Abstract
Neuroinflammation is a common feature in nearly all neurological and some psychiatric disorders. Resembling its extraneural counterpart, neuroinflammation can be both beneficial and detrimental depending on the responding molecules. The overall effect of inflammation on disease progression is highly dependent on the extent of inflammatory mediator production and the duration of inflammatory induction. The time-dependent aspect of inflammatory responses suggests that the therapeutic time window for quelling neuroinflammation might vary with molecular targets and injury types. Therefore, it is important to define the therapeutic time window for anti-inflammatory therapeutics, as contradicting or negative results might arise when different treatment regimens are utilized even in similar animal models. Herein, we discuss a few critical factors that can help define the therapeutic time window and optimize treatment paradigm for suppressing the cyclooxygenase-2/prostaglandin-mediated inflammation after status epilepticus. These determinants should also be relevant to other anti-inflammatory therapeutic strategies for the CNS diseases.
Keywords: Status epilepticus, neuroinflammation, neuronal injury, epilepsy, seizure, cyclooxygenase, prostaglandin, cytokine, interleukin, therapeutic time window, anti-inflammatory therapeutics
Inflammation was historically characterized by cardinal signs of ‘dolor, calor, rubor, tumor, and functio laesa’ by Celsus and later by Galen, and now is recognized as a protective strategy of the host to eliminate the detrimental stimuli following infection or injury. Inflammation is usually beneficial during the acute phase and subsides in days as the tissue insults are resolved. However, under some circumstances, inflammation persists and becomes out of control, and the long-lasting inflammatory processes often contribute to the pathophysiology of a variety of diseases such as rheumatoid arthritis, skin and vascular inflammation, cystic fibrosis, colitis, cancer, and metabolic syndrome [1]. The central nervous system (CNS) was traditionally considered to be immune-privileged due to the tight restriction of the blood–brain barrier (BBB). However, over the past two decades or so, inflammation in the brain, or now often termed as neuroinflammation, has been recognized to be a feature of virtually all neurological disorders and even some psychiatric impairments. Following initial brain insults such as status epilepticus, ischemia, neurotrauma, and nerve agent exposure, inflammatory responses immediately begin to proceed in the brain. The aspects of inflamed brain often involve a series of pathological alterations such as activation of glial cells and neurons, the concomitant production of bioactive mediators, including prostaglandins and cytokines, BBB disruption, and extravasation of blood proteins and cells into the brain parenchyma [2,3]. Resembling its extraneural counterpart, chronic neuroinflammation sustains and in most cases aggravates the disease progression. For instance, experimental and clinical evidence is emerging that inflammation in the brain might be a considerable cause of many forms of epilepsy, rather than just a by-product of the epileptic seizures, as once believed [4–7]. Subsequently, anti-inflammatory therapeutics targeting some of the key inflammatory molecules have been proposed to treat seizures and epilepsy [8–18]. Herein, we discuss anti-inflammatory therapeutics for the treatment of status epilepticus with a focus on the therapeutic time window that could dramatically influence the therapeutic outcomes and reproducibility of preclinical studies. Furthermore, we propose a strategy to identify the time window that could guide to optimize the treatment paradigm for improved therapeutic efficacy.
Status epilepticus
Status epilepticus is a life-threatening condition defined as a continuous epileptic seizure of greater than 30 min, or more than one seizure within a 30 min period without full recovery of consciousness between seizures [19]. However, in the clinic, a duration of 5 min is generally considered as the inclusion criterion for status epilepticus, in that most seizures stop spontaneously within 2 min and patients usually receive treatments before 30 min after seizures begin [19,20]. Status epilepticus represents the second most common neurological emergency following acute stroke, and often results in high mortality and substantial brain injuries and morbidities in survivors [21]. Moreover, status epilepticus is a leading cause for chronic epilepsy. The only effective treatment currently is to stop the seizures quickly enough to prevent severe brain damage by antiepileptic drugs [22]. However, the conventional treatments cause a broad spectrum of side effects, e.g. dizziness, fatigue, weakness, confusion, irritability, anxiety, depression, cognitive decline in children, etc. Furthermore, nearly one-third of status epilepticus patients have poor response to current therapies, and no drug has been demonstrated to prevent epileptogenesis, i.e. the process that transforms a healthy brain into one that generates spontaneous seizures after insults like prolonged seizures [9]. Thus, identifying new drug targets and developing novel therapeutics that can be delivered several hours after seizure onset are in high demand.
