Abstract
Meningiomas are one of the most common tumors affecting the central nervous system, exhibiting a great heterogeneity in grading, treatment and molecular background. This article provides an overview of the current literature regarding the molecular aspect of meningiomas. Analysis of potential biomarkers in serum, cerebrospinal fluid (CSF) and pathological tissues was reported. Applying bioinformatic methods and matching the common proteic profile, arising from different biological samples, we highlighted the role of nine proteins, particularly related to tumorigenesis and grading of meningiomas: serpin peptidase inhibitor alpha 1, ceruloplasmin, hemopexin, albumin, C3, apolipoprotein, haptoglobin, amyloid-P-component serum and alpha-1-beta-glycoprotein. These proteins and their associated pathways, including complement and coagulation cascades, plasma lipoprotein particle remodeling and lipid metabolism could be considered possible diagnostic, prognostic biomarkers, and eventually therapeutic targets. Further investigations are needed to better characterize the role of these proteins and pathways in meningiomas. The role of new therapeutic strategies are also discussed.
*These Authors contributed equally to this study
Keywords: Meningioma, proteomic, bioinformatic analysis, protein pathways
Meningiomas account for approximately 20% of all intracranial tumors in males and 38% in females (1,2).They arise from arachnoidal cells of the leptomeninges and may occur in different sites. The current World Health Organization (WHO) classification involves several variants or subtypes, divided into three grades (WHO I, II, III) (3,4). Depending on the location and WHO grading, treatment options include surgery and postoperative radiation therapy with stereotactic radiosurgery and fractionated external beam radiation therapy (5). Even though meningiomas are generally benign, higher-grade tumors demonstrate a tendency to progress and recur (6). Heterogeneity in genetic, molecular and morphological features leads to difficulties in management (7,8). Tumorigenesis and tumor progression in meningiomas are related to mutations or alterations of tumor-suppressor genes and loss of heterozygosity of different chromosomes (9-12). Common genetic alterations are the monosomy of chromosome 22, observed in about the 70% of meningiomas (13-15), and mutations of tumor suppressor neurofibromatosis type 2 (NF2) associated with over 60% of sporadic meningiomas (16-19). Progression and recurrence of meningiomas is associated with deregulation of several genes such as histone cluster 1 (6p) (20), tissue inhibitor metalloproteinases (TIMPs) (21-23), and WNT signaling pathway (24), as well as loss of heterozygosity of DAL1, a member of the 4.1 superfamily (25,26) Atypical meningiomas show chromosomal losses of 1p, 6q, 10, 14q, and 18q, as well as multiple chromosomal gains (27-29). Moreover, several reports have demonstrated the association of single nucleotide polymorphism (SNPs) and epigenetic aberrations with a higher risk for developing meningiomas (30-34). Proteomic analysis is a relatively new procedure which is highly informative for the identification of potential surrogate markers in different types of brain tumor (35,36).
Proteins and Their Related Pathways
Tissue samples. Recent articles reported a panel of proteins, such as integrin, WNT, RAS, fibroblast growth factor (FGF), epidermal growth factor (EGF), exhibiting a different expression profile within different grades of meningioma, which are implicated in the modulation of essential signal transduction of apoptosis and ubiquitin proteasome signaling in meningioma (37-39). Integrin alpha beta 5 and alpha beta 3 seemed to be strictly associated with meningioma pathogenesis (40). Thus, integrin beta 5, vasodilator-stimulated phosphoprotein, collagen alpha-3 (VI) chain, and filamin-A were found to be up-regulated in benign meningiomas. The signal-transducing component of the WNT receptor was down-regulated in benign and atypical meningiomas, except guanine nucleotide-binding protein subunit gamma-12, which was slightly up-regulated in all different grades of meningiomas (41-43). RAS-related protein R-RAS2, RAS-related C3 botulinum toxin substrate 2 were found to be associated with the EGFR pathway and represent the main part of the FGF signaling, integrin signaling and RAS pathways involved in tumor development. Neuroblast differentiation-associated protein (AHNK), protein S100-A6 and protein S100-A10 interact and mediate different key cellular processes (44,45) and are significantly up-regulated in benign and anaplastic meningiomas. In addition, elevated expression levels of tissue proteins such as caveolin, complement factor B, Y box protein, vinculin, Src homology 2 domain containing binding protein 1 and guanine nucleotide-binding protein G(i) subunit alpha were detected in benign and anaplastic meningiomas. Proteins such as serine/threonine-protein phosphatase 2B and tubulin alpha-1C chain appeared to be down-regulated in different grades of meningiomas. Apolipoprotein E (APOE), serum albumin, apolipoprotein A-I, alpha-1 antitrypsin, galectin-3, vimentin, endoplasmin, annexin A2, glutathione S-transferase P, profilin were reported differently expressed in human meningiomas (46,47). Phosphorylated vimentin was proposed as a discriminative marker for non-infiltrative and non-invasive meningiomas (48). New candidates such as gelsolin, galectin-3, neuromodulin and tumor protein D54 were found to be expressed in benign and anaplastic meningiomas.
Cerebrospinal fluid (CSF). Human CSF has been used as a significant source for protein biomarker studies (49). Recently, Kim et al. identified a small number of proteins in CSF of patients suffering from meningiomas(50). Seven spots were found for secreted proteins expressed at high levels in the majority of CSF of samples from patients with meningioma, and for three proteins expressed at lower levels (50). In greater detail, it has been reported that the content of APO E, APO J and alpha-1-antitrypsin (A1AT) was found to be increased compared to controls, while prostaglandin D2 synthase (PTGDS), transthyretin precursor (TTR) and beta 2 macroglobulin (B2M) was found to be decreased. APO E has been detected in normal human brain tissue and in human intracranial neoplasm (51). On the other hand, APO J is a major carrier protein of soluble circulating amyloid B in body fluids; it may keep the peptide in a soluble form and is considered to have an anti-amyloidogenic effect (52).
