Abstract
Background
Peutz-Jeghers syndrome (PJS) is an autosomal dominant genetic disease. It severely decreases patient quality of life and leads elevated cancer risk. Germline mutation of LKB1 is the leading cause of familial PJS.
Material/Methods
To characterize the germline mutation of LKB1 gene in Chinese familial and sporadic PJS patients, 14 PJS families, 5 sporadic PJS patients, and 250 healthy adults were collected and genomic DNAs of peripheral blood were extracted. Mutation screenings of LKB1 were performed using MLPA (multiplex ligation-dependent probe amplification), PCR, direct sequencing, and PCR-DHPLC (denaturing high-performance liquid chromatography).
Results
A total of 12 kinds of germline mutations were found in 9 familial PJS patients, most of which were point mutations (7/12); 4 large deletions of LKB1 were also observed. Of the 12 mutations, 7 were pathogenic (2 were de novo), 4 were just polymorphisms, and 1 was indefinitely pathogenic. No pathogenic mutation in exons of the LKB1 gene was detected in the 5 sporadic PJS patients. The mutation detection rate for the LKB1 gene was 85.7% in our Chinese familial PJS and 63.2% in all Chinese PJS patients. Eight familial PJS patients were identified with pathogenic germline mutations in 14 unrelated families (57.1%). Further methylation detection and analysis showed promoter methylation in carcinomatous polyps.
Conclusions
LKB1 gene germline mutation with pathogenic effect is a common cause of familial PJS in Chinese patients; however, it is not the only molecular pathogen of PJS. Methylation in the LKB1 gene promoter region may cause carcinomatous change in intestinal polyps.
MeSH Keywords: Germ-Line Mutation, Peutz-Jeghers Syndrome, Protein-Serine-Threonine Kinases
Background
Peutz-Jeghers syndrome, also known as hereditary intestinal polyposis syndrome, is a rare inherited disease, with an estimated incidence of 1 in 50 000 to 200 000 births [1]. It is an autosomal dominant genetic disease characterized by the development of benign hamartomatous polyps in the gastrointestinal tract, hyperpigmented macules on the lips and oral mucosa (melanosis), and elevated cancer risk [2–4]. Polyps may cause gastrointestinal bleeding, intussusception, obstruction, or infarction, causing pain and decreasing quality of life [5,6]. Clinicians long thought that malignant transformation of polyps is rare [7] and there is little published data on the mechanism of polyp malignancy in the Chinese population.
Genetic linkage analysis shows that the germline mutation of LKB1 is the leading cause of familial PJS. LKB1, also called serine threonine kinase 11 gene (STK11), is a tumor suppressor gene located on the autosome 19p13.3 [8–10]. Compared to familial PJS, sporadic PJS seldom shows the mutation of LKB1. With the development of medical technology and biology, systematic and high-throughput molecular genetic testing for this mutation is available clinically [11]. However, there has been little research on systematic screening of Chinese PJS patients.
The present study investigated the mutations of LKB1 gene in Chinese PJS patients by use of systematic screening methods. We combined MLPA (multiplex ligation-dependent probe amplification), PCR-DHPLC (polymerase chain reaction-denaturing high-performance liquid chromatography), and DNA sequence analysis to disclose the mutation of LKB1 and the methylation of 5′-CpG islands of its promoter in Chinese PJS patients.
Material and Methods
Participants
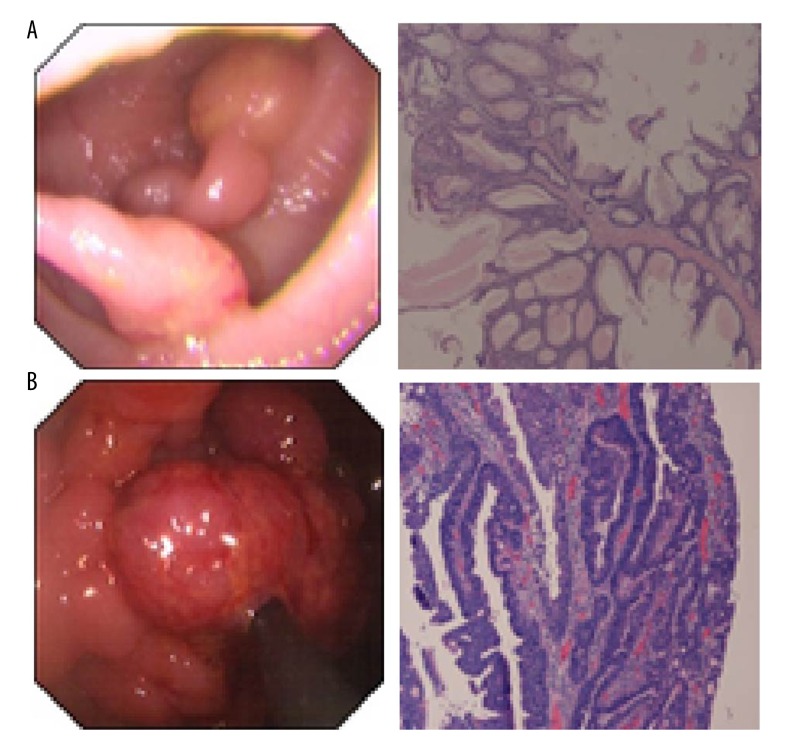
Individuals in this study were referred for genetic counseling and testing to the Department of Gastroenterology and Hepatology at Jinling Hospital, the Department of Gastroenterology at Nanjing Children’s Hospital, and Jiangsu Cancer Hospital, and the Laboratory of Genetics and Molecular Biology at Jiangsu Province Cancer Hospital in Nanjing China between 2004 and 2014. All investigations were carried out in compliance with internationally recognized guidelines. Obtained the participants’ tissues and information as well as study protocols have been approved by the ethical committee of Jinling Hospital. Written informed consent was obtained from each patient. Included in this study were 19 participants including 14 unrelated familial PJS probands and 5 sporadic PJS patients. After clinical observation and endoscopic check, the patients were noted to have classic lip, mucosa, and hand/foot pigmentations as well as hamartomatous polyps in the gastrointestinal tract (Figure 1A, 1B). The biopsies of polyps revealed malignance polyp in the colons of 2 patients, including 1 familial PJS proband and 1 sporadic PJS patient (Figure 1B). HE staining images showed that the structures of abnormal glands became complicated and local lesions exhibited diablastic and daedaleous structure.
