Abstract
Cutaneous leishmaniasis (CL) is caused by different species of the genus Leishmania. Pro- and anti-inflammatory cytokines play different roles in resistance/susceptibility and the immunopathogenesis of Leishmania infection. The balance and dynamic changes in cytokines may control or predict clinical outcome. T helper 1 (Th1) inflammatory cytokines (especially interferon-γ, tumor necrosis factor-α and interleukin-12) are the crucial factors in the initiation of protective immunity against L. major infection, whereas T helper 2 cytokines including IL-5, IL-4, and IL-13 facilitate the persistence of parasites by downregulating the Th1 immune response. On the other hand, aggravation of inflammatory reactions leads to collateral tissue damage and formation of ulcer. For this reason, immunity system such as T regulatory cells produce regulatory cytokines such as transforming growth factor-β and IL-10 to inhibit possible injures caused by increased inflammatory responses in infection site. In this article, we review the role of pro- and anti-inflammatory cytokines in the immunoprotection and immunopathology of CL.
Keywords: Leishmania, Cutaneous leishmaniasis, Immunoprotection, Immunopathology, Cytokine
Introduction
Cutaneous leishmaniasis (CL) is a significant health problem in large parts of the world, especially in underdeveloped countries.1 At least 88 countries are endemic regions,1 where about one-third of cases occur in each of three epidemiological regions, including the Americas, the Mediterranean basin, and western Asia from the Middle East to Central Asia. Afghanistan, Algeria, Colombia, Brazil, Iran, Syria, Ethiopia, North Sudan, Costa Rica, and Peru are the 10 countries with the highest incidence. It is estimated that about 0.7–1.2 million new cases of CL occur per year.1 Also, the estimated global mean age-standardised disability-adjusted life years for CL was 0.58 per 100,000 people in 2013.2 CL is caused by different species of the genus Leishmania (e.g. L. major, L. tropica, and L. aethiopica in old world and L. amazonensis, L. mexicana, and L. braziliensis in the new world).1
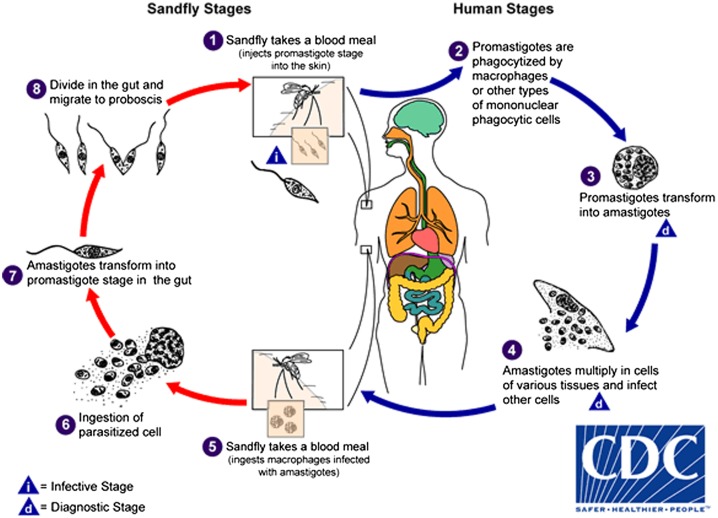
Leishmania parasite passes its life cycle in two hosts: sand flies and mammalian hosts such as humans, dogs, and rodents. When an infected sand fly feeds on a mammalian host, Leishmania metacyclic promastigotes are injected into the skin. Then, the promastigotes are phagocytosed by phagocytic cells, such as macrophages, neutrophils, and dendritic cells (DCs). The promastigotes are able to survive in macrophages (final host cells) because of complex defense mechanisms and transform into amastigote forms (Fig. 1). The Leishmania parasites proliferate in tissue macrophages and spread to other macrophages depending on various parasite and host factors. In CL, the infection is usually limited to the skin and lymphatic system, but it may influence on deeper tissues in diffuse CL or penetrate into the mucous membranes in MCL. The life cycle is completed when sand flies feed near the skin lesions and the amastigotes enter the midgut of the sand fly where they subsequently develop into promastigote forms.3–6
Figure 1.