Serum. Proteomic analysis of serum from patients with different grades of meningioma identified proteins such as vimentin, alpha-2-macroglobulin, APO B and APO A-I and antithrombin-III, which exhibited a sequential enhancement in increasing grade of malignancy of meningiomas, and were also proposed as potential predictive markers (36). Enhanced levels of a few important candidates involved in the coagulation system and hemostasis, including antithrombin-III, alpha-2-antiplasmin, vitamin K-dependent protein S, fibrinogen alpha chain, plasminogen, alpha-2-macroglobulin and coagulation factor XII, were found in different grades of meningioma. In addition, the activation of complement cascades has been demonstrated in meningiomas, with up-regulation of few complement factors including C5, C8 beta chain, C6, and C4-B. The role of complement proteins in cancer growth is still unknown, but is likely related to dysregulation of mitogenic signaling pathways, constant cellular proliferation, angiogenesis, resistance to apoptosis, and escape from the immune system (53,57). APO A-I and A-II, alpha-1-acid glycoprotein 2, hemoglobin subunit beta/alpha, leucine-rich alpha-2-glycoprotein and vimentin exhibited high expression levels in meningiomas. However, isoforms of APO A-I and A-II have also been reported as potential markers for other cancer types such as ovarian and prostatic (54,55).Expression levels of other serum proteins, including thrombispondin-I, serotransferrin, and alpha-2-macroglobulin, were found to be altered in patients with meningioma. Some of these identified proteins, such as APO E, carbonic anhydrase 1, leucine-rich a-2-glycoprotein and afamin, which showed alteration in expression levels in benign meningiomas (WHO I), may act as potential candidate markers for meningioma at their early stages of development. Different proteins such as vimentin, α-2-macroglobulin, APO B, APO A-I and antithrombin-III, which exhibited alterations in expression levels between benign, atypical or anaplastic meningiomas, can be considered as potential disease-monitoring markers.
This review aimed to evaluate the current findings regarding proteomic analysis in human meningioma, the pathways involved in tumorigenesis, and finally the common profiles derived from different samples, in order to suggest possible diagnostic and prognostic markers, and postulate potential therapeutic targets.
Materials and Methods
Data collection. A PubMed literature search including the last 10 years of all English-language publications reporting proteomic analysis and functional pathways in meningiomas was performed. Terms used in the research were "Meningioma" in multiple combinations with "proteomics", "tissue proteomics", "serum proteomics" and "cerebrospinal fluid proteomics". A total of 11 articles were retrieved and reviewed, and a total of 153 non-redundant proteins were extracted. Reports were tabulated by proteomic findings in brain tissue, CSF and serum. All proteins and genes which were significantly differently expressed (up-regulated,or down-regulated) in meningioma tissues, serum or CSF compared to controls were selected. A minimal fold-change of1.5 for univocal comparison of the same genes/proteins between different studies was considered. Selected genes/proteins were divided into two study groups: a gel-proteomics group, including all deregulated proteins arising from multiple substrates and obtained by gel-proteomic methods; and a gel-free proteomics group, collecting all the proteins which appeared to be deregulated from gel-free screening methods.
Protein interaction network construction. All these molecules were searched through the GeneMANIA Human Database (http://www.genemania.org), in order to find relationships and enrich their interaction networks with new potential partners. GeneMANIA is a web tool useful for generating hypotheses about gene function, for building gene networks, and for prioritizing genes in functional assays. Cytoscape is an open-source software platform for visualizing molecular interactions and biological pathways. In GeneMANIA networks, genes are depicted as circular nodes and their interactions by edges of different shape and colors. Edge colors and shapes reflect the type and the strength of interactions. We identified two major networks, gel-proteomic network and gel-free proteomic network, using as query genes or proteins arising from the two groups considered. GeneMANIA’s default settings were initially modified to search relationships among the components of each query list without related genes, usingpathway, co-localization, co-expression, physical interaction and similar protein domain as attributes. Gel-proteomic network and gel-free proteomic network were subsequently merged by intersection, in order to determine and maintain only the shared molecules for the further analysis. Proteins highlighted by the merging process were then resubmitted to GeneMANIA to expand their interaction network with new potential partners.GeneMANIA’s options were set according to a maximum of 40 related genes, using the same attributes described previously.
Cluster and functional analysis. The resulting network was analyzed by Molecular Complex DEtection (MCODE) clustering tool (http://apps.cytoscape.org/apps/mcode) to find highly interconnected clusters in a network. Default MCODE parameters were used on the whole network to allow the extraction ofclusters containing almost all proteins obtained from the merging process. Small clusters were discarded and the largest clusters, with the highest score, were submitted to ClueGo (http://apps.cytoscape.org/apps/cluego). By selecting “GO-terms fusion”, terms with similar associated genes (byGene Ontology) were fused in order to minimize redundancy. The options “Detailed Network” and K-value 0.45, respectively, were used to obtain specific GO-terms with few associated genes and high percentage of significance of the uploaded genes, increasing association strength between GO-terms and genes.
