Abstract
Our research team has recently isolated and characterized a new stromal stem cell line (hUCESCs) obtained from cytological smears, as routinely performed for cervical cancer screening. We have, furthermore, described that both hUCESCs directly, as well as the secretome contained in the conditioned medium used for growing them (hUCESCs-CM) have potent antitumoral, anti-inflammatory, antibiotic, antimycotic and re-epitheliasation-enhancing properties. The scientific explanation our team proposes for these pleiotropic effects are directly related to the site of origin of hUCESCs, the human cervical transition zone, which has unique features that biologically justify the different actions of hUCESCs and hUCESCs-CM. We, herein, expose our working theory for the biological activity of hUCESCs and hUCESCs-CM.
Keywords: Stem cells, stromal cells, uterus, cervix
Our research multicenter team has recently described that the secretome from stromal stem cells isolated from human uterine cervical cytological smears (hUCESCs), contained in the conditioned medium used for growing them, has potent antitumoral (1), antiinflammatory, antibiotic and re-epitheliasation-enhancing properties (2). Moreover, very recent results from our laboratories, communicated at an international Mycology congress (3), extend these properties to a growth-inhibiting effect upon yeasts belonging to the Candida species. The immediate question is: how can it be scientifically explained that one single stem cell strain obtained from such a particular region of the human body can have such varied actions? Based upon our findings, and other still unpublished results from the groups composing our team, we propose a plausible answer to this question, and venture a working theory based upon it.
Epithelial Transition Zones (also called “Transformation Zones”)
Epithelial transition zones are the meeting-point, or rather meeting line, of two completely different epithelia in the human body, resulting in a distinct frontier between them. This happens at various locations (esophago-gastric, esophago-duodenal, ileo-cecal and anorectal junctions and, more to the point in our case, also the cornea-conjunctiva and endocervix-ectocervix junctions). All these sites share additional, intriguing properties: they are almost constantly in contact either with the external medium or, at least, with potentially dangerous elements forming part of it and, more particularly, microorganisms that -in some cases- have been associated with the induction of cancer, most notably the human papilloma virus (HPV) and Helicobacter Pylori. It is now undeniably accepted that these microorganisms have a prominent role in the development of cancer at several of these sites, most notably gastroduodenal, anorectal and cervical cancer (4,5).
Transition zones are almost never immutable, fixed lines where one epithelium abruptly meets its counterpart, but rather a variable extension of territory where one epithelium is transformed into the other by means of a process called “metaplasia”. To use an unpopular, but illustrating example, it rather resembles the no-man’s-land zone between opposing trenches during the Great War, hence their other, more biologically accurate name of “transformation zones”. As happens in trench warfare, the most fierce battles, in our case biological battles, are fought in this zone and, as also happened during the Great War, the most usual state of things over time is that of a relative equilibrium or stalemate, biologically called “homeostasis”. Now, let us imagine that a certain number of soldiers from either side want to change lines (“metaplasia”). For that purpose, they enter no-man’s land, there become completely mad through the effect of, say, a poison gas cloud lingering there and, using their knowledge of war tactics and the possession of the same weapons as their “normal” comrades, penetrate either line causing havoc, ultimately leading to the destruction of the infiltrated army and its supporting state. This is exactly how cancer cells act: they are not immediately recognizable as enemies by the immune system (“they carry the same uniforms”), use the same physiological mechanisms as normal cells (“weapons”) to their own advantage, penetrate the established defense lines (the basal membrane in the case of epithelia) and invade the rearguard (the connective tissue) and, from there, using the existing traffic system of roads and railways (blood and lymph vessels), spread to neighboring locations and proselytize, creating colonies of armed individuals as mad and dangerous as them (metastases), which are in most cases capable of destroying the existing state (death).