Leishmaniasis is transmitted by the bite of infected female phlebotomine sand flies. The sand flies inject the infective stage (i.e. promastigotes) from their proboscis during blood meals  . Promastigotes that reach the puncture wound are phagocytized by macrophages
. Promastigotes that reach the puncture wound are phagocytized by macrophages  and other types of mononuclear phagocytic cells. Promastigotes transform in these cells into the tissue stage of the parasite (i.e. amastigotes)
and other types of mononuclear phagocytic cells. Promastigotes transform in these cells into the tissue stage of the parasite (i.e. amastigotes)  , which multiply by simple division and proceed to infect other mononuclear phagocytic cells
, which multiply by simple division and proceed to infect other mononuclear phagocytic cells  . Parasite, host, and other factors affect whether the infection becomes symptomatic and whether cutaneous or visceral leishmaniasis results. Sand flies become infected by ingesting infected cells during blood meals (
. Parasite, host, and other factors affect whether the infection becomes symptomatic and whether cutaneous or visceral leishmaniasis results. Sand flies become infected by ingesting infected cells during blood meals ( ,
,  ). In sand flies, amastigotes transform into promastigotes, develop in the gut
). In sand flies, amastigotes transform into promastigotes, develop in the gut  (in the hindgut for leishmanial organisms in the Viannia subgenus; in the midgut for organisms in the Leishmania subgenus), and migrate to the proboscis
(in the hindgut for leishmanial organisms in the Viannia subgenus; in the midgut for organisms in the Leishmania subgenus), and migrate to the proboscis  . Source: Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/dpdx/leishmaniasis/.
. Source: Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/dpdx/leishmaniasis/.
There are many complexities in immunity against leishmaniasis. It is well documented that resistance to leishmaniasis is related to T helper 1 (Th1) development and production of pro-inflammatory cytokines (e.g. interleukin (IL)-12, IL-1, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and/or IL-2) that lead to activation of macrophages and parasite killing.7,8 Conversely, susceptibility to the infection is linked to T helper 2 (Th2) development and production of Th2 cytokines such as IL-4, IL-5, and/or IL-13 leading to parasite replication and persistence.8–10 However, several paradoxes remain about the role of immune responses in immunoprotection and immunopathology of CL. For example, although Th1 response and production of pro-inflammatory cytokines have pivotal roles for immunoprotection against CL,8,11 their excessive production may concomitantly lead to severe immunopathology in the disease.12–15 On the contrary, Th2 development is associated with parasite persistence in the site of infection8,11 but production of anti-inflammatory cytokines at lower levels will also mitigate inflammatory reactions and accelerate wound healing process.15–17 In addition, other T cells, such as Th17 cells by production of inflammatory cytokines (e.g. IL-22, IL-17 and/or IFN-γ)18 and T regulatory (Treg) cells by production of regulatory cytokines (e.g. IL-10 and/or transforming growth factor (TGF)-β) contribute to disease progression or improvement depending on the Leishmania spp and also the genetic background of the host (Table 1).19 Hence, this review focuses on the role of pro- and anti-inflammatory cytokines in immunoprotection and immunopathology of CL.
Table 1.
Resistant and susceptible mouse strains in leishmaniasis
Pro-inflammatory cytokines in CL
IFN-γ and TNF-α
IFN-γ and TNF-α are two important pro-inflammatory cytokines involved in the immunoprotection and immunopathology of CL. IFN-γ is mainly secreted by Th1 CD4+ and CD8+ cytotoxic T lymphocytes, natural killer (NK) cells ,and natural killer T (NKT) cells. These cytokines have essential roles in control of intracellular pathogens and tumor cells, but their increased production may lead to autoimmune diseases.20 IFN-γ stimulates nitric oxide (NO) production in activated macrophages and inhibits intracellular parasite growth.21 Furthermore, IFN-γ promotes differentiation of CD4+ T cells to the Th1 subset and inhibits the development of Th2 and Th17 cells.22 It is observed that IFN-γ-deficient C57BL/6 mice are more susceptible to Leishmania infection than wild-type counterpart.23 Compared with wild-type mice, L. amazonensis infection in IFN-γ-deficient C57BL/6 mice showed larger lesions, increased parasite burden, and development of Th2-type responses associated with IL-4 elevations than wild-type mice.23
TNF-α is mostly produced by macrophages that play a crucial role in Leishmania clearance through increase in macrophage activity and NO synthesis.24 This cytokine is able to promote Th1/IFN-γ responses against L. major infection.21 TNF-α-deficient C57BL/6 mice infected with L. major showed fatal visceral infection despite the production of IFN-γ and IL-12 by macrophages.25 Treatment of BALB/c mice with TNF-α decreased the parasite burden and lesion size in CL.26 In contrast, neutralizing TNF-α receptor 1 led to non-healing lesions in resistant C57BL/6 mice following L. major infection.27
Interestingly, it was observed that IFN-γ and TNF-α have synergistic killing effects against L. major infection through stimulation of macrophages to increased NO production.28 Also in clinical studies, both IFN-γ and TNF-α have been detected in the lesions of CL patients.29–33 However, different studies demonstrated that upregulation of the pro-inflammatory cytokines (especially TNF-α and IFN-γ) are associated with increased tissue damage at the site of infection. In this regard, a positive correlation between lesion size with IFN-γ and TNF-α levels was observed in CL patients infected with L. braziliansis. Patients with greater lesions had higher levels of IFN-γ and TNF-α despite presence of IL-10 in the site of infection.31,34,35
Levels of FN-γ and TNF-α were lower in asymptomatic L. brazielienzis-infected individuals than those in patients with typical signs of CL.36 Indeed, patients with typical signs of the disease had excessive levels of IFN-γ and TNF-α with inflammatory reactions and skin ulcers at the site of infection.36 In subclinical patients, the moderate production of IFN-γ and TNF-α was associated with control of parasite growth without induction of tissue destruction.36 Th1 cells of ML patients have been reported to secrete a higher level of IFN-γ and TNF-α, and lower levels of IL-10 compared to Th1 cells of CL patients. In comparison to CL patients, lymphocytes isolated from ML patients exhibited a stronger proliferative response with higher secretion of these pro-inflammatory cytokines when stimulated with Leishmania antigen.37–39 Although IFN-γ and TNF-α production seems to be required for control of Leishmania infection, increased levels of these cytokines may lead to tissue destruction and development of progressive wounds.
