATPases in the yeast Schizosaccharmoyces pombe. Eur. J. Biochem. 1977;79:319–328. doi: 10.1111/j.1432-1033.1977.tb11812.x. [DOI] [PubMed] [Google Scholar]
- (23).Papakrivos J, Sá JM, Wellems TE. Functional characterization of the Plasmodium falciparum chloroquine-resistance transporter (PfCRT) in transformed Dictyostelium discoideum vesicles. PLoS One. 2012;7:e39569. doi: 10.1371/journal.pone.0039569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhang H, Howard EM, Roepe PD. Analysis of the antimalarial drug resistance protein PfCRT expressed in yeast. J. Biol. Chem. 2002;277:49767–49775. doi: 10.1074/jbc.M204005200. [DOI] [PubMed] [Google Scholar]
- (25).Juge N, Moriyama S, Miyaji T, Kawakami M, Iwai H, Fukui T, Nelson N, Omote H, Moriyama Y. Plasmodium falciparum chloroquine resistance transporter is a H+-coupled polyspecific nutrient and drug exporter. Proc. Natl. Acad. Sci. U. S. A. 2015;112:3356–3361. doi: 10.1073/pnas.1417102112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Echeverry D, Holmgren G, Murillo C, Higuita J, Bjorkman A, Gil J, Osorio L. Short report: Polymorphisms in the pfcrt and pfmdr1 genes of Plasmodium falciparum and in vitro susceptibility to amodiaquine and desethylamodiaquine. Am. J. Trop. Med. Hyg. 2007;77:1034–1038. [PubMed] [Google Scholar]
- (27).Gadalla NB, Malmberg M, Adam I, Oguike MC, Beshir K, Elzaki S, Mukhtar I, Gadalla AA, Warhurst DC, Ngasala B, Martensson A, El-Sayed BB, Gil JP, Sutherland CJ. Alternatively spliced transcripts and novel pseudogenes of the Plasmodium falciparum resistance-associated locus pfcrt detected in East African malaria patients. J. Antimicrob. Chemother. 2015;70:116–123. doi: 10.1093/jac/dku358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, bir Singh Sidhu A, Naudé B, Deitsch KW, Su XZ, Wootton JC, Roepe PD, Wellems TE. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wootton J, Feng X, Ferdig M, Cooper R, Mu J, Baruch D, Magill A, Su X-z. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- (30).Sidhu AB, Verdier-Pinard D, Fidock DA. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298:210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Goswami D, Dhiman S, Rabha B, Kumar D, Baruah I, Sharma DK, Veer V. Pfcrt haplotypes may not correspond with chloroquine resistance. J. Infect. Dev. Countries. 2014;8:768–773. doi: 10.3855/jidc.3398. [DOI] [PubMed] [Google Scholar]
- (32).Koleala T, Karl S, Laman M, Moore BR, Benjamin J, Barnadas C, Robinson LJ, Kattenberg JH, Javati S, Wong R, Rosanas-Urgell A, Betuela I, Siba PM, Mueller I, Davis T. Temporal changes in Plasmodium falciparum anti-malarial drug sensitivity in vitro and resistance-associated genetic mutations in isolates from Papua New Guinea. Malar. J. 2015;14:37–45. doi: 10.1186/s12936-015-0560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Wendler JP, Okombo J, Amato R, Miotto O, Kiara SM, Mwai L, Pole L, O’Brien J, Manske M, Alcock D, Drury E, Sanders M, Oyola SO, Malangone C, Jyothi D, Miles A, Rockett KA, MacInnis BL, Marsh K, Bejon P, Nzila A, Kwiatkowski DP. A genome wide association study of Plasmodium falciparum susceptibility to 22 antimalarial drugs in Kenya. PLoS One. 2014;9:e96486. doi: 10.1371/journal.pone.0096486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bray PG, Deed S, Fox E, Kalkanidis M, Mungthin M, Deady LW, Tilley L. Primaquine synergises the activity of chloroquine against chloroquine-resistant. Biochem. Pharmacol. 2005;70:1158–1166. doi: 10.1016/j.bcp.2005.07.021. [DOI] [PubMed] [Google Scholar]
- (35).Lehane AM, Kirk K. Efflux of a range of antimalarial drugs and ‘chloroquine resistance reversers’ from the digestive vacuole in malaria parasites with mutant PfCRT. Mol. Microbiol. 2010;77:1039–1051. doi: 10.1111/j.1365-2958.2010.07272.x. [DOI] [PubMed] [Google Scholar]