The Transformation Zone of the Human Uterine Cervix
The human uterine cervix is composed of two anatomically and functionally distinct portions: the exocervix, which is in constant contact with the potentially hostile external medium constituted by the vaginal environment, and the endocervical canal, through which spermatozoa have to pass on their way to the Fallopian tubes in order to be able to eventually fecundate an ovum. For obvious reasons, the exocervix must be lined by a kind of epithelium especially resistant to external insults, such as the acid, proinflammatory vaginal milieu, the bacteria and yeasts normally colonizing it and pathogens that may enter from outside the vagina, usually carried by the male penis during intercourse. In fact, the epithelium of the exocervix is a multilayer squamous epithelium, acting as a mechanical “protection shield”. The epithelium lining the endocervical canal does theoretically not have to stand up to such challenges as its exocervical counterpart because it is “hidden” from the outside and its potential threats (Figure 1). Its primary biological mission is to produce a mucus that, on the one hand, acts as a sort of plug during the infertile days of the woman or as a protective one if fertilization has occurred and, on the other one, as a promoter of the passage towards the Fallopian tubes of spermatozoa during the fertile days of the female period, as mentioned above. For these reasons, the endocervical epithelium, at variance with the ectocervical one, is constituted by just a highly specialized single layer of mucus-producing cells. Both epithelia meet at the transition or transformation zone. This is the ideal situation. However, such ideal situations do not exist or do not eternally exist in practice. Indeed, there are various occasions, some of them entirely physiological, such as the exaggerated effects of estrogens during pregnancy on the uterine cervix, where the vulnerable endocervical monolayer epithelium is not completely hidden from potential external insults within the endocervical canal, and protrudes into the outside, which is constituted by the hostile vaginal medium (Figure 2 and Figure 3).
Figure 1. Anatomical sketch of the human cervix and vagina with no transformation zone.
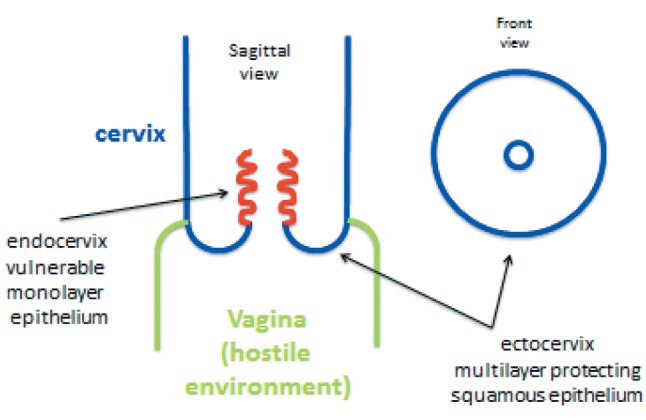
Figure 2. Everted endocervical epithelium protruding into the vaginal cavity. There is development of a transformation zone, where the vulnerable monolayer glandular epithelium is converted into the resistant multilayer squamous epithelium.
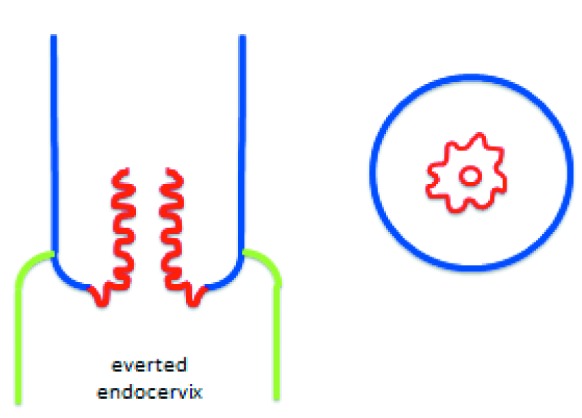
Figure 3. Longitudinal histological section (sketch) of the transformation zone.