IL-12
IL-12 acts as an essential cytokine for differentiation of Th1 cells in leishmaniasis.40,41 This cytokine is mainly produced by monocytes, macrophages, DCs, and B cells. IL-12 is involved in the development of Th1 response through IFN-γ production from NK and T cells.42 IL-12 stimulates also differentiation of naïve T cells into Th1 effectors and inhibits T cell apoptosis.43–46 The IL-12 family of cytokines, including IL-12, IL-23, and IL-27 share homology at the subunits, receptors, and signaling levels.47,48 Bioactive IL-12p70 is made up of two subunits, p35 and p40, which are essential for continued resistance to L. major infection.49 Interestingly, the absence of each of these subunits promotes the development of Th2 response and increases susceptibility to infections such as leishmaniasis.48,50 The IL-12p40 (also called IL-12/23p40) subunit is linked to IL-23p19 subunit to form IL-23. IL-27 is composed of IL-27p28 (p28) and Epstein–Barr virus-induced gene 3 (EBI3) subunits. IL-12 signals through the IL-12Rβ1 and IL-12Rβ2 subunits.51 Like IL-12, IL-23p40 subunit can bind to the IL-12Rβ1, however IL-23p19 subunit cannot bind to IL-12Rβ2 but has a second IL-23 receptor (IL-23R) subunit.51,52 However, in the JAK/STAT signalling pathway, IL-12 mainly activates STAT4 specific molecules, while IL-23 and IL-27 principally activate STAT3 and STAT1, respectively.51 IL-12 is an essential cytokine for stimulation of Th1 cells in leishmaniasis.40,41 BALB/c mice are susceptible mouse models to CL, and this susceptibility is related to the loss of genetic ability of IL-12 production. Hence, the immune responses fail to develop Th1 cells in L. major-infected BALB/c mice and in this situation IL-4-induced Th2 cells develop that resulted in progressive skin lesions with visceral invasion.40 Conversely, genetically resistant mice lacking IL-12 developed Th2 response with high IL-4 and low IFN-γ levels along with progressive skin lesions similar to susceptible BALB/c mice following L. major infection.50 IL-12 has been successfully used as an adjuvant for L. major vaccination.53–55 Moreover, immunotherapy with IL-12 led to resolution of L. major infection in BALB/c mice with concomitant reduction in parasite burden and lesion size,and increased IFN-γ and decreased IL-4 production.42,56 Notably, neutralization of IL-12 during primary infection with L. major led to deterioration with progressive lesions.41 It is of interest that neutralization of IFN-γ repealed the treatment effect of IL-12 and restored Th2 cytokine responses.56 Therefore, IL-12 plays a crucial role in the shift of T cell into Th1 or Th2 immune responses in CL.
IL-2
IL-2 is a growth factor that is mainly synthesized by CD4+Th cells and also in smaller amounts by CD8+ T cells, NK cells and NKT cells.57,58 IL-2 signals affect various lymphocyte subsets during differentiation, immune responses, and homeostasis. This cytokine promotes immune responses by increase in proliferation, cytokine secretion, and cytolytic activity in CD4+, CD8+, and NK cells.59,60 For example, IL-2 stimulates the production of IFN-γ by Th1 cells and activates propagation of cytotoxic T cells via binding to IL-2 receptors on lymphocytes.61 By contrast, IL-2 promotes autoimmune diseases through the death of activated T cells due to IL-2 deprivation, initiation of pro-apototic pathways by increase in FasL expression on activated T cells and development of CD4+CD25+ Tregs.59,62–65
Different studies have shown that IL-2 is involved in the protective immune response of CL.66–68 Together with IFN-γ, IL-2 facilitates Th1 response and macrophage activation for killing Leishmania parasite.22 It has been observed that the sera of CL patients with primary infection contain higher concentrations of IFN-γ and IL-2 in comparison to uninfected individuals or those with secondary infection.69 In humans, genetic mutations that lead to reduced IL-2 production are associated with exacerbated human CL.68 Co-administration of recombinant IL-2/diphtheria toxin fusion protein (rIL-2/DTx) depletes Treg cells.70 In this regard, Divanovic et al.70 used rIL-2/DTx as adjunctive therapy for experimental L. major infection in a murine model. They observed that rIL-2/DTx therapy suppressed lesional Tregs, increased IFN-γ production, decreased parasite burden, and enhanced wound healing process. They also found an additive therapeutic effect when rIL-2/DTx combined with sodium stibogluconate (a choice drug for leishmaniasis treatment) that lead to a reduction in dose and duration of sodium stibogluconate therapy.70 Conversely, IL-2 can also stimulate proliferation of Th2 cells through generation of IL-4.71 The IL-2 receptor is composed of multiple subunits and the common gamma chain is shared between IL-2 and IL-4.72,73 It is reported that neutralization of IL-2 in L. major infected BALB/c mice leads to decreased IL-4 and increased IFN-γ production in their lymph nodes.74 Collectively, IL-2 seems to play as a bifunctional cytokine that may promote susceptibility or resistance to CL.