Figure 1.
Polyps of PJS patients. (A) The polyps in the duodenum of 1 PJS patient and its HE staining image. The glands lose polarity and show disordered arrangements. Glandular cavities had aberrant morphology or presented mild dilatation. The epithelial lining demonstrated normal epithelium without signs of dysplasia. These phenotypes demonstrated prototypic hamartomatous polyps. (B) The polyps in the colon of 1 PJS patient and its HE staining image. HE staining shows that the structures of abnormal glands were complicated, and local lesions exhibited diablastic and daedaleous structure (white arrow). The epithelial cells with mild dysplasia demonstrate cancerous transformation.
The Extraction of DNA
The total DNAs were extracted from peripheral blood using the QIAamp DNA Tissue Mini Kit (QIAGEN, USA). The procedures were performed according to manufacturer instructions. The extracted DNA was stored at −20°C for future experiments.
PCR primer pairs and synthesis
The 9 primer pairs used in the experiments were from a published paper [9]. The sequences of primer pairs are listed in Table 1 and were synthesized by Takara Biotechnology (Dalian) Co. Ltd.
Table 1.
The primer pairs for LKB1 gene.
| Exons | Forward primer (5′→3′) | Reward primer (5′→3′) |
|---|---|---|
| 1 | GGAAGTCGGAACACAAGGAA | GGGAGGAGAGAAGGAAGGAA |
| 2 | GAGGTACGCCACTTCCACAG | CTTCAAGGAGACGGGAAGAG |
| 3 | CTCCAGAGCCCCTTTTCTGG | CAGTGTGGCCTCACGGAAAG |
| 4–5 | GGCCCCAGGACGGGTGTGTG | AGTGTGCGTGTGGTGAGTGC |
| 6 | TGACTGACCACGCCTTTCTT | CCCCCAACCCTACATTTCTG |
| 7 | CTCCTCGCCGGCTTCTCCTC | CCCACCACGCCCTGCTCTA |
| 8 | GACAGGCGCCACTGCTTCTG | GGACATCCTGGCCGAGTCAG |
| 9 | CTTGCCGTCTCCCTCCCA | GCATCCAGGCGTTGTCCC |
PCR amplification
The ingredients of the 25-μl PCR system are as follows: 5 μl of 5 X PrimeSTAR buffer (Mg2+ plus), 1 μl of forward primer, 1 μl of reward primer, 2 μl of dNTPs, 0.2 μl of PrimeSTAR DNA polymerase, 1 ul of sample DNAs, and 14.8 ul of ddH2O. The PCR reactions were performed on a Biometra PCR machine. The amplification steps were carried out at 95°C for 5 min followed by 35 cycles of denaturing at 95°C for 40 s, annealing at 62°C for 40 s, and extension at 72°C for 30 s, and then a final extension at 72°C for 5 min on a PCR machine. PCR products were separated by use of electrophoresis in 1.2% agarose gel. Images were acquired and analyzed using the Gel image system (GIS-2500, Tanon).
Sequencing
The PCR products of sample DNAs from 19 patients were sent to BGI for sequencing. The sequencing results were shown in Chroma software and blast analyses of gene sequences were carried out for the identification of germline mutations. The heterozygous mutants were tested by use of further reverse sequencing.
Multiplex ligation-dependent probe amplification (MLPA) assay
We used the SALSA MLPA kit P101-B111 (MRC-Holland, Amsterdam, The Netherlands), containing SALSA MLPA buffer, Ligase-65, Ligase-65 buffer A and B, SALSA PCR buffer, SALSA PCR primer mix, SALSA polymerase, SALSA enzyme dilution buffer, and SALSA probe mix. The probe mix, including probes for 10 exons and promoter region of LKB1 gene and 14 reference probes, can be used to screen whole LKB1 gene.
We denatured 100 ng of extracted genomic DNA by incubating at 98°C on a PCR machine. The MLPA probes were added to the DNA extract and hybridized at 60°C. The subsequent ligation reaction was performed at 54°C for 15 min after the addition of the ligase and ligase buffer. The ligase was inactivated by incubating the reaction tube at 98°C. PCR of the ligation product was performed following the addition of universal PCR primers, dNTPs, and polymerase. The amplified products were then analyzed using the ABI 3100 Avant sequencer (Applied Biosystems, Foster City, CA, USA). MLPA assay results were analyzed using GeneScan 3.1 software (Applied Biosystem, Foster City, CA, USA) to determine the deletions or the number of duplications. A peak ratio less than 0.7 was interpreted as a ‘deletion’, while a peak ratio greater than 1.3 was interpreted as a ‘duplication’.
Polymerase chain reaction denaturing high-performance liquid chromatograph (PCR-DHPLC) assay
Excluding known polymorphic sites, other mutation sites were selected to be tested in 250 normal people. The PCR products of these sites in 250 normal people were loaded into a DHPLC machine (WAVE system, Transgenomics) for analysis. The fluid was 0.1 M of triethyl ammonium acetate (TEAA), which was used for DNA binding to cartridge matrix. Then, different concentrations of acetonitrile buffer were used for elution. The fluid velocity was 0.9 ml/min. Finally, the binding products were analyzed by use of 260 nM UV. The elution maps of patients with mutation sites were taken as positive controls. The PCR products of 250 normal peoples were screened to exclude mutation sites with unknown gene polymorphism.