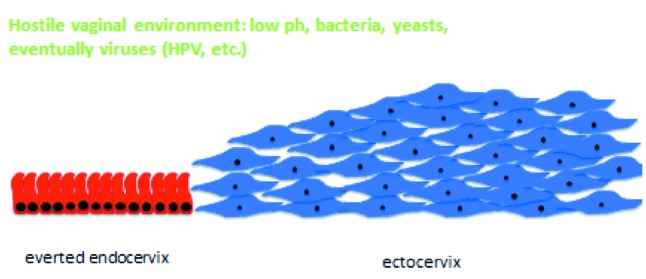
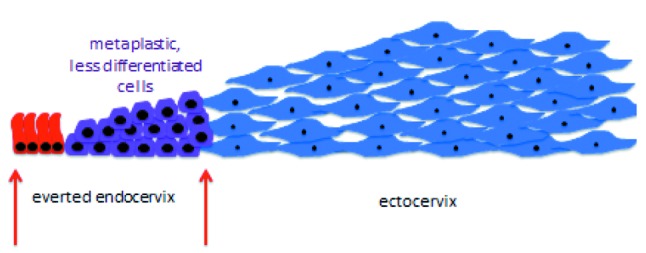
The vagina hosts a microenvironment in which bacteria, yeasts and eventually other microorganisms cohabitate within an acid milieu and is, furthermore, exposed to the penetration of other potentially dangerous elements, most usually through sexual intercourse. The delicate endocervical epithelial lining is not prepared to resist the challenges posed by this hostile outside environment and, as soon as it is exposed to it, endocervical tissue extending from the border with the squamous multilayer epithelial protective shield of the exocervix to the protective endocervical canal is converted into a transformation zone, where the exposed monolayer glandular endocervical epithelium is actively converted to the resistant multilayer squamous epithelium of the ectocervix by means of a process called “squamous metaplasia” (Figure 4).
Figure 4. Conversion of the vulnerable endocervical epithelium into resistant squamous epithelium by means of squamous metaplasia.
Squamous Metaplasia
Metaplasia is the process by which one type of cell is converted into another, most usually, as in the case of the uterine transformation zone, because the former is more vulnerable in a particularly hostile environment than the latter. That is the reason for which squamous metaplasia, like the one taking place in the cervix, is rather usual in other settings, such as the bladder or the epithelial linings of the airways of smokers. However, squamous metaplasia is not a direct, immediate process and is rather composed by a succession thereof, some of which may pose significant biological risks.
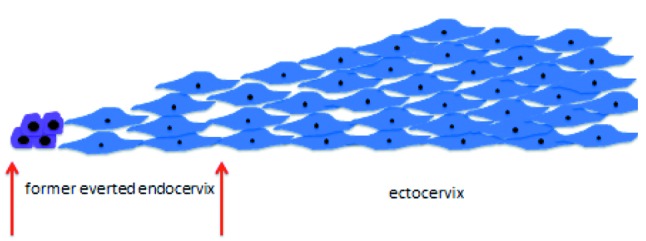
In a very succinct summary, the original cell, in order to be able to convert into the other, entirely different one, must first undergo a rather high degree of dedifferentiation and from the undifferentiated cell the new one emerges. The dedifferentiation process does not go down all the steps until reaching the status of a stem cell from which potentially any kind of cell can be derived. This is probably true because the transformation that takes place in squamous metaplasia is from one kind of epithelial cell into another kind of epithelial cell and, therefore, many traits defining epithelial cells can be kept intact, despite a necessary high degree of dedifferentiation. At a given point, thus, three distinct types of cells cohabitate within the transformation zone: the differentiated original glandular and squamous epithelial cells, on the one hand, and the undifferentiated metaplastic ones on the other (Figure 5). All of them lie on a “bed” composed of stromal cells beneath the basal membrane. Ultimately, the resistant ectocervical multilayer squamous epithelium is reconstituted over the whole area facing the vaginal environment (Figure 6). At this point, if the glandular endocervical cell is vulnerable to the external insults of the vaginal environment, the undifferentiated metaplastic cell is equally vulnerable to oncogenic transformation, both intrinsically so, and also through external influence. In the first place, it is highly proliferative, with high proliferation being the first step in oncogenic transformation. Moreover, high proliferation requires, among other things, much more frequent DNA replication than is the case for quiescent or normally proliferating cells. DNA replication is in itself risky because errors, such as spontaneous mutations, occur at a constant rate during the process and, furthermore, because the open DNA chain is more prone to virus integration, in this particular case, the greatest threat being posed by the HPV virus. Once the process of metaplasia is finally completed, enhanced proliferation, but within tolerable limits, is necessary so that the “tissue defect” is covered as fast as possible by the new, more resistant one. All this requires: a) A protective mechanism against all potential insults harming the vulnerable original epithelium exposed to an environment it is not accustomed to (low pH with the ensuing inflammatory response, bacteria, yeasts, etc.). b) More importantly, still, a protective mechanism that allows cells to become undifferentiated and then to proliferate above normal levels without, however, reaching critical ones or overstepping all the red lines that lead to oncogenic transformation.