IL-1
The IL-1 family consists of two main agonistic proteins, including IL-1α and IL-1β that are involved in various immunopathologies and inflammatory disorders, as well as protective immune responses against infectious pathogens. IL-1 is an important pro-inflammatory cytokine that is mainly secreted by macrophages and like TNF-α act as ‘alarm cytokine.’75–79 IL-1 is a critical regulator for early differentiation of Th17 cells and Th17-mediated autoimmunity. Also, IL-1 along with IL-6 and IL-23 regulates Th17 differentiation and maintains cytokine expression in effectors Th17 cells.80 IL-1 can induce protective or pathogenic effects during Leishmania infection. For example, the production of IL-1α in lymph nodes of L. major-infected BALB/c mice decreased three times in comparison to resistant C57BL/6 mice. Local treatment with IL-1α significantly decreased lesion size and parasite burden in infected animals and led to enhancement of the Th1 response via high production of IFN-γ and low production of IL-4.7 Leishmania infection promotes Nod-like receptor protein 3 (NLRP3) inflammasome-derived IL-1β productions that leads to host resistance to infection by NO production.81
Similar to IL-12, IL-1 acts as an adjuvant that supports the generation of IFN-γ-secreting T cells and helps in IgG2a production.82 Short-term treatment of L. major-infected C57BL/6 mice using IL-1β during early phases of infection promotes Th1 response and protects against leishmaniasis.83 In contrast, continuous treatment of L. major-infected C57BL/6 mice with IL-1α induces Th2 response and exacerbates the disease outcome.83 NLRP3 inflammasome-derived IL-1β production is responsible for non-healing lesions in C57BL/6 mice infected with L. major Seidman strain. NLRP3 inflammasome promotes susceptibility to L. major infection by production of IL-18 and IL-1β so that BALB/c mice lacking the inflammasome components NLRP3, ASC, or caspase 1 were resistant to L. major infection and produced high levels of IFN-γ and low levels of IL-4 and IL-5 leading to smaller footpad swelling and lower parasite burden in comparison to control mice.84 IL-1β also promotes pathology and the formation of exacerbated lesions in C57BL/6 mice infected with L. major through the development of Th17 cells and regulation of IL-17 levels.85 Also, IL-1 induces inflammatory responses in L. major infected BALB/c mice that leads to progressive disease and lack of IL-1 genes delays development of the disease and induces more attenuated systemic inflammatory responses.86 Another study showed that IL-1 signaling is dispensable for protection against CL in C57BL/6 mice.87 Human studies have demonstrated that IL-1 can also contribute to disease progression by promoting TNF-α production.88 Therefore, IL-1β promotes differentiation of protective CD4+ T cells, while excessive production of IL-1β during the chronic phase of infection leads to progression disease.
IL-18
IL-18 is a pleiotropic cytokine also named IFN-γ-inducing factor. IL-18 is secreted by different cells such as activated macrophages, DCs and Kupffer cells. IL-18 induces Th1 responses via IFN-γ production in collaboration with IL-12.89,90 Another study demonstrated that IL-18-deficient C57BL/6 mice show high susceptibility to L. major infection with decreased levels of IFN-γ and increased levels of IL-4 in comparison with wild-type mice.91 Monteforte et al.92 reported that IL-18−/− C57BL/6 mice promotes Th1 responses in L. major infection. In this study, although IL-18−/− C57BL/6 mice developed larger lesions during early phase of infection, disease resolved eventually in IL-18-deficient mice by production of IL-12 and IFN-γ but no IL-4 similar to IL-18+/+ mice. However, it seems that the genetic background and cytokine milieu influence on induction of Th1 or Th2 responses by IL-18.93 Treatment of L. major-infected BALB/c mice with recombinant IL-18 promotes Th2 responses in the absence of IL-4 and leads to exacerbated disease in comparison with untreated animals.93 In another study, NLRP3-dependent IL-18 production promotes Th2 responses during L. major infection so that neutralizing IL-18 reduces production of Th2 cytokines such as IL-4 and induces protection against L. major infection in BALB/c mice.94 Overall, it seems that IL-18 promotes Th1 or Th2 responses during CL depending upon cytokine milieu and genetic background.