Methylation-specific PCR (MSP) Assay
Participant DNA was treated with sodium bisulfite and analyzed by MSP as described by Herman et al. [12]. This process converts nonmethylated cytosine residues to uracil, whereas methylated cytosines remain unchanged. Bisulfite-modified samples were aliquoted and stored at −80°C. The sequences of primers used for amplifying unmethylated and methylated CpG islands were synthesized as described by the Trojan group [13]. The sequences of primer for amplifying methylated CpG island were as follows: forward (fw) primer, ACGAAGTTGATTTTGATCGGGTC and reward (rw) primer, CGATACAAAATCTACGAACCGACG. The sequences of primer for amplifying unmethylated CpG island were as follows: fw primer, GGATGAAGTTGATTTTGATTGGGTT and rw primer, ACCCAATACAAAATCTACAAACCAACA. The total 25-μl PCR system contains 100 ng of sodium bisulfite-treated DNAs, 0.2 mmol/L dNTPs, 0.5 μmol/L of fw and rw primers, and 2 U of Taq DNA polymerase. The PCR amplification cycles were carried out at 95°C for 5 min followed by 35 cycles of denature at 95°C for 20 s, annealing at 58°C for 20 s and extension at 72°C for 20 s, and then a final extension at 72°C for 5 min on a PCR machine. The PCR products were separated by use of electrophoresis in 1.2% agarose gel. Images were acquired and analyzed using the Gel image system (GIS-2500, Tanon).
Results
Patient characteristics
Total 14 unrelated familial PJS patients and 5 sporadic PJS patients were screened for mutation analysis in LKB1 gene. Among 14 unrelated familial PJS patients, there were 9 males and 5 females, with a mean age of 21.8 years. The total 5 sporadic PJS patients includes 3 males and 2 females, with a mean age of 21.6 years, and they were also genetically unrelated. As mentioned above, the patients were noted to have classic lip, mucosa, and hand/foot pigmentations, as well as hamartomatous polyps in the gastrointestinal tract (Table 2, Figure 1A, 1B). The biopsies of polyps revealed that malignant polyps in the colons of 2 patients: 1 familial PJS proband (Table 2) and 1 sporadic PJS patient (Figure 1B). Samples of peripheral blood of 250 healthy adults were collected for screening to avoid polymorphism in the LKB1 gene.
Table 2.
The clinic features of the probands with familial PJS.
| Probands | Age (years) | Gender | Age of onset/diagnose (years) | Family history | Distribution of polyp |
|---|---|---|---|---|---|
| 1# | 19 | Female | 18 | Grandfather* and father | Jejunum and colon |
| 2# | 10 | Male | 10 | Father | Stomach and colon |
| 3# | 45 | Male | 40 | Father and son | Colon |
| 4# | 22 | Female | 21 | Father | Colon |
| 5# | 26 | Male | 26 | Father | Colon |
| 6# | 26 | Male | 21 | Mother and brother | Colon |
| 7# | 44 | Male | 8 | Father, daughter | Colon |
| 8# | 41 | Female | 35 | Matrilineal grandmother, mother, mother’s brother and sister | Duodenum, jejunum, colon, adenocarcinoma |
| 9# | 14 | Male | 8 | Mother | Jejunum and colon |
| 10# | 31 | Female | 31 | Matrilineal grandmother, mother and mother’s sister | Colon and rectum |
| 11# | 24 | Male | 16 | Father | Colon and rectum |
| 12# | 26 | Male | 16 | Grandmother*, father and son | Stomach and colon |
| 13# | 19 | Male | 18 | Father | Stomach and colon |
| 14# | 37 | Female | 37 | Brother | Colon and rectum |
Represents patrilineal lineage.
The PCR optimization and amplification of samples
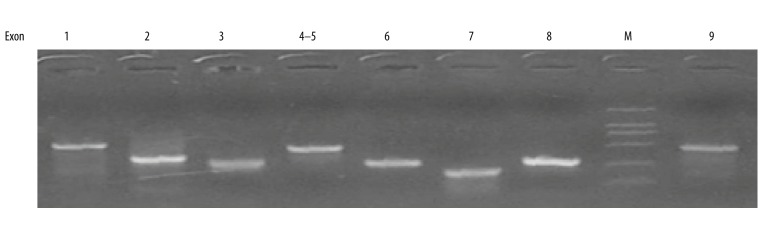
We first optimized the conditions for PCR reactions, including the concentration of template DNA from samples and cycles. After several rounds of tests, the PCR products of all samples for 9 exons of LKB1 gene showed only 1 specific and clear band after separation through 1.2% agarose gel (Figure 2). By use of primer pairs listed in Table 1 and the PCR procedure described above, PCR reactions were carried out with different concentrations of template DNA. The optimized final concentration was 100 ng. The optimized PCR system was then used in all samples. The PCR products of LKB1 exons all presented 1 clear and specific band in 1.2% agarose gel with ethidium bromide (EB) after electrophoresis. The results demonstrated that these pure PCR products could be used for further sequencing and analyzing.
Figure 2.
Representative gel image exhibiting PCR products of 9 exons of LKB1 genes in patients. M, DNA marker. Number stands for exons of LKB1 gene from 1 to 9. 1, exon 1; 2, exon 2; 3, exon 3; 4–5, exon 4 and 5; 6, exon 6; 7, exon 7; 8, exon 8; 9, exon 9.