Results
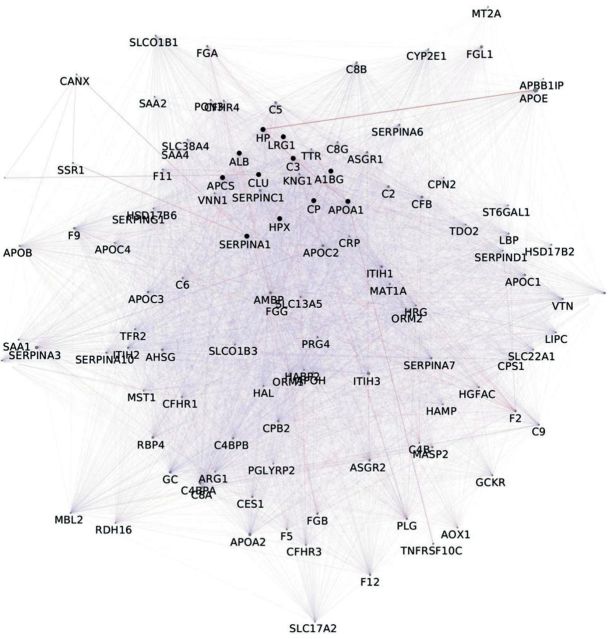
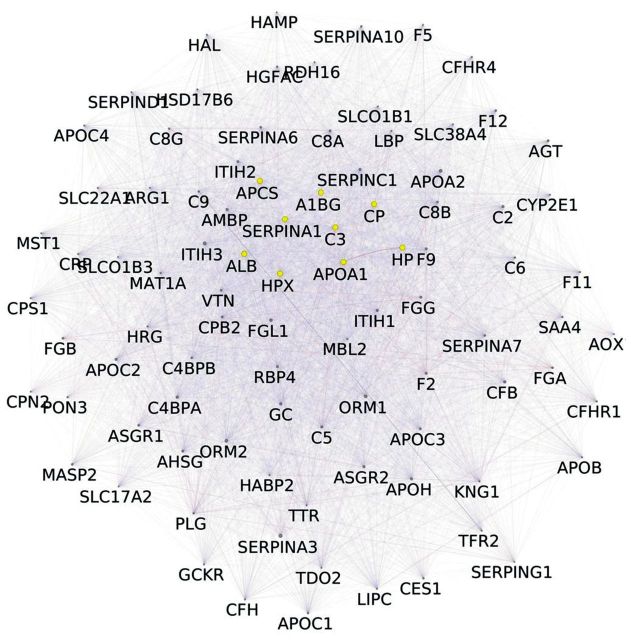
A total of 153 non-redundant proteins in meningiomas, arising from our reviewed articles, were analyzed. Results obtained by merging gel-free proteomic data and in-gel proteomic data, revealed 11 proteins common to both approaches and detected in all samples considered: serpin peptidase inhibitoralpha 1 (SERPINA1), ceruloplasmin (CP), hemopexin (HPX), albumin (ALB), complement component 3 (C3), apolipoprotein A1 (APO A1), haptoglobin (HP), amyloid-P-component serum (APCS) and alpha-1-beta-glycoprotein (A1BG), clusterin (CLU), leucine-rich alpha-2-glycoprotein 1 (LRG1) (Figure 1). Gene-enrichment by GeneMANIA allowed the expansion of original networkto 111 nodes and 6,410 unique edges (Figure 2). Nodes indicate the proteins from the original dataset and those directly interacting with them, while edges, of different shape and color, represent the specific type of interaction (e.g.co-expression, and co-localization). By MCODE analysis, a large cluster of 92 nodes and 5,483 unique edges, with a score of 82,901, was extracted from the enriched network (Figure 3). Another potential cluster, comprising 10 nodes and 15 edges, was discarded due to its low score value (score=2,889).
Figure 1. Associated proteins in meningiomas, depicted as circular nodes, extracted after merging networks derived from in-gel proteomics with those from gel-free proteomics. Edges: Co-expression (violet), colocalization (light blue) and physical interactions (pink).
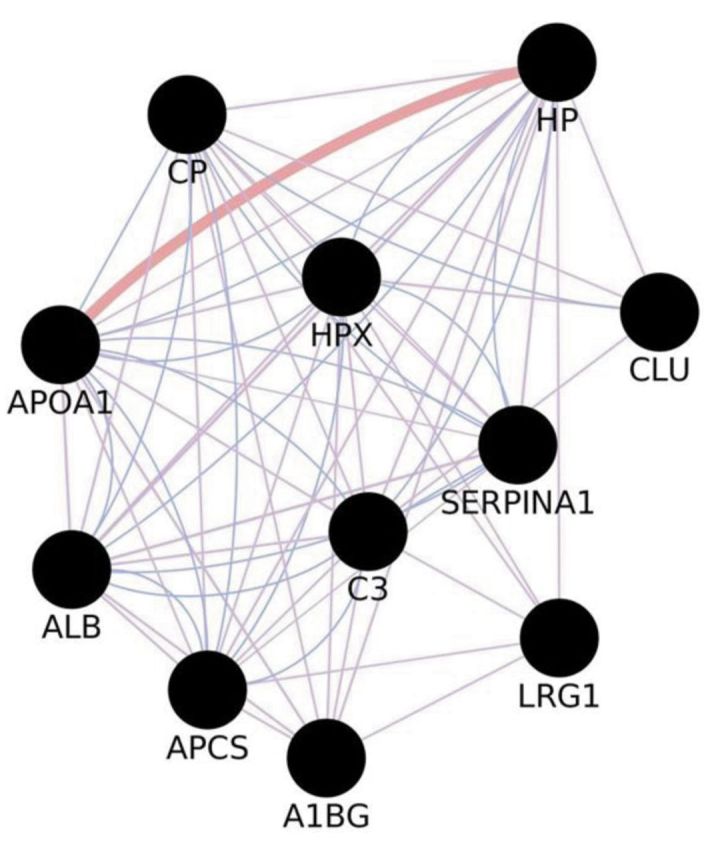
Figure 2. Network arising after gene enrichment on proteins common to both in-gel proteomics and gel-free proteomics of meningiomas. Query genes/proteins are represented by black nodes, newly found interacting partners are depicted as grey nodes.
Figure 3. Cluster arising from the enriched network reported in Figure 2. The original dataset is reduced to nine proteins highlighted in yellow.