IL-15
IL-15 is a pleiotropic cytokine that plays the role in the homeostasis of the innate and adaptive immunity through various mechanisms.95 IL-15 is produced mainly by DCs, monocytes, macrophage, and epithelial cells.96 This cytokine in collaboration with IL-12 facilitates IFN-γ and TNF-α production by NK and T cells.97 IL-15 also enhances protective immune responses against intracellular pathogens.98 Some studies also demonstrate that IL-15 is involved both in the development of Th1 responses by inducing IFN-γ production99–101 as well as Th2 responses by increase in IL-5 and IL-13 production.98,100,102 D’Agostino et al.103 showed that similar to IFN-γ, IL-15 induces leishmanicidal activity in macrophages via both IL-12-dependent and IL-12-independent pathways.103 In the recent study, endogenous IL-15 suppresses Th2 cytokines such as IL-4 in L. infantum infection without production of Th1 cytokines.104
IL-8
IL-8 is a strong proinflammatory cytokine that plays an essential role in the recruitment and activation of neutrophils in the course of inflammation.105 This cytokine is secreted by tissue-resident macrophages in response to Leishmania infections and plays a key role in the initial stages of infection or tissue damage.22 Monocytes isolated from L. major-infected individuals exhibited a high level of IL-8 expression.106 IL-8 (a chemoattractant for neutrophils) and neutrophils are involved in early defense against Leishmania parasite in the site of infection.105,107 In leishmaniasis, neutrophils play different roles in stimulation of the immune response to infection. Neutrophils may kill the parasites or protect them depending on the parasite species and the host. For example, neutrophils can contribute to kill L. amazonensis and L. braziliensis promastigotes by neutrophil extracellular traps or by the activation of infected macrophages to kill parasites.108–111 Upon inoculation of L. major promastigotes, neutrophils are the first cells that migrate to the infected site.112,113 Two CXC chemokines, namely macrophage inflammatory protein-2 and KC (murine homologues of IL-8) are quickly produced by distinct cell types in the skin, that act as neutrophil chemoattractants and lead to the early neutrophil accumulation.107 Infected neutrophils with L. major secrete high levels of IL-8 that lead to increased infiltration of neutrophils for phagocytosis of the parasite.114,115 Neutrophils increase the production of DCs by CC-chemokine ligand 3-dependent mechanism.116 L. major-infected neutrophils express apoptotic factors in their surface that promotes their preferential elimination by DCs and inhibits cross-presentation function in DCs. Consequently, reduction in DC activation leads to suppression of Th1 cell and CD8+ T cell function.117,118 Also, the capture of Leishmania infected neutrophils by macrophages can limit the activation of macrophages and leads to parasite survival.119 Collectively, IL-8 plays a significant role in death or survival of Leishmania parasites through the production of neutrophils.
IL-17
IL-17 is a highly inflammatory cytokine that is produced by Th17 cells and mediates tissue inflammation. IL-17 also induce different pro-inflammatory cytokines (such as IL-6 and TNF-α) and chemokines.120 IL-1β and IL-23 promote developments of Th17 cells. Also, TGF-β plus IL-6 differentiates naive T cells into Th17 cells. Furthermore, IL-6 leads to upregulation of IL-21 and IL-23, which promotes further Th17 development.121,122 IFN-γ suppresses differentiation of Th17 cells and IL-17 production by downregulation of TGF-β and IL-6 or IL-1β and IL-23.123–126 Th2 cytokines such as IL-4 and IL-13 suppress IL-6 and TGF-β-induced differentiation of Th17 cells.123,124,127 IL-27 and IL-25 also regulate Th 17 cells by development of Th1 and Th2 responses.128–130 Also different studies demonstrate that IL-17 is involved in the immunopathology of CL.85,131 For example, lack of IL-10 in L. major-infected C57BL/6 mice induced severe immunopathology associated with elevated IL-17 and neutrophil production.85 Lopez Kostka et al.131 observed that neutrophil-derived IL-17 promotes susceptibility to L. major infection in BALB/c mice. They found that IL-17-deficient BALB/c mice infected with L. major develop smaller cutaneous lesions with fewer parasite burden associated with a decreased number of neutrophils and decreased CXCL2-accumulation in the lesion site. Gonzalez-Lombana et al.85 found that increased IL-17 production is responsible for immunopathology in IL-10-deficient C57BL/6 mice infected with L. major by infiltration of neutrophils at the site of infection. Bacellar et al.132 showed that lymphocytes of patients with mucosal leishmaniasis and CL produced a significantly higher level of IL‐17 in comparison with uninfected control subjects. In contrast, several studies demonstrate that Leishmania vaccines in mouse and human models induce elevated IL-17 and IL-22 levels that play complementary roles with Th1 cytokines in protection against CL.19,133,134 Taken together, there are contradictory results about the role of IL-17 in pathogenesis and protection against leishmaniasis.