Figure 5. Presence of three main types of cells in the transformation zone.
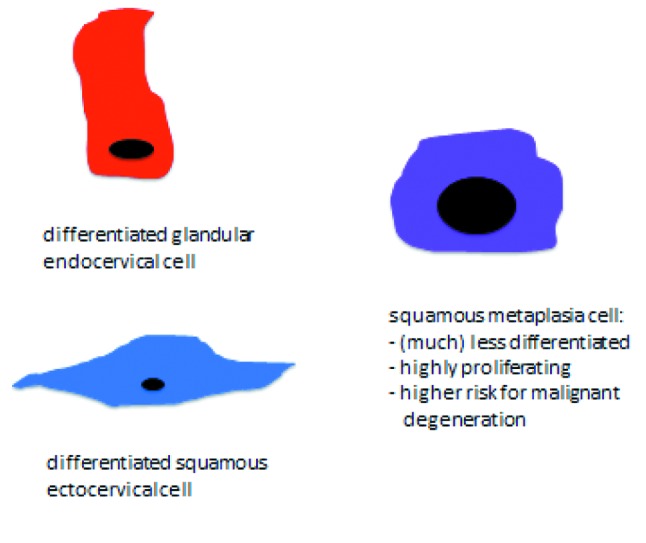
Figure 6. Conversion of the vulnerable endocervical epithelium into squamous epithelium almost completed.
Antitumor Effects of hUCESCs
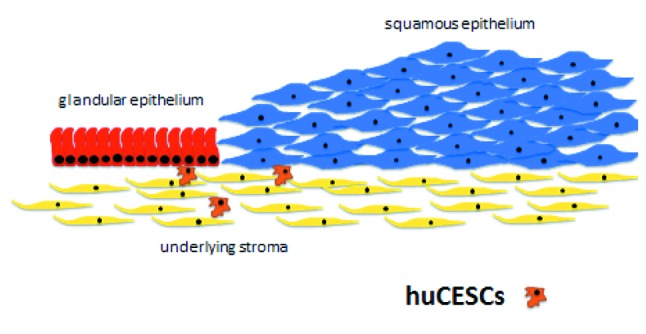
Due to the experimental results obtained by our research group in different in vitro and animal models with the conditioned medium produced by the human cervical stromal stem cells (hUCESCs-CM) isolated and characterized by us (1), we believe that hUCESCs underlying the cervical transformation zone are the mastermind regulating all these processes (Figure 7) through a paracrine mechanism involving, among other elements, the production of a cocktail of hundreds of cytokines we have now begun to study in depth.
Figure 7. Human cervical stromal stem cells (hUCESCs). The possible mastermind regulating the processes taking place in the cervical transformation zone through a paracrine mechanism (conditioned medium).
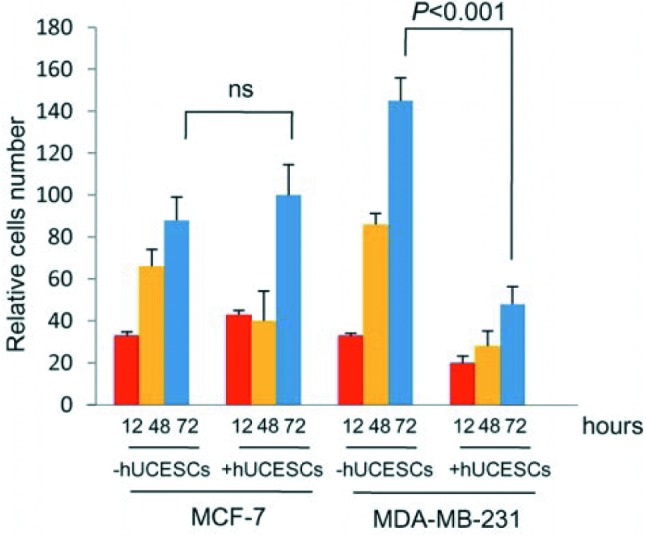
Our experimental findings demonstrate that, regarding point a) of the previous section, the conditioned medium produced by hUCESCs (hUCESCs-CM) not only has antibiotic, antiinflammatory and antifungal properties (1-3) but, more importantly, is inducing a controlled hyperproliferation of normal squamous epithelium in order to cover epithelial defects, such as in the case of corneal ulcers (2) (Figure 8), whereas, much more importantly, still, as it concerns point b) hUCESC-CM has potent antitumor effects that are more pronounced with highly proliferating tumors (1), thus leading to the hypothesis that the control mechanism exerted by hUCESCs does indeed allow a certain degree of hyperproliferation that, once it reaches a critical threshold, acts aggressively to counteract the oncogenic properties of tumor cells. In fact, as shown in Figure 9, we tested in vitro the effect of hUCESCs in co-culture on the proliferation of the non-invasive breast cancer cell line MCF-7 and the highly invasive breast cancer cell line MDA-MB-231. We did not find significant effects on proliferation in MCF-7 