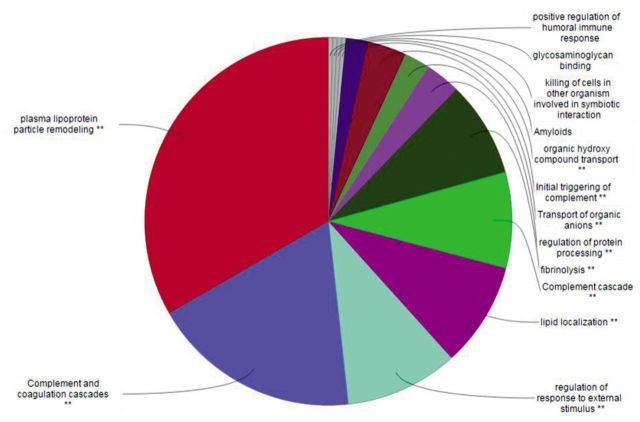
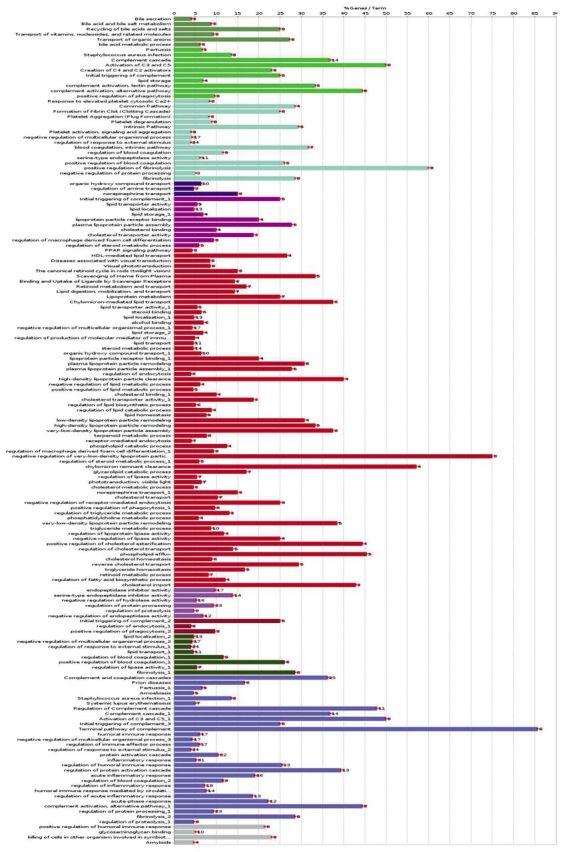
After gene enrichment and cluster analysis, this list was further reduced to nine proteins still present in the cluster of 92 nodes, with exclusion of CLU and LRG1 because of their lack of interactions. All these molecules seem to be apparently highly interconnected with each other by edges, indicating coexpression and co-localization. Functional analysis using ClueGO (http://apps.cytoscape.org/apps/cluego), followed by removal of redundant terms, showed a significant association (pV≤0.05, k-value=0.45) of the 92-node cluster with the following Gene-Ontology terms: amyloids, complement and coagulation cascades, complement cascade, initial triggering of complement, transport of organic anions, fibrinolysis, glycosaminoglycan binding, killing of cells in other organism involved in symbiotic interaction, lipid localization, organic hydroxyl compound transport, plasma lipoprotein particle remodeling, positive regulation of humoral immune response, regulation of protein processing, regulation of response to external stimulus. Genes associated with each functional group are reported in the Table I. A detailed graphical overview of ClueGO results is reported in Figure 4 and Figure 5.
Table I. ClueGO functional groups with associated genes.
Figure 4. ClueGO pie chart of principal Gene-Ontology (GO) functions associated to the 92 node cluster. In order to avoid redundancy, functions reported in the pie chart are those with the highest numbers of related genes. **Indicates significant association between the 92 node cluster and represented GO terms (ρV≤0.05).
Figure 5. Number of cluster genes associated with each Gene-Ontology function. Please refer to Figure 4 for color designations.
Discussion
The proteomic characterization of different grades of meningioma, through a bioinformatic approach, offers the possibility of investigating their molecular hetereogeneity. Few previous studies, focusing on the analysis of various biological samples, have been conducted to explore the protein spectrum of different grades of these tumors and its correlation with functional pathways, in order to find potential prognostic and therapeutic biomarkers (46, 50, 56, 57). Our analysis highlighted the dysregulation of nineproteins in all samples considered: SERPINA1, CP, HPX, APOA1, ALB, C3, HP, APCS and A1BG belonging to the pathways which showed major involvement in meningioma development and progression, plasma complement/coagulation cascades and lipoprotein particle remodeling (58,59).
Several studies have demonstrated that the activation of the coagulation cascade is implicated in tumor development, however, the exact mechanism(s) by which coagulation proteins promote tumorigenesis are not fully understood, and are likely related to peritumoral deposition of fibrin and to the alteration of hemostatic factors, hence favoring proliferation, angiogenesis and metastasis (58,60-62). Serine proteinases are capable of degrading the extracellular matrix (ECM) and basement membranes and have been implicated in human brain tumors, playing a decisive role in this malignant process by degradation of brain ECM components, secreting adhesion molecules, regulating the activity of growth and chemotactic factors and providing space for movement and infiltration (63). In detail, expression of SERPINA1, an inhibitor of serine proteases, was found to be enhanced from benign to anaplastic meningioma, suggesting its role as prognostic biomarker (64-68). Overexpression of SERPINA1 has been associated with the invasive and metastatic behavior in lung, colorectal, and gastric carcinoma (64-68). In our analysis, the significant association between higher SERPINA1 levels and meningioma grade suggests a possible role of this protein as a therapeutic target for monoclonal antibodies, in order to limit ECM degradation and infiltrative behavior, similarly to the mechanism of antiangiogenetic therapy with monoclonal antibodies to vascular endothelial growth factor in meningioma treatment (69). Further development of targeted therapies designed to inhibit tumor infiltration, and to evaluate these new agents in clinical trials, will be needed to improve survival and quality of life for patients with brain tumors (70).
Moreover, increased levels of ceruloplasmin have also been reported in different types of cancers, such as ovarian, breast, renal, colonic and brain, as well as in cancers stem-like cells of glioblastoma multiforme (71). Accordingly, in our analysis, expression of ceruloplasmin was found to be enhanced from low to higher grade meningioma. However, little is known on the role of this protein in cancerogenesis and its potential application in anticancer drug development (72,73).