Mutation analysis of LKB1 gene
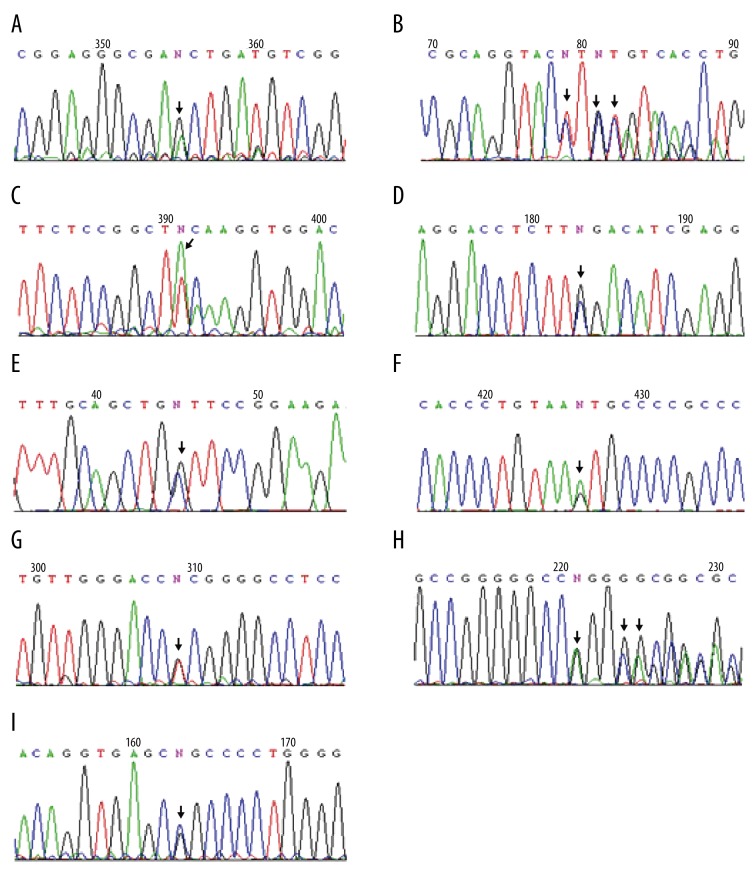
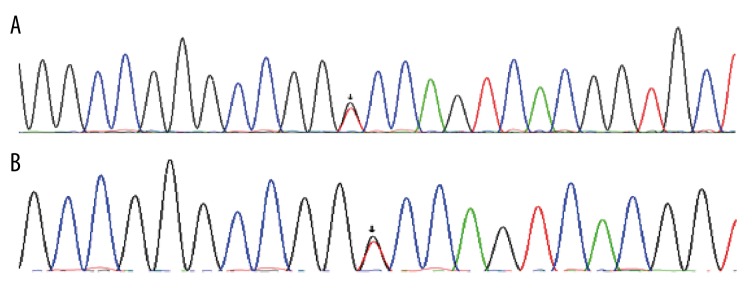
The direct sequencing results of PCR products from 14 probands with familial PJS showed that 12 kinds of germline mutations of LKB1 gene were found in 12 PJS patients, most of which were point mutations (58.3% in all mutations, Table 3, Figure 3), 4 were base deletions, and 1 was base insertion of small fragments (Table 3, Figure 4). Figure 3 depicts the representative germline mutations. Through the presentation and blast analysis using Chrome software, point mutations were identified clearly. The mutation (c.48G>A) was clearly shown in exon 1 (Figure 3A). There was the mutation (c.701T>A) in exon 4 (Figure 3C). Interestingly, there were 2 point mutations in exon 8 of LKB1 gene in 1 patient: 1 was the mutation (c.1062C>G) (Figure 3D) and the other was the mutation (c.924G>C) (Figure 3E). The sequencing results also demonstrated point mutations in introns. There was 1 mutation (c.734+5G>A) in intron 5 (Figure 3F), 1 mutation (c.374+24G>T) in intron 2 (Figure 3G), and 1 mutation (c.920+7G>C) in intron 7 (Figure 3I). In 1 patient, 30-bp deletion was detected in exon4 (Figure 3B) and an insertion mutation (c.464+47_48insGGGGGCC) was also found in intron3 (Figure 3H).
Table 3.
The LKB1 germline mutation patterns of 14 familial PJS probands.
| Probands | Mutation patterns | Mutation sites | Mutation types | Anticipated effects |
|---|---|---|---|---|
| 1# | Deletion in exon1 | Exon1 | Deletion | Pathogenic |
| 7# | c.48G>A | Exon 1 | Nonsense | Glu16Glu, indefinitely |
| 6# | c.471_501delTTCTGTCAGCTGATTGACGGCCTGGAGTAC | Exon 4 | Small Deletion | Loss of 10 aa, pathogenic |
| 8# | deletion in exon3–10 | Exon3–10 | Deletion | Pathogenic |
| 11# | c.701T>A | Exon 4 | Missense | Phe267Tyr, pathogenic |
| c.1062C>G | Exon 8 | Missense | Phe354Leu, polymorphism | |
| 2#, 13# | c.924G>C | Exon 8 | Missense | Trp308Cys, pathogenic |
| 14# | Deletion in exon 2–4 | Exon 2–4 | Deletion | Pathogenic |
| 9# | c.734+5G>A | Intron 5 | Intron mutation | Splicing alteration, pathogenic |
| 7#, 8#, 9#, 11#, 12#, 13# | c.374+24G>T | Intron 2 | Intron mutation | Polymorphism, dbsnpID rs2075604 |
| 8#9#11# | c.464+47_48insGGGGGCC | Intron 3 | Intron mutation | Polymorphism, dbsnpID rs66999113 |
| 4#7#10# | c.920+7 G>C | Intron 7 | Intron mutation | Polymorphism, dbsnpID rs2075607 |
Figure 3.
Direct sequencing results of LKB1 gene PCR Products. Arrow, mutation sites. (A) The mutation (c.48G>A) in exon 1. (B) The deletion (c.471_501delTTCTGTCAGCTGATTGACGGCCTGGAGTAC) in exon 4. (C) The mutation (c.701T>A in exon 4. (D) The mutation (c.1062C>G) in exon 8. (E) The mutation (c.924G>C) in exon 8. (F) The mutation (c.734+5G>A) in intron 5. (G) The mutation (c.374+24G>T) in intron 2. (H) The insertion (c.464+47_48insGGGGGCC) in intron 3. (I) The mutation (c.920+7G>C) in intron 7.