Cyclooxygenase-2 cascade as anti-inflammatory therapeutic targets
A large number of inflammation-associated genes are rapidly and persistently induced by status epilepticus with cyclooxygenase-2 (COX-2) and pro-inflammatory cytokine – interleukin-1β (IL-1β) – induction temporally leading others [23]. Thus, COX-2 and IL-1β have been suggested to be the dominant driving forces of seizure-induced neuroinflammation and are widely considered front-running candidate targets for anti-inflammatory therapeutics [9]. COX-2 is the rate-limiting enzyme that is necessary for the synthesis of prostanoids, consisting of prostaglandin D2 (PGD2), PGE2, PGF2α, prostacyclin PGI2, and thromboxane TXA2. These bioactive molecules function via binding and activating their downstream G protein-coupled receptors (GPCRs) [24,25]. COX-2 is rapidly and robustly induced and plays pivotal roles during the early inflammatory responses following acute brain insults such as prolonged seizures via producing prostaglandins [23,26]. However, recent studies in experimental animals have provided evidence that prostaglandins – primarily PGE2 – also function in the transition to and preservation of the prolonged inflammation through multiple mechanisms, i.e. inducing cytokines and recruiting immune cells to the inflammatory sites [1,27]. As the major COX-2 product in the brain, PGE2 can bind and activate four GPCRs – EP1, EP2, EP3, and EP4 – to mediate a variety of physiological and pathological functions. Recent evidence from animal studies using genetic and pharmacological strategies suggest that PGE2 mediates brain inflammation and injury largely via its receptor EP2 subtype following acute brain insults such as status epilepticus, [16,28,29] exposure to organophosphorus agent, [30] and intracerebral hemorrhage [31,32]. These results indicate that the major deleterious effects of COX-2 induction in the CNS are largely attributed to EP2 receptor activation by PGE2 and demonstrate the promising potential of EP2 receptor as a candidate drug target for anti-inflammatory intervention to treat seizures and epilepsy [33].
Time window for anti-inflammatory therapeutics
A number of COX-2 selective inhibitors, including celecoxib, etoricoxib, parecoxib, rofecoxib, SC58125, NS398, nimesulide, etc., have been tested in several seizure models till date, [17,34–44] and the vast majority of these experimental studies demonstrate that the blockade of COX-2 inflammatory cascade is overall beneficial following status epilepticus [15]. However, some studies also suggest that some COX-2 inhibitors, e.g. SC58236, do not show adequate efficacy or even exacerbate seizure-promoted pathogenesis in rats when the animals are treated either too early or too late [45,46]. Moreover, treatment by COX-2 inhibitor NS398 beginning 5 h after but not 30 min before kainate injection is neuroprotective in rats [41]. Likewise, treatment with nonselective COX inhibitor aspirin beginning hours after pilocarpine-induced status epilepticus reduces hippocampal neuronal loss and spontaneous seizures in rats, [47] whereas aspirin treatment starting days before status epilepticus does not show any beneficial effect in pilocarpine-treated mice but increases the seizure susceptibility in mice [48]. Furthermore, conditional ablation of COX-2 in forebrain neurons shows a trend of aggregating neuronal injury 1 day after pilocarpine-induced status epilepticus in mice, whereas affords a significant neuroprotection 4 days after status epilepticus [29]. These seemingly controversial outcomes indicate that the dosage amount and dosing time, as well as the pharmacokinetic and pharmacodynamic properties of tested drugs could potentially influence the therapeutic outcomes. Whether antiepileptic drugs are used to terminate seizures along with COX-2 inhibition in these models is another potential contributor to the incongruent results [39,41,46].