IL-22
IL-22 is secreted from Th cell subsets, including T helper 22 (Th22), Th17 and Th1 cells, as well as innate lymphocytes.135 This cytokine has antimicrobial properties and plays a pivotal role in tissue repair. Although IL-22 is a beneficial cytokine for host, it is involved in many infectious and inflammatory disorders and can be pathogenic due to its inherent pro-inflammatory properties, especially when it is released together with other pro-inflammatory cytokines such as IL-17.136 IL-22 plays a protective role against tissue damage during CL. For example, Gimblet et al.137 reported that IL-22 deficient C57BL/6 mice infected with L. major develop more severe pathological changes with a higher parasite burden than wild-type mice.137 In another study, IL-22 improves the efficacy of DNA vaccines against L. major in BALB/c mice138,139 so that IL-22 plus a DNA vaccine encoding LACK antigen resulted in increased IFN-γ and decreased IL-4 levels than LACK gene alone.139 However, conflicting results have been reported indicating an independence of IL-22 in host resistance of C57BL/6 mice to L. major.140 Overall, although there are a few studies about the role of IL-22 in leishmaniasis, it seems that this cytokine has a protective role against Leishmania infection.
Anti-inflammatory cytokines in CL
IL-6
IL-6 is a pleiotropic cytokine that acts as both a pro-inflammatory and anti-inflammatory cytokine.141 IL-6 is produced by several cell types, including macrophages, DCs, and T cells.141 Also, this cytokine acts as a B-cell growth factor.142 IL-6 plus TGF-β stimulate the development of Th17 responses and produce IL-17 and IL-10 cytokines and lead to restrain pathogenic function of Th17 cells.122 Animal experimentations demonstrated that IL-6 promotes Th2 responses in CL.143,144 Moskowitz et al.144 showed that IL-6-deficient C57BL/6 mice infected with L. major can control infection and promote strong Th1 responses as efficiently as wild-type C57BL/6 mice. Titus et al.142 reported that the production of both Th1 and T2 cytokines decreased in L. major-infected IL-6−/− BALB/c mice but there were no significant difference between lesion size and parasite burden in IL-6-deficient and wild-type mice.142 Hatzigeorgiou et al.145 found that pretreatment of macrophages with IL-6 suppressed IFN-γ and TNF-α production against L. amazonensis in vitro. Taken together, IL-6 is a susceptibility factor in CL.
IL-27
IL-27 is a pleiotropic cytokine produced by activated APCs such as macrophages and DCs.146 IL-27 plays two-sided roles in immune responses. It acts as an inflammatory cytokine that initiates Th1-type responses, but also suppresses inflammatory T-cell responses.147,148 IL-27 inhibits production of pro-inflammatory cytokines, including IL-17 and IL-23 and promotes production of IL-10 from CD4+ T Cells.149,150 IL-27 initiates Th1 responses and induces protection against Leishmania infection so that IL-27R-deficient mice were susceptible to early L. major infection that associated with impaired Th1 response by decrease in IFN-γ production.151 Another study showed that IL-27 induces IFN-γ production and controls the infection only in the presence of IL-4.152 Therefore, IL-27 seems to play a bifunctional factor that may promote susceptibility or resistance to leishmaniasis.