The complement cascade represents the other pathway involved in tumorigenesis and progression of meningiomas emerging from our review. The reviewed articles, through comparative bioinformatic proteomic approaches, supported the activation of complement pathway in meningioma development, probably due to its role in cellular proliferation and regeneration. The exact mechanism through which complement proteins influence cancer growth is still unknown, but dysregulation of mitogenic signaling pathways, constant cellular proliferation, angiogenesis, resistance to apoptosis, and escape from the immune-system have been postulated(53). Bouwens et al. investigated the involvement of the three complement cascade-initiating pathways and their consequences in terms of complement pathway continuation in glioblastoma multiforme by determining preoperative serum levels and tissue localizations of C1q, mannose binding lectin (MBL), factor B, as well as of C3 and C5b-9 (74). The three initiating pathways of the complement system converge at the level of proteolytic cleavage of C3 that ultimately may lead to full-blown activation of the complement cascade and to the formation of the C5b-9 complex. Consequently, the presence of C3 in tumor tissue is essential for the propagation of the complement cascade. Indeed, in our investigation, we found an enhancement of C3 levels from benign to anaplastic meningioma, supporting its role as a predictive marker. Moreover, C3 expression was found abundantly present in both necrotic and non-necrotic areas of glioblastoma multiforme tumor tissues, and C5b-9 complex was detected on individual cells in glioblastoma multiforme tumor tissue (74).
Lipid metabolism and lipoprotein particle remodeling pathway appeared particularly involved in atypical and anaplastic meningiomas (75). In the networks considered, before and after cluster analysis, one marked physical interaction was always observed regarding ALB and APOA1. Apolipoproteins are polypeptides implicated in a variety of diseases and play a significant role in diagnosis and prognosis of several conditions, especially brain tumors. APOA1, the major protein component of high-density lipoprotein, is known to play a central role in regulation of the efflux and transport of cholesterol from peripheral tissues to the liver, and as a co-factor for lecithin. Recently, Hashemi et al. reported the up-regulation of serum albumin, as a carrier, and APOA1 in malignant gliomas, reflecting the ability of both these proteins to pass into the interstitium of malignant glioma because of either the disruption ofthe brain–blood barrieror its absence in tumor capillaries, and suggesting its major involvement in the vascular microenvironment, tumor development, migration and angiogenesis (76). Regarding meningioma, Sharma et al. reported an up-regulation of both albumin and APOA1 increasing from benign to anaplastic meningioma, due to the same mechanism of alteration of the brain–blood barrier (77). Current evidence also suggests the involvement of APOA1 as a promising diagnostic marker and a potential target for therapeutic strategies in neurodegenerative disorders. Additionally, we can postulate that these proteins and their pathways, could represent promising targets for brain cancer therapy (78,79), strictly related to the innovative use of nanoparticles, small molecules which facilitate drug transport into the brain, with a lower rate of toxicity (80). Furthermore overexpression of HPX, HP, APCS, and A1BG was demonstrated, however, the lack of relevant literature does not allow us to explain their possible role and implications in brain tumorigenesis and progression.
Conclusion
Bioinformatic methods were applied in our review of literature to identify the most common proteins and pathways leading to meningioma development and progression. The results obtained by matching genes and proteins expressed in tissues, serum and CSF samples highlighted the following proteins: SERPINA1, CP, HPX, APOA1, ALB, C3, A1BG, HP and APCS, mainly implicated in complement/coagulation cascades and pathways of lipid metabolism. Moreover, the presence of high levels of all these proteins could represent a molecular tool for prediction of clinical outcome in patients with meningioma and future targets for brain cancer therapies. Future investigations might address the study and discovery of therapies targeting these pathways at different levels in order to modify cancer behavior.
Conflicts of Interest
None to declare.
References
- 1.Claus BE, Bondy LM, Schildkraut JM, Wiemels LJ, Wrench M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–1095. doi: 10.1227/01.neu.0000188281.91351.b9. [DOI] [PubMed] [Google Scholar]
- 2.Davis FG, Kupelian V, Freels S, McCarthy B, Surawicz T. Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro Oncol. 2001;3:152–158. doi: 10.1093/neuonc/3.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saraf S, McCarthy BJ, Villano JL. Update on Meningiomas. Oncologist. 2011;16:1604–1613. doi: 10.1634/theoncologist.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99:379–391. doi: 10.1007/s11060-010-0342-2. [DOI] [PubMed] [Google Scholar]
- 5.Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, Schiff D, Weber DC, Wen PY, Vogelbaum MA. Meningiomas knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122:4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallinan JT, Hegde AN, Lim WE. Dilemmas and diagnostic difficulties in meningioma. Clin Radiol. 2013;68:837–844. doi: 10.1016/j.crad.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann A, Ooi J, Launay S, Searcy JL, Deighton RF, McCulloch J, Whittle IR. Proteomic data meningiomas in post-proteomic analysis can reveal novel pathophysiologicalpathways. J Neurooncol. 2011;104:401–410. doi: 10.1007/s11060-010-0526-9. [DOI] [PubMed] [Google Scholar]