Similar to COX-2, PGE2 signaling through EP2 receptor exerts both beneficial and deleterious effects, [25,27,33,49,50] mirroring the Jekyll and Hyde nature of inflammation. Intriguingly, EP2 receptor activation by its selective agonist – butaprost – immediately after pilocarpine status epilepticus moderately reduces degenerating neurons in rats, [29] a finding that appears to conflict with the broad benefits from delayed administration of EP2 antagonists in similar seizure models [16,28,30]. However, these results might reflect the complexity of seizure-promoted inflammatory processes in the brain and indicate a dual outcome of COX-2 induction and EP2 receptor activation – early neuroprotection succeeded by later neurotoxicity involving persistent inflammation. The dichotomous actions of inflammation in the brain, highlighted by COX-2 and EP2 receptor, insinuate therapeutic time windows for anti-inflammatory therapeutics. The inconsistent efficacy from blocking the COX-2/PGE2/EP2-mediated inflammation might be resolved if the therapeutic time windows are defined and the treatment protocols are optimized for these animal models.
Strategy to identify the therapeutic time window and optimize the treatment regimen
The therapeutic time windows are governed by a number of factors, but mainly by spatiotemporal expression of the therapeutic targets and the pharmacodynamic and pharmacokinetic properties of the therapeutic compounds. Below, we elaborate how these contributory factors potentially determine the therapeutic time windows via discussing the application of selective EP2 antagonist – TG6-10-1 – to treat status epilepticus induced by pilocarpine in mice (Figure 1) [16,23,28].
Figure 1. Schematic of seizure induction and drug treatment procedure.
Mice are pretreated with methylscopolamine and terbutaline (2 mg/kg each, i.p.) to minimize the peripheral effects of pilocarpine, and 30 min later, treated with pilocarpine (280 mg/kg, i.p.) to induce status epilepticus. The status epilepticus is allowed to persist for 1 h and terminated by pentobarbital (30 mg/kg, i.p.). Mice are then randomly split into groups and treated with vehicle or EP2 antagonist TG6-10-1 (5 mg/kg, i.p.) for multiple doses beginning hours after status epilepticus onset. Modified from Jiang et al., 2015.
COX-2/PGE2 induction after prolonged seizures
Following pilocarpine-induced status epilepticus, many inflammatory proteins and molecules are upregulated in the brain. In general, the induction of pro-inflammatory genes is faster than that of anti-inflammatory genes, [23] indicating a time-course transition of inflammatory processes. Among the pro-inflammatory mediators, COX-2 and IL-1β are most quickly induced, suggestive of their leading roles in seizure-induced pathogenesis. EP2 receptor in the brain is activated by its endogenous ligand PGE2, which is quickly synthesized by COX-2 following seizures. The action of PGE2 is highly dynamic due to its short half-life in vivo (<1 min); therefore, COX-2 induction and PGE2 synthesis profiles can be utilized as surrogates for the expected period of EP2 receptor activation by PGE2. COX-2 protein begins to significantly increase about 1 h after status epilepticus onset, maximizes 1 day after status epilepticus, and then gradually subsides to a lower induction level (Figure 2) [23]. This COX-2 time-course expression pattern essentially controls the therapeutic time window for using EP2 antagonists to treat status epilepticus.
Figure 2. COX-2 induction after status epilepticus onset.
COX-2 protein level in mouse hippocampi after pilocarpine-induced status epilepticus was measured by western blot analysis with GAPDH as the loading control. Three representative samples from each time point after status epilepticus onset are shown on the blots. Modified from Jiang et al., 2015.