IL-10
Although IL-10 is known to be a potent immunoregulatory cytokine, it is produced by different innate immunity cells (e.g. DCs, macrophages, mast cells, NK cells, eosinophils, and neutrophils) and adaptive immunity cells (e.g. Th1, Th2 and Th17 cell subsets, Treg cells, CD8+ T cells, and B cells) .153–158 In addition, IL-10 suppresses macrophage activation and maturation of DCs. IL-10 production by Th1 cells limits immune responses against intracellular parasite infections such as L. major and Toxoplasma gondii.159 This cytokine is associated with the susceptibility to leishmaniasis and parasite persistence in infection site.160 While infection of IL-10−/− C57BL/6 Mice with L. major led to inhibit production of IL-10 by Treg cells during the chronic phase of CL that consequently lead to parasite clearance and wound healing.161 IL-10 and Treg cells limit the effective function of Th1 responses to control infection in the skin.161 Indeed, treatment of L. major-infected mice with anti-IL-10 receptor antibodies led to sterile cure and parasite clearance.160 Castellano et al.162 reported that CL patients with active lesions had higher levels of Th1 and Th2 cytokines including IFN-γ, TNF-α, IL-12, IL-4, and IL-10 in comparison with cured patients, while cured patients had higher level of IFN-γ. Salhi et al.163 reported that L. braziliensis-infected individuals with active lesions had polarized Th2 or mixed Th1/Th2 responses, both associated with increased IL-10 levels.163 These results suggested that downmodulation of IL-10 and IL-4 and elevation of IFN-γ is associated with clinical cure in CL patients.162 Buxbaum and Scott164 found that L. Mexicana-infected C57BL/6 wild-type mice had minimal immune response and chronic lesions, but 10−/− mice resolved their lesions and had increase production of IFN-γ, NO and delayed-type hypersensitivity.164 The quantitative IFN-γ/IL-10 ratio is important to the result of vaccination against CL. A low ratio has been found to result in vaccine failure whereas a high ratio provided vaccine success.165 IL-10 production by Treg cells has been found to suppress the magnitude and quality of the Th1 response, while neutralization of IL-10 increased magnitude and quality of the Th1 response after vaccination.166 Despite suppressive effects of IL-10 that leads to disease progression and parasite persistence, this cytokine is a vital immuneregulator that modulates immunopathology and tissue damage caused by excessive Th1 immune response and their inflammatory cytokines, especially IFN-γ in CL.167
Based on cell surface markers or secreted cytokines, Treg cells are divided into two subsets: first, natural (n) Tregs that develop in thymus and express CD4, CD25 (IL-2 receptor α chain) and also Forkhead box protein 3 (Foxp3) (a specific marker for nTregs). nT reg (CD4+CD25+Treg) subset represents 5–10% of the adult peripheral CD4 T cells and are vital for self-tolerance and avoid autoimmune diseases.168–171 Second, acquired (a)/induced (i) Tregs develop from conventional T cells and are grouped two subsets: T regulatory1 (Tr1) and T-helper 3 (Th3). Tr1 cells do not express Foxp3172 and are able to produce high levels of IL-10 and TGF-β,173 while Th3 cells express Foxp3174 and produce elevated levels of TGF-β.175 Overall, Treg cells with high levels of Foxp3 (Foxp3high) suppress the function of effector T cells (Th1 and Th2) and DCs by IL-10 production but promote differentiation of Th17 cells by TGF-β production. Treg cells with low levels of Foxp3 (Foxp3low) promote Th2 responses by IL-4 and IL-10 production. Treg cells lacking Foxp3 (Foxp3 null) may transform into different types of effectors T cells (Th1, Th2, and Th17).170
During infection with L. major, CD4+CD25+ T cells accumulate in the dermis and suppress function of CD4+CD25− effectors T cells to eliminate the parasite from infection site by both IL-10-dependent and IL-10-independent mechanisms. CD4+CD25+ T cells are responsible for the persistence of L. major in healed lesions that leads to concomitant immunity and host resistance to reinfection.153 In another study, increased level of IL-10 in CD4+CD25−Foxp3−Th1 cells is responsible for development of nonhealing lesions following L. major infection.176
Taken together, IL-10 acts as a double-edged sword that suppresses cellular immune response and production of inflammatory cytokines (IFN-γ and TNF-α) that lead to parasite persistence in the infection site. On the other hand, IL-10 inhibits an exacerbated immunopathology and tissue damage following increased production of inflammatory cytokines and plays a central role in the regulation of tissue remodeling during wound healing.177
IL-4
IL-4 plays an important role in the differentiation of Th0 cells into Th2 cells.178 IL-4 is mainly produced by Th2, mast cells, basophils, and activated eosinophils.179–181 IL-4 production drives upregulation of arginase and poly amine biosynthesis that inhibits leishmanicidal activity of macrophages and prolonged survival of parasites.10 IL-4 limits the generation of Th1 cytokines through downregulation IL-12 production.182 Furthermore, IL-4 downregulates the production of chemokines that recruit Th1-type cells to the infection site.183 Pro-inflammatory cytokines such as IFN-γ stimulate M1 macrophages that lead to NOS2 activation, NO release, and parasite death, while Th2 cytokines such as IL-4 and IL-13 stimulate M2 macrophages to induce arginase activity which result in parasite survival and inhibition of inflammation by