- (36).Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science. 2009;325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- (37).Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin. Infect. Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- (38).Tan LL, Lau TY, Timothy W, Prabakaran D. Full-Length Sequence Analysis of Chloroquine Resistance Transporter Gene in Plasmodium falciparum Isolates from Sabah, Malaysia. Sci. World J. 20142014:935846. doi: 10.1155/2014/935846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Patel JJ, Thacker D, Tan JC, Pleeter P, Checkley L, Gonzales JM, Deng B, Roepe PD, Cooper RA, Ferdig MT. Chloroquine susceptibility and reversibility in a Plasmodium falciparum genetic cross. Mol. Microbiol. 2010;78:770–787. doi: 10.1111/j.1365-2958.2010.07366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mu J, Ferdig M, Feng X, Joy D, Duan J, Furuya T, Subramanian G, Aravind L, Cooper R, Wootton J, Xiong M, Su X-z. Multiple transporters associated with malaia parasite response to chlorquine and quinine. Mol. Microbiol. 2003;49:977–989. doi: 10.1046/j.1365-2958.2003.03627.x. [DOI] [PubMed] [Google Scholar]
- (41).Summers RL, Dave A, Dolstra T, Bellanca S, Marchetti RV, Nash MN, Richards SR, Schenk RL, Stein WD, Kirk K, Sanchez CP, Lanzer M, Martin RE. Diverse mutational pathways converge on saturable chloroquine transport via the malaria parasites chloroquine resistance transporter. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E1759–E1767. doi: 10.1073/pnas.1322965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lakshmanan V, Bray PG, Verdier-Pinard D, Johnson DJ, Horrocks P, Muhle RA, Alakpa GE, Hughes RH, Ward SA, Krogstad DJ, Sidhu ABS, Fidock DA. A critical role for PfCRT K76T in Plasmodium falciparum verapamil-reversible chloroquine resistance. EMBO J. 2005;24:2294–2305. doi: 10.1038/sj.emboj.7600681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Chaijaroenkul W, Ward S, Mungthin M, Johnson D, Owen A, Bray P, Na-Bangchang K. Sequence and gene expression of chloroquine resistance transporter (pfcrt) in the association of in vitro drugs resistance of Plasmodium falciparum. Malar. J. 2011;10:42–47. doi: 10.1186/1475-2875-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Johnson D, Fidock D, Mungthin M, Lakshmanan V, Sidhu A, Bray P, Ward S. Evidence for a Central Role for PfCRT in Conferring Plasmodium falciparum Resistance to Diverse Antimalarial Agents. Mol. Cell. 2004;15:867–877. doi: 10.1016/j.molcel.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Ursing J, Kofoed P, Rodrigues A, Rombo L, Gil JP. Plasmodium falciparum genotypes asscociated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am. J. Trop. Med. Hyg. 2007;76:844–848. [PubMed] [Google Scholar]
- (46).Lim P, Chy S, Ariey F, Incardona S, Chim P, Sem R, Denis M, Hewitt S, Hoyer S, Socheat D, Merecreau-Puijalon O, Fandeur T. pfcrt Polymorphism and Chloroquine Resistance in Plasmodium falciparum Strains Isolated in Cambodia. Antimicrob. Agents Chemother. 2003;47:87–94. doi: 10.1128/AAC.47.1.87-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Mittra P, Vinayak S, Chandawat H, Das M, Singh N, Biswas S, Dev V, Kumar A, Ansari M, Sharma Y. Progressive Increase in Point Mutations Associated with Chloroquine Resistance in Plasmodium falciparum from India. J. Infect. Dis. 2006;193:1304–1312. doi: 10.1086/502979. [DOI] [PubMed] [Google Scholar]
- (48).Randrianarivelojosia M, Fidock DF, Belmonte O, Valderramos SG, Mercereau-Puijalon O, Ariey F. First evidenct of pfcrt mutants Plasmodium falciparum in Madagascar. Trans. R. Soc. Trop. Med. Hyg. 2006;100:826–830. doi: 10.1016/j.trstmh.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]