Dosage
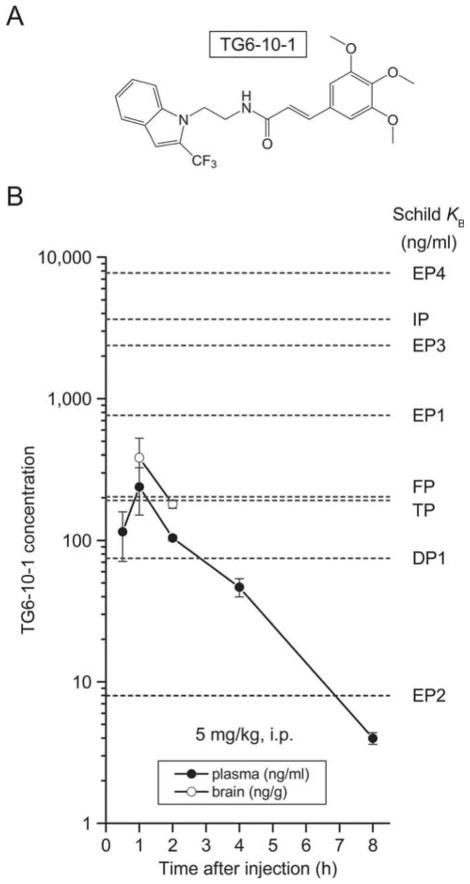
Although off-target activity assays show that TG6-10-1 has a negligible effect on a panel of 40 enzymes, ion channels, receptors, and neurotransmitter transporters (IC50 > 10 μM), it has a relatively moderate selectivity for EP2 receptor against other canonical prostanoid receptors [28]. In a comparison of Schild KB values, compound TG6-10-1 shows at least 300-fold selectivity for the EP2 receptor over EP3, EP4, and IP, 100-fold selectivity against EP1, 25-fold selectivity against FP and TP, and 10-fold selectivity against DP1 receptors [28]. Given its pharmacokinetics, with a dosage of 5 mg/kg (i.p.), TG6-10-1 brain concentration should be more than twice its EP2 receptor KB for nearly 7 h following each dose, whereas its inhibition on DP1 receptor should be very weak and brief (Figure 3). This is important because PGD2 has been demonstrated to show antiseizure effect via DP1 receptor in pentylenetetrazole (PTZ)-induced seizures in both rats and mice; [51–53] therefore, a significant inhibition on DP1 receptor could exacerbate seizure severity and duration. Furthermore, none of other prostanoid receptors is sensitive enough to TG6-10-1 to be appreciably inhibited by this dose (Figure 3). Thus, a moderate dosage such as 5 mg/kg or lower should be used to avoid any significant inhibition on other prostanoid receptors that might mediate some beneficial functions.
Figure 3. Pharmacokinetics of EP2 antagonist TG6-10-1.
(A) The chemical structure of compound TG6-10-1. (B) TG6-10-1 was administered to mice (5 mg/kg, i.p.) for pharmacokinetic studies. Compound concentrations in the plasma (ng/ml) and brain (ng/g) were measured at different time points. TG6-10-1 displayed a plasma terminal half-life of 1.6 h and brain/plasma ratio of 1.6. Data are shown as mean ± SEM (n = 3 mice per time point). The compound Schild KB values for 8 canonical prostanoid receptors were also indicated: 74.6, 762, 7.98, 2380, 7740, 202, 3640, and 193 ng/ml for DP1, EP1, EP2, EP3, EP4, FP, IP, and TP receptors, respectively. Modified from Jiang et al., 2013.
Time of first dose
COX-2 protein level begins to considerably increase 2–4 h after pilocarpine status epilepticus onset and continues to upsurge until it peaks 1 day post-status epilepticus (Figure 2). Therapeutic compound TG6-10-1 has plasma half-life of 1.6 h in mice; thus, administration of TG6-10-1 before or right after seizure onset may miss the first COX-2 expression peak. On the other hand, a delayed treatment right before COX-2 is substantially induced, i.e. 2–4 h after status epilepticus onset in this model, is demonstrated to be able to quell the most pathological consequences caused by overwhelming COX-2 activities following status epilepticus [23,28].
Dose number and duration
Multiple doses of TG6-10-1 are required to block EP2 receptor-mediated brain inflammation due to its moderately short plasma half-life (1.6 h), although it has a favorable brain-to-plasma ratio (1.6) after systemic administration in mice (Figure 3) [28]. The first two doses should be designated to counteract the COX-2 induction peaks from 4 to 24 h after status epilepticus onset; the third dose can further suppress EP2 receptor activity during the subsiding phase of COX-2 induction (Figure 2). COX-2 protein may remain noticeably elevated in some regions of the brain, such as hippocampal CA3, for weeks after status epilepticus [23]. Therefore, a daily subdose of TG6-10-1 for a few additional days might help further reduce the pathological effects of COX-2 induction.