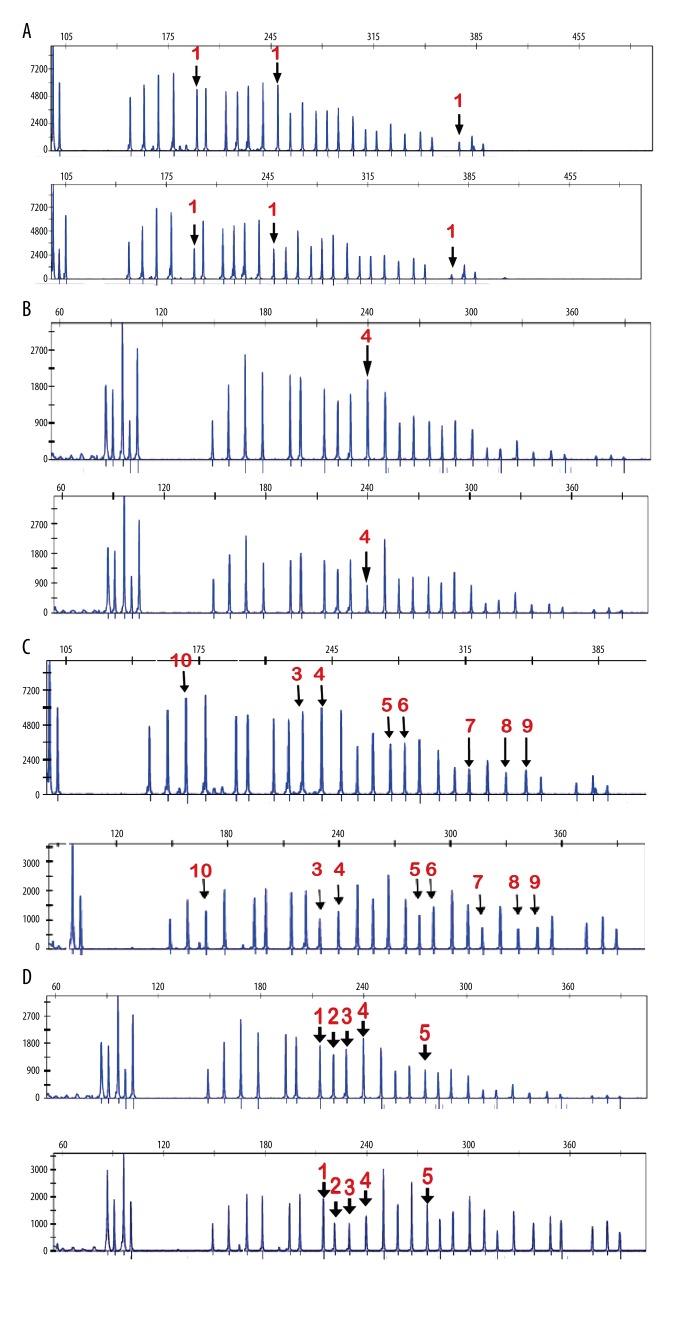
Figure 4.
Peak patterns of exons from LKB1 gene in MLPA assay. The x-axis shows the size of PCR products, and the y-axis reflects the relative quantity of PCR products. (A) Deletion in exon 1 was detected in 1 case. Top shows the amount of every PCR product from normal controls. Bottom shows the amount of every PCR product from familial PJS patients. (B) Deletion in exon 4 was detected in 1 case. Top shows the amount of every PCR product from normal controls. Bottom shows the amount of every PCR product from familial PJS patients. (C) Deletions in exon 3–10 were detected in 1 case. Top shows the amount of every PCR product from normal controls. Bottom shows the amount of every PCR product from familial PJS patients. (D). Deletions in exon 2–4 were detected in 1 case. Top shows the amount of every PCR product from normal controls. Bottom shows the amount of every PCR product from familial PJS patients. Red numbers represent exons.
We used MLPA assay to detect deletions or insertions of genomic fragments in PJS patients. By use of the assay, we found 4 large base fragment deletions in PJS patients (Table 3, Figure 4). Representative peak patterns are showed in Figure 4. The peak patterns of patients were compared to those of healthy adults, showing significant differences in patterns. The deletions in exon 1 and exon 4 were discovered in 2 patients (Figure 4A, 4B). Intriguingly, 1 patient had the deletions in exon 3–10 (Figure 4C) and another patient had the deletions in exon 2–4 (Figure 4D).
We thought that missense mutations and frame shifts due to nonsense mutation, base deletions, or insertions were pathogenic mutations. These pathogenic mutations were not detected in healthy adults, and the ones detected in healthy adults were thought to be non-pathogenic mutations, regardless of mutation type. Of the 12 mutations, 7 were considered to be pathogenic, of which 2 were de novo, 4 were polymorphic sites detected in healthy adults, and 1 was indefinite in pathogenesis (Table 3, Figure 3, 4).
We also sequenced the PCR products of samples from 5 patients with sporadic PJS. The direct sequencing results showed no mutations in the region of 9 exons of LKB1 gene, and only 1 point mutation (c.G>T) was detected in the intron between exon1 and exon2, which might be associated with RNA splicing, or just the polymorphic site of LKB1 gene (Figure 5).
Figure 5.
Direct sequencing results of LKB 1 gene PCR products in sporadic PJS patients. Arrows, mutation sites. (A) and (B). Only 1 (c.G>T) polymorphic site was observed in intron between exon 1 and exon 2 in 2 sporadic PJS patients. No mutation was detected in any exons of LKB1 gene in the 5 sporadic PJS patients.
The detection of methylation modification of LKB1 gene promoter in sporadic PJS patients
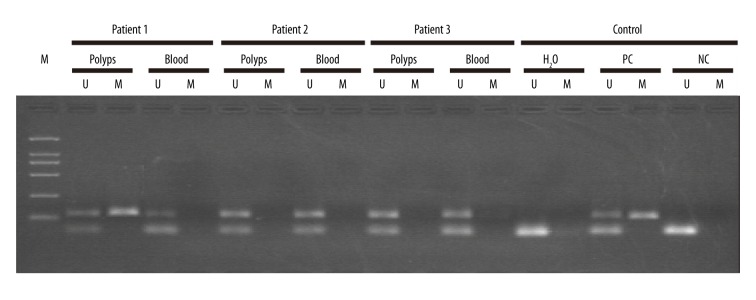
We detected the methylation modification of the promoter region of LKB1 gene in the polyps and blood of 3 sporadic PJS patients (Figure 6). The methylation of LKB1 gene promoter was detected in 1 sporadic PJS patient whose polyps showed cancerous transformation (Figure 6)., suggesting that the methylation of the promoter of LKB1 gene inhibited the role of LKB1 in tumor formation and cancer initiation as a tumor suppressor. This might be the cause of the elevated cancer risk in PJS patients.
Figure 6.