cells. However, in MDA-MB-231 cells, we observed a significant decrease of cell proliferation when the cells were co-cultivated with hUCESCs as compared to controls. Exactly the same results were obtained by treating MCF-7 and MDA-MB-231 cultures with hUCESCs-CM if compared to the same cultures treated with conditioned medium from adipose stem cells (1). We next evaluated cell cycle and apoptosis as possible mediators of the effect of hUCESCs-CM on cell proliferation. We demonstrated that hUCESCs-CM-treated cells underwent cycle delay or arrest, decreasing cyclin A, B and D1 protein expression and inducing an increase of Annexin+/PI+, suggesting that hUCECs-CM induces late apoptosis. In addition, in MDA-MB-231 cells treated with hUCECs-CM, we observed an increased expression of the pro-apoptotic caspases-8, -12, -9, activated caspase-3, the pro-apoptotic cleaved PARP protein and a decreased expression of anti-apoptotic proteins BID and BIM. We also evaluated if hUCESCs-CM affected invasion of MDA-MB-231 cells, a key feature in breast cancer progression and metastasis. We observed that MDA-MB-231 treated with hUCESCs-CM significantly decreased their invading capacity as compared with cells in presence of medium without fetal bovine serum (1).
Figure 8. Conditioned medium from human cervical stromal stem cells (hUCESCs) promotes re-epitheliazation of corneal lesions more efficiently than commercial ophtalmic drops (2).
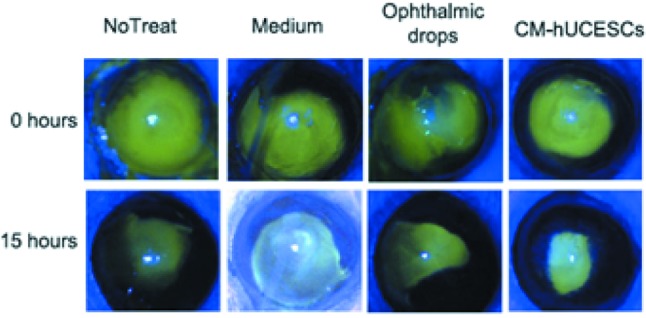
Figure 9. Co-culture of hUCESCs with low-proliferating MCF-7 breast cancer cells exerts no significant effect on growth, but a drastic effect on high-proliferating MDA-MB-231 cells.
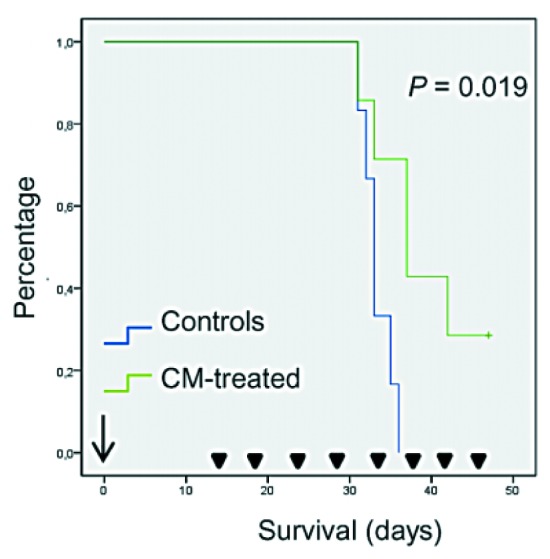
We next explored the effect of intratumoral treatment with hUCESCs-CM in vivo in a xenograft model of severe combined immunodeficiency (SCID) mice that were injected with MDA-MB-231 cells. A significant decrease in tumor volume was observed after 15 days of treatment with hUCESCs-CM as compared with mice treated with incomplete medium (Figure 10). Indeed, we demonstrated that mice treated with hUCESCs-CM had a longer overall survival (Figure 11).