Dose interval
The basal activation of EP2 receptor by PGE2 plays several important physiological functions, such as synaptic transmission, [54] immunoregulation, [55] and neuronal survival [25,27,56]. Thus, dosing interval needs to be considered to avoid continuous undesired effects that might occur when multiple doses are used. The second dose of TG6-10-1 at 21 h is designated to counteract the peak of COX-2 induction that occurs 16–24 h after status epilepticus onset (Figure 2), and also allows the necessary dosing interval. An additional dose of TG6-10-1 is administered at 30 h to cover the remitting phase of COX-2 induction.
This therapeutic regimen (2–4, 21, and 30 h after seizure onset, 5 mg/ml/dose; a few subdoses thereafter) has been proven to reduce neuroinflammation, neuronal death, functional deficits, and BBB disruption following pilocarpine-induced status epilepticus in mice [23,28] and acute exposure to organophosphorus compounds such as diisopropyl fluorophosphate (DFP) in rats [30]. The therapeutic paradigm can be further fine-tuned by shifting the treatment frame back and forth until improved outcomes are yielded. In addition, the therapeutic time window might change if EP2 antagonist TG6-10-1 is used to treat other acute brain insults such as nerve agent attack or intracerebral hemorrhage, as the COX-2/prostaglandin induction pattern in the brain might be different under these conditions. Furthermore, since the compound pharmacokinetic and pharmacodynamic properties play critical roles in the determination of dosage and dose frequency in order to achieve desirable efficacy and avoid adverse effects, the therapeutic time window needs to be redefined if analogs with improved plasma half-life and brain penetration [57–59] or other compounds with distinct chemical scaffolds are used [60].
Expert commentary & five-year view
In sum, brain inflammation is now recognized as a substantial contributor to the pathophysiology of many neurological disorders, and a variety of anti-inflammatory agents have been proposed as adjunctive strategies, along with first-line treatments, to reduce brain injuries and functional deficits. However, it is also well known that inflammatory responses can provide some beneficial effects in the brain, suggesting that the therapeutic time windows for anti-inflammatory therapies need to be defined to achieve adequate efficacy and avoid potential side effects. The therapeutic time windows are mainly determined by the intervention targets and the pharmacokinetic and pharmacodynamic profiles of the therapeutic compounds. In addition, the inter-species and inter-model differences should also be considered, because the inflammatory induction pattern is often species- or model-specific. Nonetheless, the case of using EP2 antagonist TG6-10-1 to reduce brain inflammation and injury after status epilepticus sets an example that could guide to identify the therapeutic time windows and optimize the treatment paradigms for other anti-inflammatory strategies.
Translation of the preclinical therapeutic time window to the clinical setting might be challenging, particularly in status epilepticus, where urgent care is required to stop seizures. However, the anti-inflammatory therapeutics discussed here aim to reduce seizure-triggered neuronal death and functional loss, although they might also show antiepileptic or antiepileptogenic effects. Therefore, the anti-inflammatory therapeutic time window is not necessarily tightly bound to that of antiepileptic drugs, which usually need to be delivered as quickly as possible. Within the next 5 years, it is very likely that we will witness more positive and reproducible outcomes in the battle of controlling inflammation-mediated pathogenesis in the brain as our knowledge about the time window of anti-inflammatory therapeutics increases and the treatment paradigms are optimized for the current anti-inflammatory agents.
Key Issues.
Inflammation in the brain now is widely recognized as an important contributor to the pathophysiology of many neurological conditions, including status epilepticus.
Many inflammation-associated genes are rapidly and persistently induced by status epilepticus with COX-2 and IL-1β induction temporally leading others, suggesting their dominant roles in seizure-promoted neuroinflammation.
As the major prostaglandin product of COX-2 in the brain, PGE2 plays a pivotal role in COX-2 cascade-mediated pathogenesis via its EP2 receptor subtype.
Blockade of EP2 receptor by recently developed small-molecule antagonists provides a novel anti-inflammatory therapeutic strategy to treat status epilepticus.
Inflammatory responses can also provide some beneficial effects in the brain, indicating that the therapeutic time windows for anti-inflammatory therapies need be defined to achieve adequate efficacy and avoid potential undesired effects.