The methylation detection of LKB1 gene promoter in sporadic PJS patients. The representative results of 3 patients are shown here. The detected samples include polyps tissue and peripheral blood, respectively. U – unmethylated; M – methylated; PC – positive control; NC – negative control.
PCR-DHPLC analysis of mutation sites of LKB1 gene in healthy adults
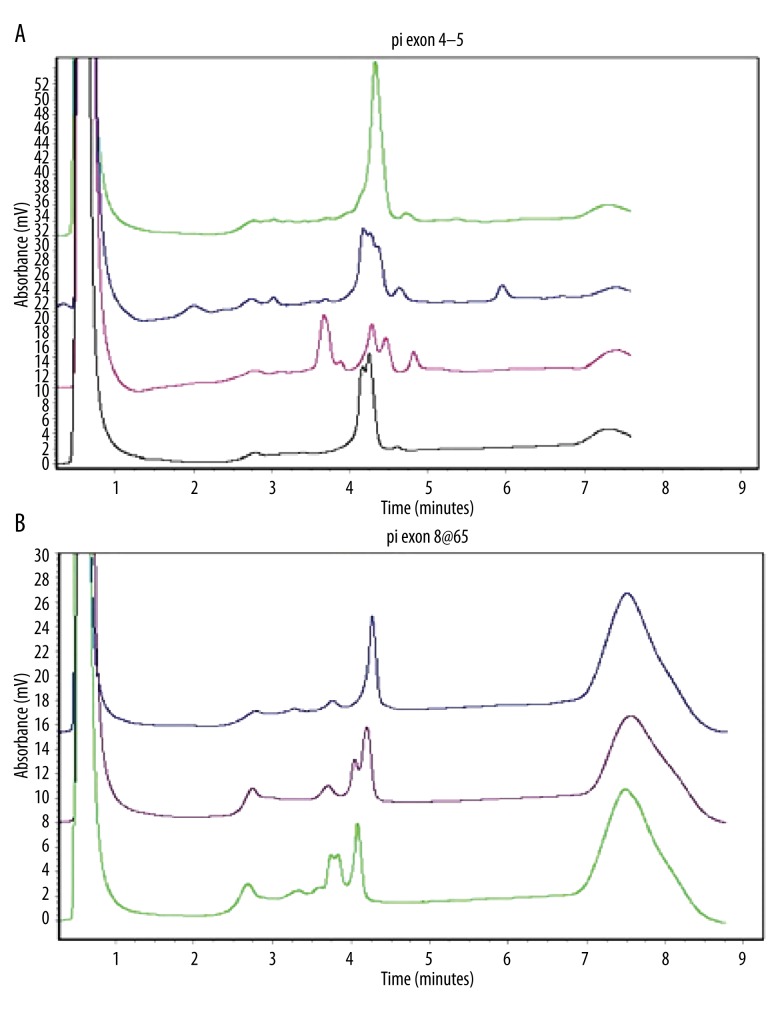
To further confirm the mutations of LKB1detected in PJS patients and to identify polymorphic sites in these mutations, we detected corresponding sites in healthy adults by use of PCR-DHPLC (Figure 7). The results indicated that no elution peak patterns in healthy adults were identical to the mutations (c.48G>A, c.701T>A, c.469-498delTTCTGTCAGCTGATTGACGGCCTGGAGTAC, c.924G>C, c.734+5G>A, the deletion in exon 1, the deletion in exon 4, the deletions in exon 2–4 and the deletions in exon 3–10) detected in familial PJS patients. The evidence demonstrates that these 9 germline mutations were real mutations rather than polymorphic sites. However, there were elution peak patterns of 5 healthy adults that were identical to the mutation (c.1062C>G) detected in familial PJS patients. These result show that the mutation (c.1062C>G) should be 1 polymorphic site (Table 3, Figure 7B).
Figure 7.
The representative PCR-DHPLC elution maps. (A) The elution maps from top to bottom for normal controls, the 734+5G>A mutation in intron 5, the deletion 471_501delTTCTGTCAGCTGATTGACGGCCTGGAGTAC in exon 4, and the 701T>A mutation in exon 4. (B) The elution maps for normal controls: the 924G>C mutation in exon 8 and the 1062C>G mutation in exon 8, respectively.
Therefore, by using both direct sequencing and MLPA assay following by verified by PCR-DHPLC, the total mutation detection rate for the LKB1 gene was 85.7% in Chinese familial PJS (12 of 14 familial PJS patients, Table 3) and 63.2% in total Chinese PJS patients (12 of 19 PJS patients).
Discussion
Peutz-Jeghers syndrome (PJS), first described by Peutz in 1921 and by Jegher in 1944, is an autosomal dominantly inherited disorder. The incidence of the syndrome is estimated to be between 1 in 50 000 and 1 in 200 000 births [1]. The syndrome is characterized by hamartomatous polyps of the gastrointestinal tract (GIT) and melanin pigmentation on the lips, buccal mucosa, and hands or feet [14–16]. The syndrome usually leads to abdominal pain, rectal blood loss, intestinal obstruction, bleeding or chronic anemia, and clinical intussusception [17–19]. In addition, patients with the syndrome have an increased risk of GIT malignancies, with an estimated risk 18 times higher than in the general population [20,21]. As an autosomal dominantly inherited disease, PJS is strictly associated with mutation of LKB1/STK11 gene, which is a serine/threonine kinase tumor suppressor. The penetrance of LKB1/STK11 mutation is 100% in familial PJS patients [9,22,23]. Thus, genetic screening of LKB1mutation aids in mapping and selecting high-risk populations, helps family members with germline mutation of LKB1 gene via performing routine endoscopic observation, aids in clinical diagnosis and PJS disease control of young LKB1-mutation carriers, and reduces the incidence of tumor initiation. In addition, genetic screening of LKB1 mutation can also promote healthy birth and child development.