Figure 10. Nude mouse MDA-MB-231 breast cancer xenograft model. Reduction of tumor volume after treatment with hUCESCs-CM.

Figure 11. Nude mouse MDA-MB-231 breast cancer xenograft model. Survival after treatment with hUCESCs-CM.
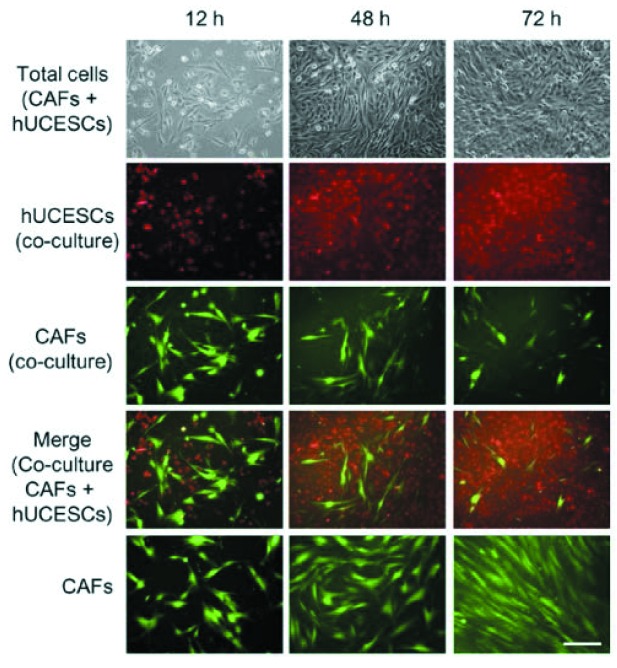
Finally, accumulating clinical and experimental evidence reveals that tumor development is intimately related to the complex interactions of carcinomas with several distinct stromal cell types that together create the microenvironment of the cancer cells (6-8). The breast microenvironment consists of the extracellular matrix (ECM) and numerous stromal cell types, including endothelial and immune cells, fibroblasts and adipocytes. The two best-studied and most common cellular components of tumor stroma are cancer-associated fibroblasts (CAFs) and cancer-associated macrophages (CAMs). Recent studies, some by our own group, have reported that CAFs and CAMs promote cancer initiation, angiogenesis, invasion and metastasis (6,7). In breast cancer, CAFs not only promote tumor progression but also induce therapeutic resistance. Accordingly, targeting CAFs provides a novel way to control tumors with therapeutic resistance. Consequently, there is an increasing interest in developing novel therapies targeting the microenvironment, particularly as it relates to invasiveness and metastatic progression. In relation to this point, we studied whether hUCESCs-CM could act on the breast cancer microenvironment. We demonstrated that co-culture with hUCESCs, as well as treatment with hUCESCs-CM, significantly reduced CAFs’ cell proliferation and invasion and increased early apoptosis (Figure 12).
Figure 12. Effect of hUCESCs in co-culture on cancer-associated fibroblasts (CAFs). Reduction of cell proliferation and induction of apoptosis.
Furthermore, we demonstrated that treatment of monocytes with hUCESCs-CM significantly impaired their differentiation to macrophages.