The therapeutic time windows are primarily determined by the target molecules and the pharmacokinetics and pharmacodynamics of the therapeutic agents; however, animal species and disease models are other potential contributory factors.
Optimization of the treatment paradigm, i.e. dosage, time of first dose, dose number and duration, and dose interval, might yield improved therapeutic outcomes of anti-inflammatory therapeutics as well as increase the reproducibility of preclinical studies.
Footnotes
orcid
Jianxiong Jiang http://orcid.org/0000-0003-3955-8928
Financial & Competing Interests Disclosure
J. Jiang is supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grant R00NS082379 and NARSAD Young Investigator Grant 20,940 from the Brain & Behavior Research Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33(6):304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2•.Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends Neurosci. 2014;37(2):55–65. doi: 10.1016/j.tins.2013.11.002. This excellent review summarizing recent advances in the understanding and treatment of epileptic seizures where inflammatory pathways involving the blood-brain barrier dysfunction play essential roles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janigro D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia. 2012;53(Suppl 1):26–34. doi: 10.1111/j.1528-1167.2012.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchi N, Granata T, Freri E, et al. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One. 2011;6(3):e18200. doi: 10.1371/journal.pone.0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vezzani A, French J, Bartfai T, et al. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. doi: 10.1111/j.1528-1167.2011.03033.x. A great review mainly introducing the recent clinical observations that support the involvement of the inflammatory responses in the development of epilepsy. [DOI] [PubMed] [Google Scholar]
- 8.Vezzani A. Anti-inflammatory drugs in epilepsy: does it impact epileptogenesis? Expert Opin Drug Saf. 2015;14(4):583–592. doi: 10.1517/14740338.2015.1010508. [DOI] [PubMed] [Google Scholar]
- 9••.Varvel NH, Jiang J, Dingledine R. Candidate drug targets for prevention or modification of epilepsy. Annu Rev Pharmacol Toxicol. 2015;55:229–247. doi: 10.1146/annurev-pharmtox-010814-124607. A recent review discussing the potential drug targets for the disease modification or prevention of the human epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Vezzani A, Dingledine R, Rossetti AO. Immunity and inflammation in status epilepticus and its sequelae: possibilities for therapeutic application. Expert Rev Neurother. 2015;15(9):1081–1092. doi: 10.1586/14737175.2015.1079130. An excellent review focusing on the detrimental roles for inflammation during and after status epilepticus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Ambrosio R, Eastman CL, Fattore C, et al. Novel frontiers in epilepsy treatments: preventing epileptogenesis by targeting inflammation. Expert Rev Neurother. 2013;13(6):615–625. doi: 10.1586/ern.13.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maroso M, Balosso S, Ravizza T, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 13.Ravizza T, Lucas SM, Balosso S, et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia. 2006;47(7):1160–1168. doi: 10.1111/j.1528-1167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 14.Marchi N, Fan Q, Ghosh C, et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33(2):171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas A, Jiang J, Ganesh T, et al. Cyclooxygenase-2 in epilepsy. Epilepsia. 2014;55(1):17–25. doi: 10.1111/epi.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Jiang J, Ganesh T, Du Y, et al. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A. 2012;109(8):3149–3154. doi: 10.1073/pnas.1120195109. This research article reported the first-generation of selective antagonists for human EP2 receptor by high-throughput screening and demonstrated that administration of EP2 antagonist TG4-155 is neuroprotective after pilocarpine-induced status epilepticus in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung KH, Chu K, Lee ST, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006;23(2):237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Ivens S, Kaufer D, Flores LP, et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130(Pt 2):535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 19.Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40(1):120–122. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 20.DeLorenzo RJ, Garnett LK, Towne AR, et al. Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia. 1999;40(2):164–169. doi: 10.1111/j.1528-1157.1999.