To date, a total of about 200 different LKB1 germline mutations have been identified and reported. Most of mutations are missense or nonsense mutations. Insertion and deletion and abnormal splicing have also been reported. Most mutation cause frameshift and premature stop codon [24]. In our report, 12 kinds of mutations were presented in 14 probands with familial PJS. Most were point mutations, which were about 58.3% (7/12) of all mutations. There was also 1 case that carried small fragment base deletion in the region of exon4 of LKB1 gene. One more mutation showed base insertion of small flanking sequences of exon 4. There were 3 cases that carried base deletion of exons. Among 12 mutations, 7 were thought to be pathogenic, 4 were polymorphic sites, and 1 could not be defined as pathogenic. In the 7 kinds of pathogenic mutations, 6 were located in the region of exons and 1 was in the intron region. One proband with familial PJS bore 2 kinds of mutations: (c.701T>A) and (c.1062C>G), located in exon 4 and exon 8, respectively. The mutation (c.701T>A) led to the missense mutation (p.Phe267Tyr). The mutation site localized in the kinase domain of LKB1, and the genetic mutation was not detected in any of the 250 healthy adult controls, suggesting it was a pathogenic mutation. The mutation (c.1062C>G) resulted in the missense mutation (p.The354Tyr). Yang et al. [1] also detected this mutation, but the mutation site was not localized in the function domains, and it was also detected in 5 healthy adult controls. The mutation was thus thought to be a polymorphic site rather than a pathogenic mutation. The LKB1 gene of 1 proband with familial PJS lost the 30-bp fragment in exon 4, leading to loss of 10 amino acids and truncated protein. This mutation can severely disturb the function of LKB1, affect LKB1 mRNA splicing, and promote mRNA degradation. The mutation (c.924G>C) in exon 8 of LKB1 gene in 1 familial PJS patient led to the missense mutation (p.Trp308Cys). The mutation also localized in the kinase domain and was not detected in the 250 healthy adults. Mehenni et al. also detected the mutation in a patient with familial PJS, but they did not detect it in 100 healthy persons [25]. Thus, the mutation was considered pathogenic in this report. Another mutation (c.734+5G>A) in intron 5 of LKB1 gene in 1 patient with familial PJS was also discovered by Mehenni et al. They thought that the mutation resulted in abnormal mRNA splicing and truncated LKB1 protein. DHPLC results did not show the elution peak pattern of the mutation in 250 healthy adults. In addition, bioinformatics analysis (http://rulai.cshl.edu/new_alt_exon_db2/HTML/score.html) indicated that the splice site score decreased to 3.4 from 6.8. Papp et al. also found a decrease in splice site score from 9.4 to 1.6 due to an intron mutation of LKB1 using this web-based bioinformatics software to analyze a mutation in intron 4 of LKB1. cDNA analysis revealed that the intron 4 nucleotide substitution results in skipping of exon 4 [26]. Thus, it was concluded that the mutation (c.734+5G>A) affects normal mRNA splicing. In all, a total of 8 familial PJS patients were identified with pathogenic germline mutations in 14 unrelated families (57.1%). Two germline mutations herein were reported for the first time. In addition, there was 1 nonsense mutation (c.48G>A) in exon1 of LKB1 gene in 1 familial PJS patient. This nonsense mutation might be associated with mRNA splicing and lead to disease, although it did not change the structure of LKB1 protein. Thus, it was considered indefinite in pathogenesis.
The amplification and sequencing results of the flanking sequences presented 3 kinds of polymorphisms in introns of LKB1 gene: (c.374+24G>T, c.464+47_48inGGGGGCC, and c.920+7G>C). An online database also showed that these 3 mutations were all polymorphic sites, and their dbsnpID were rs2075604, rs66999113, and rs2075607, respectively.
The sequencing results of LKB1 gene in 5 sporadic PJS patients did not show abundant pathogenic mutations in familial PJS patients. The results are identical to previous reports that sporadic PJS seldom shows the mutation of LKB1. Thus, not all PJS patients carry the mutations of LKB1 [27]. In addition, many mutations of LKB1 in PJS patients were not detected due to the limitation of technologies. For example, direct sequencing of PCR product is not able to identify and detect deletion of large genomic sequences. Thus, MLPA were utilized by Papp et al. to detect the deletion of large genomic sequences in 13 unrelated families. They discovered and identified 5 families (38%, 5/13) with the large deletion [26]. Yang et al. also found 5 probands with the large deletion in 17 Korea PJS children by use of the same technique. We also found 4 deletions of genomic fragments using MLPA in our patients, and we also used MSP to screen methylation of CpG island in the promoter of LKB1. The methylation was detected in the carcinomatous polyps of 1 sporadic PJS patient. The result indicated that LKB1 gene germline mutation might not be the only molecular pathogen of PJS, and methylation in LKB1 gene promoter might cause malignancy of intestinal polyps. Some researchers have also reported that there are other genetic mutation sites, such as 19q13.4 [28,29].
Although Peutz-Jegher syndrome is rare, patients usually have severe clinic symptoms and elevated cancer risk. The mechanism of PJS thus needs to be elucidated in more detail. Effective screening and identification of the disease using knowledge of its mechanism would benefit prevention and treatment of PJS disease. In our study, we combined MLPA, PCR-DHPLC, MSP, and DNA sequence analysis to disclose the mutation of LKB1 and the methylation of 5′-CpG islands of its promoter in 19 Chinese PJS patients, including 14 familial and 5 sporadic PJSs. We discovered 12 germline mutations, of which 7 were pathogenic and 2 were considered de novo. Our study enriches knowledge of germline mutations of LKB1 in familial PJS and elucidates the relationship between sporadic PJS and LKB1 epigenetic modification. It also shows the importance of screening and treating Chinese PJS patients.
Conclusions
These results and analyses demonstrate that the pathogenic effect of LKB1 gene germline mutation is a common cause of familial PJS in Chinese patients. However, it may not be the only molecular cause of Peutz-Jeghers syndrome. Most mutations in Chinese familial PJS patients were point mutations. The epigenetic analysis presented here shows that methylation in the LKB1 gene promoter region may cause carcinomatous change in intestinal polyps. It should be considered a future target for treatment of PJS.
Acknowledgements
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared no financial conflicts of interests.