- 8.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrobel G, Roerig P, Kokocinski F, Neben K, Hahn M, Reifenberger G, Lichter P. Microarray-based gene expression profiling of benign, atypical and anaplastic meningiomas identifies novel genes associated with meningioma progression. Int J Cancer. 2005;114:249–256. doi: 10.1002/ijc.20733. [DOI] [PubMed] [Google Scholar]
- 10.Wibom C, Mörén L, Aarhus M, Knappskog PM, Lund-Johansen M, Antti H, Bergenheim AT. Proteomic profiles differ between bone invasive and noninvasive benign meningiomas of fibrous and meningothelial subtype. Neurooncol. 2009;94:321–331. doi: 10.1007/s11060-009-9865-9. [DOI] [PubMed] [Google Scholar]
- 11.Aydemir F, Yurtcu E, Balci TB, Sahin FI, Gulsen S, Altinors N. Identification of promoter region methylation patterns of MGMT, CDKN2A, GSTP1, and THBS1 genes in intracranial meningioma patients. Genet Test Mol Biomarkers. 2012;16:335–340. doi: 10.1089/gtmb.2011.0245. [DOI] [PubMed] [Google Scholar]
- 12.Bello MJ, Amiñoso C, Lopez-Marin I, Arjona D, Gonzalez-Gomez P, Alonso ME, Lomas J, de Campos JM, Kusak ME, Vaquero J, Isla A, Gutierrez M, Sarasa JL, Rey JA. DNA methylation of multiple promoter-associated CpG islands in meningiomas relationship with the allelic status at 1p and 22q. Acta Neuropathol. 2004;108:413–421. doi: 10.1007/s00401-004-0911-6. [DOI] [PubMed] [Google Scholar]
- 13.Seizinger BR, de la Monte S, Atkins L, Gusella JF, Martuza RL. Molecular genetic approach to human meningioma: Loss of genes on chromosome 22. Proc Natl Acad Sci USA. 1987;84:5419–5423. doi: 10.1073/pnas.84.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry A, Louis D, Scheithauer B, Budka H, von Deimling A. Molecular genetic approach to human meningioma: Loss of genes on chromosome 22. Proc Natl Acad Sci USA. 2007;Lyon:IARC Press–504. doi: 10.1073/pnas.84.15.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Glez V, Franco-Hernandez C, Alvarez L, Alvarez L, De Campos JM, Isla A, Vaquero J, Lassaletta L, Casartelli C, Rey JA. Meningiomas and schwannomas: molecular subgroup classification found by expression arrays. Int J Oncol. 2009;34:493–504. [PubMed] [Google Scholar]
- 16.Ruttledge MH, Sarrazin J, Rangaratnam S, Phelan CM, Twist E, Merel P, Delattre O, Thomas G, Nordenskjöld M, Collins VP, Dumanski JP, Rouleau GA. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994;6:180–184. doi: 10.1038/ng0294-180. [DOI] [PubMed] [Google Scholar]
- 17.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO Classification of Tumors of the Nervous System. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Glez V, Franco-Hernández C, Peña-Granero C, Rey JA. Oncogenes and tumor suppresor genes expression in meningiomas. MAPFRE MEDICINA. 2007;18:227–233. [Google Scholar]
- 19.Striedinger K, VandenBerg SR, Baia GS, McDermott MW, Gutmann DH, Lal A. The neurofibromatosis 2 tumor-suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10:1204–1212. doi: 10.1593/neo.08642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-Magán E, Rodríguez de Lope A, Ribalta T, Ruano Y, Campos-Martín Y, Pérez-Bautista G, García JF, García-Claver A, Fiaño C, Hernández-Moneo JL, Mollejo M, Meléndez B. Differential expression profiling analyses identifies down-regulation of 1p, 6q, and 14q genes and overexpression of 6p histone cluster 1 genes as markers of recurrence in meningiomas. Neuro Oncol. 2010;12:1278–1290. doi: 10.1093/neuonc/noq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–2252. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- 22.Paek SH, Kim DG, Park CK, Phi JH, Kim YY, Im SY, Kim JE, Park SH, Jung H. The role of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in microcystic meningiomas. Oncol Rep. 2006;16:49–56. [PubMed] [Google Scholar]
- 23.Linsler S, Kraemer D, Driess C Oertel J, Kammers K, Rahnenführer J, Ketter R, Urbschat S. Molecular biological determinations of meningioma progression and recurrence. PLoS One. 2014;9:e94987. doi: 10.1371/journal.pone.0094987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Magán E, Campos-Martín Y, Mur P, Fiaño C, Ribalta T, García JF, Rey JA, Rodríguez de Lope A, Mollejo M, Meléndez B. Genetic alterations associated with progression and recurrence in meningiomas. J Neuropathol Exp Neurol. 2012;71:882–893. doi: 10.1097/NEN.0b013e31826bf704. [DOI] [PubMed] [Google Scholar]
- 25.Gutmann DH, Donahoe J, Perry A Lemke N, Gorse K, Kittiniyom K, Rempel SA, Gutierrez JA, Newsham IF. Loss of DAL-1, a protein 4.1-related tumor suppressor, is an important early event in the pathogenesis of meningiomas. Hum Mol Genet. 2000;9:1495–1500. doi: 10.1093/hmg/9.10.1495. [DOI] [PubMed] [Google Scholar]
- 26.Nunes F, Shen Y, Niida Y, Beauchamp R, Stemmer-Rachamimov AO, Ramesh V, Gusella J, MacCollin M. Inactivation patterns of NF2 and DAL-1/4.1B (EPB41L3) in sporadic meningioma. Cancer Genet Cytogenet. 2005;162:135–139. doi: 10.1016/j.cancergencyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Bello M, de Campos J, Vaquero J, Kusak M, Sarasa J, Rey J. High-resolution analysis of chromosome arm 1p alterations in meningioma. Cancer Genet Cytogenet. 2000;120:30–36. doi: 10.1016/s0165-4608(99)00249-6. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Glez VL, Alvarez L, Franco-Hernández C, Torres-Martin M, de Campos JM, Isla A, Vaquero J, Lassaletta L, Castresana JS, Casartelli C, Rey JA. Genomic deletions at 1p and 14q are associated with an abnormal cDNA microarray gene expression pattern in meningiomas but not in schwannomas. Cancer Genet Cytogenet. 2010;196:1–6. doi: 10.1016/j.cancergencyto.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Lusis E, Gutmann DH. Meningioma: an update. Curr Opin Neurol. 2004;17:687–692. doi: 10.1097/00019052-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Sadetzki S, Flint-Richter P, Starinsky S, Novikov I, Lerman Y, Goldman B, Friedman E. Genotyping of patients with sporadic and radiation-associated meningiomas. Cancer Epidemiol Biomarkers Prev. 2005;14:969–976. doi: 10.1158/1055-9965.EPI-04-0366. [DOI] [PubMed] [Google Scholar]