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 21.DeLorenzo RJ, Kirmani B, Deshpande LS, et al. Comparisons of the mortality and clinical presentations of status epilepticus in private practice community and university hospital settings in Richmond, Virginia. Seizure. 2009;18(6):405–411. doi: 10.1016/j.seizure.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossetti AO, Lowenstein DH. Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol. 2011;10(10):922–930. doi: 10.1016/S1474-4422(11)70187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Jiang J, Yang MS, Quan Y, et al. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol Dis. 2015;76:126–136. doi: 10.1016/j.nbd.2014.12.032. This study identified a therapeutic time window for anti-inflammatory treatment via inhibition of the EP2 receptor that opens approximately two hours after onset of pilocarpine-induced status epilepticus in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata T, Narumiya S. Prostanoid receptors. Chem Rev. 2011;111(10):6209–6230. doi: 10.1021/cr200010h. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013;34(7):413–423. doi: 10.1016/j.tips.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcheselli VL, Bazan NG. Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J Biol Chem. 1996;271(40):24794–24799. doi: 10.1074/jbc.271.40.24794. [DOI] [PubMed] [Google Scholar]
- 27.Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat. 2010;91(3–4):104–112. doi: 10.1016/j.prostaglandins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Jiang J, Quan Y, Ganesh T, et al. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A. 2013;110(9):3591–3596. doi: 10.1073/pnas.1218498110. This research paper reported the second-generation of selective antagonists for EP2 receptor with improved pharmacokinetics by medicinal chemistry and showed that delayed treatment by EP2 antagonist TG6-10-1 reduces the seizure-induction neuroinflammation, neuronal injury, blood-brain barrier dysfunction and functional deficits after pilocarpine-induced status epilepticus in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrano GE, Lelutiu N, Rojas A, et al. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011;31(42):14850–14860. doi: 10.1523/JNEUROSCI.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Rojas A, Ganesh T, Lelutiu N, et al. Inhibition of the prostaglandin EP2 receptor is neuroprotective and accelerates functional recovery in a rat model of organophosphorus induced status epilepticus. Neuropharmacology. 2015;93:15–27. doi: 10.1016/j.neuropharm.2015.01.017. This study on rats showed that EP2 receptor inhibition within a time window that coincides with the cyclooxygenase-2 induction by organophosphorus compound diisopropyl fluorophosphate is neuroprotective and reduces functional deficits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan S, Ahmad AS, Glushakov AV, et al. Putative role of prostaglandin receptor in intracerebral hemorrhage. Front Neurol. 2012;3:145. doi: 10.3389/fneur.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leclerc JL, Lampert AS, Diller MA, et al. Prostaglandin E2 EP2 Receptor deletion attenuates intracerebral hemorrhage-induced brain injury and improves functional recovery. ASN Neuro. 2015;7:2. doi: 10.1177/1759091415578713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang J, Dingledine R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther. 2013;344(2):360–367. doi: 10.1124/jpet.112.200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gobbo OL, O’Mara SM. Post-treatment, but not pre-treatment, with the selective cyclooxygenase-2 inhibitor celecoxib markedly enhances functional recovery from kainic acid-induced neurodegeneration. Neuroscience. 2004;125(2):317–327. doi: 10.1016/j.neuroscience.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 35.Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13(3):569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira MS, Furian AF, Royes LF, et al. Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res. 2008;79(1):14–21. doi: 10.1016/j.eplepsyres.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Akula KK, Dhir A, Kulkarni SK. Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor increases pentylenetetrazol seizure threshold in mice: possible involvement of adenosinergic mechanism. Epilepsy Res. 2008;78(1):60–70. doi: 10.1016/j.eplepsyres.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Azevedo FA, Carvalho LR, Grinberg LT, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513(5):532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 39.Polascheck N, Bankstahl M, Loscher W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol. 2010;224(1):219–233. doi: 10.1016/j.expneurol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Kawaguchi K, Hickey RW, Rose ME, et al. Cyclooxygenase-2 expression is induced in rat brain after kainate-induced seizures and promotes neuronal death in CA3 hippocampus. Brain Res. 2005;1050(1–2):130–137. doi: 10.1016/j.brainres.