Footnotes
Source of support: This works was financially supported by the Jiangsu Natural Science Foundation (Grant No. BK20141490) and the National Natural Science Foundation of China (Grant No. 81301765)
References
- 1.Yang HR, Ko JS, Seo JK. Germline mutation analysis of STK11 gene using direct sequencing and multiplex ligation-dependent probe amplification assay in Korean children with Peutz-Jeghers syndrome. Dig Dis Sci. 2010;55:3458–65. doi: 10.1007/s10620-010-1194-5. [DOI] [PubMed] [Google Scholar]
- 2.William J, Timothy B, Dirk E. Andrews’ diseases of the dkin: Clinical Dermatology. 10th ed. Saunders; 2005. [Google Scholar]
- 3.Boardman LA, Pittelkow MR, Couch FJ, et al. Association of Peutz-Jeghers-like mucocutaneous pigmentation with breast and gynecologic carcinomas in women. Medicine. 2000;79:293–98. doi: 10.1097/00005792-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Mallory SB, Stough DB. Genodermatoses with malignant potential. Dermatol Clin. 1987;5:221–30. [PubMed] [Google Scholar]
- 5.Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: A systematic review and recommendations for management. Gut. 2010;59:975–86. doi: 10.1136/gut.2009.198499. [DOI] [PubMed] [Google Scholar]
- 6.Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209–15. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 7.Noriega-Iriondo MF, Colon-Otero G, Kipp BR, et al. High-grade endometrial stromal sarcoma as the initial presentation of an adult patient with Peutz-Jeghers Syndrome: A case report. Hered Cancer Clin Pract. 2015;13:6. doi: 10.1186/s13053-015-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemminki A, Tomlinson I, Markie D, et al. Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet. 1997;15:87–90. doi: 10.1038/ng0197-87. [DOI] [PubMed] [Google Scholar]
- 9.Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 10.Hemminki A, Avizienyte E, Roth S, et al. [A serine/threonine kinase gene defective in Peutz-Jeghers syndrome]. Duodecim. 1998;114:667–68. [in Finnish] [PubMed] [Google Scholar]
- 11.McGarrity TJ, Amos CI, Frazier ML, Wei C. Peutz-Jeghers Syndrome. In: Pagon RA, Adam MP, Bird TD, et al., editors. Gene Reviews. Seattle: University of Washington; 2013. [Google Scholar]
- 12.Herman JG, Graff JR, Myohanen S, et al. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–26. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trojan J, Brieger A, Raedle J, et al. 5′-CpG island methylation of the LKB1/STK11 promoter and allelic loss at chromosome 19p13.3 in sporadic colorectal cancer. Gut. 2000;47:272–76. doi: 10.1136/gut.47.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson IP, Houlston RS. Peutz-Jeghers syndrome. J Med Genet. 1997;34:1007–11. doi: 10.1136/jmg.34.12.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGarrity TJ, Kulin HE, Zaino RJ. Peutz-Jeghers syndrome. Am J Gastroenterol. 2000;95:596–604. doi: 10.1111/j.1572-0241.2000.01831.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. WHO classification of tumours. [Google Scholar]
- 17.Rufener SL, Koujok K, McKenna BJ, Walsh M. Small bowel intussusception secondary to Peutz-Jeghers polyp. Radiographics. 2008;28:284–88. doi: 10.1148/rg.281075092. [DOI] [PubMed] [Google Scholar]
- 18.Harned RK, Buck JL, Sobin LH. The hamartomatous polyposis syndromes: Clinical and radiologic features. Am J Roentgenol. 1995;164:565–71. doi: 10.2214/ajr.164.3.7863873. [DOI] [PubMed] [Google Scholar]
- 19.Latchford A, Greenhalf W, Vitone LJ, et al. Peutz-Jeghers syndrome and screening for pancreatic cancer. Br J Surg. 2006;93:1446–55. doi: 10.1002/bjs.5609. [DOI] [PubMed] [Google Scholar]
- 20.McGarrity TJ, Amos C. Peutz-Jeghers syndrome: Clinicopathology and molecular alterations. Cell Mol Life Sci. 2006;63:2135–44. doi: 10.1007/s00018-006-6080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Lier MG, Westerman AM, Wagner A, et al. High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut. 2011;60:141–47. doi: 10.1136/gut.2010.223750. [DOI] [PubMed] [Google Scholar]
- 22.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–87. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 23.Aretz S, Stienen D, Uhlhaas S, et al. High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Hum Mutat. 2005;26:513–19. doi: 10.1002/humu.20253. [DOI] [PubMed] [Google Scholar]
- 24.Ausavarat S, Leoyklang P, Vejchapipat P, et al. Novel mutations in the STK11 gene in Thai patients with Peutz-Jeghers syndrome. World J Gastroenterol. 2009;15:5364–67. doi: 10.3748/wjg.15.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehenni H, Resta N, Guanti G, et al. Molecular and clinical characteristics in 46 families affected with Peutz-Jeghers syndrome. Dig Dis Sci. 2007;52:1924–33. doi: 10.1007/s10620-006-9435-3. [DOI] [PubMed] [Google Scholar]
- 26.Papp J, Kovacs ME, Solyom S, et al. High prevalence of germline STK11 mutations in Hungarian Peutz-Jeghers Syndrome patients. BMC Med Genet. 2010;11:169. doi: 10.1186/1471-2350-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang CY, Esufali S, Berk T, et al. STK11/LKB1 germline mutations are not identified in most Peutz-Jeghers syndrome patients. Clin Genet. 1999;56:136–41. doi: 10.1034/j.1399-0004.1999.560207.x. [DOI] [PubMed] [Google Scholar]
- 28.Mehenni H, Gehrig C, Nezu J, et al. Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am J Hum Genet. 1998;63:1641–50. doi: 10.1086/302159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchet-Poyau K, Mehenni H, Radhakrishna U, Antonarakis SE. Search for the second Peutz-Jeghers syndrome locus: exclusion of the STK13, PRKCG, KLK10, and PSCD2 genes on chromosome 19 and the STK11IP gene on chromosome 2. Cytogenet Genome Res. 2002;97:171–78. doi: 10.1159/000066620. [DOI] [PubMed] [Google Scholar]