- 31.Rajaraman P, Hutchinson A, Rothman N, Black PM, Fine HA, Loeffler JS, Selker RG, Shapiro WR, Linet MS, Inskip PD. Oxidative response gene polymorphisms and risk of adult brain tumors. Neuro Oncol. 2008;10:709–715. doi: 10.1215/15228517-2008-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajaraman P, Brenner AV, Neta G Pfeiffer R, Wang SS, Yeager M, Thomas G, Fine HA, Linet MS, Rothman N, Chanock SJ, Inskip PD. Risk of meningioma and common variation in genes related to innate immunity. Cancer Epidemiol Biomarkers Prev. 2010;19:1356–1361. doi: 10.1158/1055-9965.EPI-09-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajaraman P, Hutchinson A, Wichner S, Black PM, Fine HA, Loeffler JS, Selker RG, Shapiro WR, Rothman N, Linet MS, Inskip PD. DNA repair gene polymorphisms and risk of adult meningioma, glioma, and acoustic neuroma. Neuro Oncol. 2010;12:37–48. doi: 10.1093/neuonc/nop012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jun P, Hong C, Lal A, Wong JM, McDermott MW, Bollen AW, Plass C, Held WA, Smiraglia DJ, Costello JF. Epigenetic silencing of the kinase tumor suppressor WNK2 is tumor-type and tumor-grade specific. Neuro Oncol. 2009;11:414–422. doi: 10.1215/15228517-2008-096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108:479–485. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma S, Ray S, Moiyadi A, Sridhar E, Srivastava S. Quantitative proteomic analysis of meningiomas for the identification of surrogate protein markers. Sci Rep. 2014;4:7140. doi: 10.1038/srep07140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuevas IC, Slocum AL, Jun P, Costello JF, Bollen AW, Riggins GJ, McDermott MW, Lal A. Meningioma transcript profiles reveal deregulated Notch signaling pathway. Cancer Res. 2005;65:5070–5075. doi: 10.1158/0008-5472.CAN-05-0240. [DOI] [PubMed] [Google Scholar]
- 38.Laurendeau I, Ferrer M, Garrido D D'Haene N, Ciavarelli P, Basso A, Vidaud M, Bieche I, Salmon I, Szijan I. Gene expression profiling of the hedgehog signaling pathway in human meningiomas. Mol Med. 2010;16:262–270. doi: 10.2119/molmed.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson MD, Okediji E, Woodard A. Transforming growth factor-beta effects on meningioma cell proliferation and signal transduction pathways. JNeurooncol. 2004;66:9–16. doi: 10.1023/b:neon.0000013461.35120.8a. [DOI] [PubMed] [Google Scholar]
- 40.Bello L, Zhang J, Nikas D, Strasser JF, Villani RM, Cheresh DA, Carroll RS, Black PM. Alpha(v)beta3 and alpha(v)beta5 integrin expression in meningiomas. Neurosurgery. 2000;47:1185–1195. doi: 10.1097/00006123-200011000-00035. [DOI] [PubMed] [Google Scholar]
- 41.Tsai BP, Hoverter NP, Waterman ML. sBlending hippo and WNT: sharing messengers and regulation. Cell. 2012;151:1401–1403. doi: 10.1016/j.cell.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Svenningsson P, Greengard P. p11 (S100A10)–an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto H, Li J, Vortmeyer AO, Jaffe H, Lee YS, Gläsker S, Sohn TS, Zeng W, Ikejiri B, Proescholdt MA, Mayer C, Weil RJ, Oldfield EH, Zhuang Z. Comparative proteomic profiles of meningioma subtypes. Cancer Res. 2006;66:10199–10204. doi: 10.1158/0008-5472.CAN-06-0955. [DOI] [PubMed] [Google Scholar]
- 47.Cui GQ, Jiao AH, Xiu CM, Wang YB, Sun P, Zhang LM, Li XG. Proteomic analysis of meningiomas. Acta Neurol Belg. 2014;114:187–194. doi: 10.1007/s13760-013-0253-z. [DOI] [PubMed] [Google Scholar]
- 48.Bouamrani A, Ramus C, Gay E, Pelletier L, Cubizolles M, Brugière S, Wion D, Berger F, Issartel JP. Increased phosphorylation of vimentin in noninfiltrative meningiomas. PLoS One. 2010;5:e9238. doi: 10.1371/journal.pone.0009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan S, Zhu D, Quinn JF, Peskind ER, Montine TJ, Lin B, Goodlett DR, Taylor G, Eng J, Zhang J. A combined dataset of human cerebrospinal fluid proteins identified by multi-dimensional chromatography and tandem mass spectrometry. Proteomics. 2007;7:469–473. doi: 10.1002/pmic.200600756. [DOI] [PubMed] [Google Scholar]
- 50.Kim JH, Lee SK, Yoo YC, Park NH, Park DB, Yoo JS, An HJ, Park YM, Cho KG. Proteome analysis of human cerebrospinal fluid as a diagnostic biomarker in patients with meningioma. Med Sci Monit. 2012;18:450–460. doi: 10.12659/MSM.883538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murakami M, Ushio Y, Morino Y, Ohta T, Matsukado Y. Immunohistochemical localization of apolipoprotein E in human glial neoplasms. J Clin Invest. 1988;82:177–188. doi: 10.1172/JCI113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zlokovic BV. Cerebrovascular transport of Alzheimer’s amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 1996;59:1483–1497. doi: 10.1016/0024-3205(96)00310-4. [DOI] [PubMed] [Google Scholar]
- 53.Rutkowski MJ, Sughrue M E, Kane A J, Mills SA, Parsa AT. Cancer and the complement cascade. Mol Cancer Res. 2010;8:1453–1465. doi: 10.1158/1541-7786.MCR-10-0225. [DOI] [PubMed] [Google Scholar]
- 54.Moore LE, Fung ET, McGuire M, Rabkin CC, Molinaro A, Wang Z, Zhang F, Wang J, Yip C, Meng XY, Pfeiffer RM. Evaluation of apolipoprotein A1 and posttranslationally modified forms of transthyretin as biomarkers for ovarian cancer detection in an independent study population. Cancer Epidemiol Biomarkers Prev. 2006;15:1641–1646. doi: 10.1158/1055-9965.EPI-05-0980. [DOI] [PubMed] [Google Scholar]
- 55.Zali H, Rezaei Tavirani M. Meningioma protein–protein interaction network. Arch Iran Med. 2014;17:262–272. [PubMed] [Google Scholar]