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 41.Takemiya T, Maehara M, Matsumura K, et al. Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res. 2006;56(1):103–110. doi: 10.1016/j.neures.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Takemiya T, Suzuki K, Sugiura H, et al. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid Mediat. 2003;71(3–4):205–216. doi: 10.1016/s1098-8823(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 43.Citraro R, Leo A, Marra R, et al. Antiepileptogenic effects of the selective COX-2 inhibitor etoricoxib, on the development of spontaneous absence seizures in WAG/Rij rats. Brain Res Bull. 2015;113:1–7. doi: 10.1016/j.brainresbull.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Trandafir CC, Pouliot WA, Dudek FE, et al. Co-administration of subtherapeutic diazepam enhances neuroprotective effect of COX-2 inhibitor, NS-398, after lithium pilocarpine-induced status epilepticus. Neuroscience. 2015;284:601–610. doi: 10.1016/j.neuroscience.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holtman L, Van Vliet EA, Edelbroek PM, et al. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobe epilepsy. Epilepsy Res. 2010;91(1):49–56. doi: 10.1016/j.eplepsyres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Holtman L, Van Vliet EA, Van Schaik R, et al. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res. 2009;84(1):56–66. doi: 10.1016/j.eplepsyres.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Ma L, Cui XL, Wang Y, et al. Aspirin attenuates spontaneous recurrent seizures and inhibits hippocampal neuronal loss, mossy fiber sprouting and aberrant neurogenesis following pilocarpine-induced status epilepticus in rats. Brain Res. 2012;1469:103–113. doi: 10.1016/j.brainres.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 48.Jeong KH, Kim JY, Choi YS, et al. Influence of aspirin on pilocarpine-induced epilepsy in mice. Korean J Physiol Pharmacol. 2013;17(1):15–21. doi: 10.4196/kjpp.2013.17.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad AS, Zhuang H, Echeverria V, et al. Stimulation of prostaglandin EP2 receptors prevents NMDA-induced excitotoxicity. J Neurotrauma. 2006;23(12):1895–1903. doi: 10.1089/neu.2006.23.1895. [DOI] [PubMed] [Google Scholar]
- 50.McCullough L, Wu L, Haughey N, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24(1):257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaushik MK, Aritake K, Kamauchi S, et al. Prostaglandin D (2) is crucial for seizure suppression and postictal sleep. Exp Neurol. 2014;253:82–90. doi: 10.1016/j.expneurol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Mello CF, Oliveira MS. Commentary on Kaushik et al.: Prostaglandin D2 is crucial for seizure suppression and postictal sleep. Novel evidence supporting a role for prostanoid receptors in seizure control. Exp Neurol. 2014;257:157–161. doi: 10.1016/j.expneurol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Akarsu ES, Mamuk S, Comert A. Inhibition of pentylene-tetrazol-induced seizures in rats by prostaglandin D2. Epilepsy Res. 1998;30(1):63–68. doi: 10.1016/s0920-1211(97)00092-2. [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Zhang J, Breyer RM, et al. Altered hippocampal long-term synaptic plasticity in mice deficient in the PGE2 EP2 receptor. J Neurochem. 2009;108(1):295–304. doi: 10.1111/j.1471-4159.2008.05766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L, Yamagata N, Yadav R, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111(5):727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang J, Ganesh T, Du Y, et al. Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci U S A. 2010;107(5):2307–2312. doi: 10.1073/pnas.0909310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ganesh T, Jiang J, Dingledine R. Development of second generation EP2 antagonists with high selectivity. Eur J Med Chem. 2014;82:521–535. doi: 10.1016/j.ejmech.2014.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganesh T, Jiang J, Yang MS, et al. Lead optimization studies of cinnamic amide EP2 antagonists. J Med Chem. 2014;57(10):4173–4184. doi: 10.1021/jm5000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang J, Ganesh T, Du Y, et al. WO2012177618; US20140179750; EP2721011; CA2839956. Prostaglandin receptor EP2 antagonists, derivatives, compositions, and uses related thereto. 2014
- 60.Ganesh T, Jiang J, Shashidharamurthy R, et al. Discovery and characterization of carbamothioylacrylamides as EP selective antagonists. ACS Med Chem Lett. 2013;4(7):616–621. doi: 10.1021/ml400112h. [DOI] [PMC free article] [PubMed] [Google Scholar]