All the aforementioned effects on both tumor cells and the peritumoral stroma could be, at least, partly explained in theory by ways of a paracrine mechanism, involving the production of a cytokine cocktail by hUCESCs. We explored this possibility using a human cytokine antibody array and, indeed, we found that, on the one hand, the secretome of hUCESCs overexpresses anti-tumoral factors, such as tumor necrosis factor superfamily member 14 (TNFSF14) also known as LIGHT, Fms-related tyrosine kinase 3 (FLT-3) ligand, C-X-C motif chemokine 10 (CXCL10) and liver-enriched transcriptional activator protein (LAP). On the other hand, several markers of tumor progression, such as epithelial growth factor receptor (EGFR), fibroblast growth factor (FGF), intercellular adhesion molecule 3 (ICAM3), interleukin-6 (IL-6), c-c motif ligand 7 (CCL-7), macrophage migration inhibitory factor (MIF), soluble glycoprotein 130 (sgp130) and vascular endothelial growth factor D (VEGF-D) are not detected or are lower compared with the control secretome of adipose stem cells (ASCs). This cocktail of factors could regulate cancer progression through a paracrine mechanism in both cancer cells and in tumor stroma (CAFs and CAMs), which is not surprising considering the biological events that take place in the transformation zone of the human cervix, where hUCESCs act preventing adult cells from acquiring a malignant phenotype through modulation of their proliferation rate and induction of apoptosis if they become dangerous for the host. Similarly, on breast cancer, hUCESCs do indeed allow a certain degree of hyperproliferation, so that they do not act on the low-proliferating and non-invasive MCF-7 cell line, whereas, once a critical threshold is reached, they exert a very potent and effective anti-tumor activity against highly proliferating and metastasis-producing cancer cells, such as the MDA-MB-231 breast cancer cells tested. All these effects were reproduced in an almost identical fashion on cell lines produced from samples obtained in the clinic from real patients (1).
In conclusion, the conditioned medium obtained from the culture of the human uterine stromal cervical stem cell line identified and characterized by our group (1) may constitute an absolutely new modality of biological anti-tumor therapy, using the weapons provided by nature itself along the path of evolution of our species.
Acknowledgements
This work has been financially supported by grants SAF2015-69221-R from Ministerio de Economía y Competitividad, PI13702745 from Instituto de Salud Carlos III and GRUPIN14-116 from Consejería de Economía y Empleo del Pricipado de Asturias y FEDER and by Fundación para la Investigación de Células Madre Uterinas (FICEMU), Gijón, Spain.
References
- 1.Eiró N, Sendón-Lago J, Seoane S, Bermúdez MA, Lamelas ML, García-Caballero T, Schneider J, Perez-Fernandez R, Vizoso FJ. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget. 2014;5:10692–10708. doi: 10.18632/oncotarget.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez MA, Sendon-Lago J, Eiro N, Treviño M, Gonzalez F, Yebra-Pimentel E, Giraldez MJ, Manuel Macia M, Lamelas ML, Saa J, Vizoso FJ, Perez-Fernandez R. Corneal Epithelial Wound Healing and Bactericidal Effect of Conditioned Medium From Human Uterine Cervical Stem Cells. Invest Ophthalmol Vis Sci. 2015;56:983–992. doi: 10.1167/iovs.14-15859. [DOI] [PubMed] [Google Scholar]
- 3.Marcos-Arias C, Mateo-Alesanco E, Eiro N, Vizoso F, Pérez-Fernández R, Eraso E, Schneider J, Quindós G. Antifungal activity of the human uterine cervical stem cells conditioned medium against medically important species of Candida. Mycoses. 2015;58:205–206. [Google Scholar]
- 4.Zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Wadstrom T. An update on Helicobacter pylori. Current Opinion on Gastroenterology. 1995;11:69–75. [Google Scholar]
- 6.Mao Y, Keller ET, Gardfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González L, Eiro N, Fernandez-Garcia B, González LO, Dominguez F, Vizoso FJ. Gene expression profile of normal and cancer-associated fibroblasts according to intratumoral inflammatory cells phenotype from breast cancer tissue. Mol Carcinog. 2015 doi: 10.1002/mc.22403. Sep 9. doi: 10.1002/mc.22403. [DOI] [PubMed] [Google Scholar]
- 8.Delinasios JG, Angeli F, Koumakis G, Kumar S, Kang WH, Sica S, Iacopino F, Lama G, Lamprechi S, Sigal-Batikoff I, Tsangaris GT, Farfarelos GD, Farfarelos MC, Vairaktaris E, Vassiliou S, Delinasios GJ. Proliferating fibroblasts and HeLa cells co-cultured in vitro reciprocally influence growth patterns, protein expression, chromatin features and cell survival. Anticancer Res. 2015;35:1881–1916. [PubMed] [Google Scholar]