- 56.Wiemels J, Wrensch M, Claus BE. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saydam O, Senol O, Schaaij-Visser TB, Pham TV, Piersma SR, Stemmer-Rachamimov AO, Wurdinger T, Peerdeman SM, Jimenez CR. Comparative protein profiling reveals minichro-mosome maintenance (MCM) proteins as novel potential tumor markers for meningiomas. J Proteome Res. 2010;9:485–494. doi: 10.1021/pr900834h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boccaccio C, Medico E. Cancer and blood coagulation. Cell Mol Life Sci. 2006;63:1024–1027. doi: 10.1007/s00018-005-5570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rickles FR, Levine MN. Epidemiology of thrombosis in cancer. Acta Haematol. 2001;106:6–12. doi: 10.1159/000046583. [DOI] [PubMed] [Google Scholar]
- 60.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao M, Li Z, Qu H. An evidence-based knowledgebase of metastasis suppressors to identify key pathways relevant to cancer metastasis. Sci Rep. 2015;5:15478. doi: 10.1038/srep15478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11:223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 63.Mentlein R, Hattermann K, Held-Feindt J. Lost in disruption: role of proteases in glioma invasion and progression. J Biochim Biophys Acta. 2012;1825:178–185. doi: 10.1016/j.bbcan.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Ikota H, Nakazato Y. A case of metaplastic meningioma with extensive xanthomatous change. Neuropathology. 2008;28:422–426. doi: 10.1111/j.1440-1789.2008.00865.x. [DOI] [PubMed] [Google Scholar]
- 65.Higashiyama M, Doi O, Kodama K, Yokouchi H, Tateishi R. An evaluation of the prognostic significance of alpha-1-antitrypsin expression in adenocarcinomas of the lung: an immunohistochemical analysis. BrJ Cancer. 1992;65:300–302. doi: 10.1038/bjc.1992.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karashima S, Kataoka H, Itoh H, Maruyama R, Koono M. Prognostic significance of alpha-1-antitrypsin in early stage of colorectal carcinomas. Int J Cancer. 1990;45:244–250. doi: 10.1002/ijc.2910450207. [DOI] [PubMed] [Google Scholar]
- 67.Tahara E, Ito H, Taniyama K, Yokozaki H, Hata J. Alpha 1- antitrypsin, alpha 1-antichymotrypsin, and alpha 2-macroglobulin in human gastric carcinomas: a retrospective immunohistochemical study. Hum Pathol. 1984;15:957–964. doi: 10.1016/s0046-8177(84)80125-2. [DOI] [PubMed] [Google Scholar]
- 68.Kwon CH, Park HJ, Lee JR, Kim HK, Jeon TY, Jo HJ, Kim DH, Kim GH, Park DY. Serpin peptidase inhibitor clade A member 1 is a biomarker of poor prognosis in gastric cancer. BrJ Cancer. 2014;11:1993–2002. doi: 10.1038/bjc.2014.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caruso G, Elbabaa SK, Gonzalez-Lopez P, Barresi V, Passalacqua M, Caffo M. Innovative therapeutic strategies in the treatment of meningioma. Anticancer Res. 2015;35(12):6391–6400. [PubMed] [Google Scholar]
- 70.Newton HB. Molecular neuro-oncology and the development of targeted therapeutic strategies for brain tumors. Part 3: brain tumor invasiveness. Expert Rev Anticancer Ther. 2004;4(5):803–21. doi: 10.1586/14737140.4.5.803. [DOI] [PubMed] [Google Scholar]
- 71.McCarthy RC, Kosman DJ. Activation of C6 glioblastoma cell ceruloplasmin expression by neighboring human brain endothelia-derived interleukins in an in vitro blood–brain barrier model system. Cell Commun Signal. 2014;12:65. doi: 10.1186/s12964-014-0065-7. doi:10.1186/s12964-014-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klomp LW, Gitlin JD. Expression of the ceruloplasmin gene in the human retina and brain: implications for a pathogenic model in aceruloplasminemia. HumMol Genet. 1996;5:1989–1996. doi: 10.1093/hmg/5.12.1989. [DOI] [PubMed] [Google Scholar]
- 73.Tye SL, Gilg AG, Tolliver LB, Wheeler WG, Toole BP, Maria BL. Hyaluronan regulates ceruloplasmin production by gliomas and their treatment multipotent progenitors. J Child Neurol. 2008;23(10):1221–1230. doi: 10.1177/0883073808321066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bouwens TA, Trouw LA, Veerhuis R, Dirven CM, Lamfers ML, Al-Khawaja H. Complement activation in glioblastoma multiforme pathophysiology: evidence from serum levels and presence of complement activation products in tumor tissue. J Neuroimmunol. 2015;278:271–276. doi: 10.1016/j.jneuroim.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Liu M, Zhang K, Zhao Y, Guo Q, Guo D, Zhang J. Evidence for involvement of steroid receptors and coactivators in neuroepithelial and meningothelial tumors. Tumour Biol. 2015;36(5):3251–3261. doi: 10.1007/s13277-014-2954-1. [DOI] [PubMed] [Google Scholar]
- 76.Hashemi ML, Pooladi M, Razi Abad SK. Apolipoprotein A1 and albumin in malignant astrocytoma brain tumor. J Cancer Res Ther. 2014;10(1):107–111. doi: 10.4103/0973-1482.131413. [DOI] [PubMed] [Google Scholar]
- 77.Sharma S, Ray S, Mukherjee S, Moiyadi A, Sridhar E, Srivastava S. Multipronged quantitative proteomic analyses indicate modulation of various signal transduction pathways in human meningiomas. Proteomics. 2015;15:394–407. doi: 10.1002/pmic.201400328. [DOI] [PubMed] [Google Scholar]
- 78.Prasanna P, Thibault A, Liu L, Samid D. Lipid metabolism as a target for brain cancer therapy: synergistic activity of lovastatin and sodium phenyl acetate against human glioma cells. J Neurochem. 1996;66:710–716. doi: 10.1046/j.1471-4159.1996.66020710.x. [DOI] [PubMed] [Google Scholar]
- 79.Kreuter J, Hekmatara T, Dreis S, Vogel T, Gelperina S, Langer K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J Control Release. 2007;118:54–58. doi: 10.1016/j.jconrel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 80.Caruso G, Caffo M, Alafaci C, Raudino G, Cafarella D, Lucerna S, Salpietro FM, Tomasello F. Could nanoparticle systems have a role in the treatment of cerebral gliomas? Nanomedicine. 2011;7(6):744–752. doi: 10.1016/j.nano.2011.02.008. [DOI] [PubMed] [Google Scholar]