counteracting the effects of NOS2 activation and nitric. Indeed, the balance between classically activated macrophage (M1) and alternatively activated macrophage (M2) regulates inflammatory responses and leads to homeostasis in immunity system and wound healing.184–187 Also, IL-4 suppresses IL-6 and TGF-β-induced differentiation of Th17 cells that leads to inhibition of immunopathology caused by IL-17.123,124,127 Studies using IL-4 transgenic and knockout mice have shown an important role of IL-4 in susceptibility to Leishmania infection. Kopf et al.188 found that IL-4-deficient BALB/c mice are resistant to L. major infection. Also, they observed that IL-4 transgenic C57BL/6 mice were more susceptible to L. major infection in comparison to wild-type mice.188 Radwanska et al.189 showed that deletion of IL-4Rα on CD4 T cells lead to resistance of BALB/c mice to L. major infection. Sadick et al.190 found that neutralization of IL-4 by anti-IL-4 mAb inhibits development of Th2 response in L. major-infected BALB/c mice so that IFN-γ mRNA expression increased fourfold in the lymph nodes of infected mice. They also found that neutralization of IL-4 led to complete cure in 85% of infected mice and attenuation of infection in 100% of animals.190
Heinzel et al.191 found that IL-4 mRNA was expressed only in BALB/c mice infected with L. major but not in infected C57BL/6. However, IFN-γ mRNA increased in draining nodes and spleen of C57BL/6 mice than that in BALB/c mice except at 4 and 6 weeks of infection, when splenic IFN-γ levels were transiently comparable. In this study, neutralization of IL-4 by anti-IL-4 mAb led to disease healing by a reduction in serum IgE, lesion size, and parasite burden in infected BALB/c mice.191 Another study showed although both IL-4 and IL-4Rα-deficient BALB/c mice were more resistant to L. major infection in comparison to wild-type mice, IL-4Rα−/− mice efficiently controlled infection by reduction of Th2 responses, while IL-4−/− mice partially controlled the infection.192 In contrast, Noben-Trauth et al.193 showed that disruption of the IL-4 gene in L. major-infected BALB/c mice did not promote polarization of Th1 response and had no effect on wound healing and parasite clearance. Although IL-4 plays a critical role in susceptibility to Leishmania infection, several contributing factors help in its susceptibility. For example, IL-4−/− and IL-13−/− L. mexicana-infected BALB/c mice revealed that IL-4 plays a pivotal role in initiation of lesion development, but IL-13 plays a crucial role in development of chronic and non-healing infection.194 Failure of IL-12 production is another contributing factor that leads to susceptibility to L. major infection.195 Also study in IL-4-deficient BALB/c mice demonstrated that different parasite isolates, and differences in the age of the mice and in the arginase activity are other influencing factors of susceptibility to L. major infection of IL-4−/− BALB/c mice.10,196 Therefore, IL-4 is an important cytokine in susceptibility to Leishmania infection.
IL-13
IL-13 shares signaling pathway with IL-4.197 Like IL-4, IL-13 is produced by Th2, mast cells, and basophils.181 IL-13 decreases the inflammatory responses via down regulation of pro-inflammatory cytokines such as IL-1, IL-6, TNF-α, and IL-12.198,199 IL-13 inhibits the production of IL-12 by macrophages and limits L. major killing.200,201 Therefore, upregulation of IL-13 in transgenic animals delays the onset of a Th1 response subsequent to L. major infection.202 IL-13 can limit NO production in human mesangial cells through downregulation of iNOS.203 Subsequently, NO suppression decreases the parasiticidal activity of macrophage.199 IL-13 makes specific T cells unresponsive to IL-12 by downregulation of the IL-12Rβ2 chain.204 As observed by Bourreau et al.204 IL-13 was the predominant Th2 cytokine in peripheral blood mononuclear cells of L. guyanensis-infected patients, while the absence of IL-12Rβ2 chain in lesions indicated the pivotal role of IL-13 in susceptibility to CL caused by L. guyanensis.204 Matthews et al.202 found that IL-13 is a susceptible factor for L. major infection, while IL-13-deficient BALB/c mice are resistant to the infection. Overexpression of IL-13 in C57BL/6 mice leads to suppression of IFN-γ and IL-12 expression and increases susceptibility to L. major infection, even in the absence of IL-4 expression.202 Additionally, transgenic mice expressing IL-13 failed to control L. major infection and showed 1000-fold higher parasite load than wild-type C57BL/6 mice. In contrast, Sosa et al.205 showed that IL-13-deficient C57BL/6 mice were susceptible to infection and indicated progressive and non-healing wounds as the wild-type mice infected to L. mexicana, but IL-4−/−/IL-13−/− mice were highly resistant with lower parasite burden and higher levels of IL-12 and IFN-γ than wild-type and IL-13−/− mice. The study shows that IL-13 is not a major susceptible factor to L. mexicana but IL-4 is a dominant cytokine for pathogenesis of cutaneous L. mexicana infection. Indeed, IL-4 may compensate lack of IL-13 and promotes susceptibility to L. mexicana in C57BL/6 mice and led to pathogenesis of L. mexicana infection. Alexander et al.194 using L. mexicana-infected-IL-4/IL-13−/− mice demonstrated that IL-13 is a crucial factor to maintaining non-healing forms of CL in chronic phase but IL-4 plays a crucial role in primary lesion formation. Collectively, IL-13 plays a vital in susceptibility to leishmaniasis, especially in promoting chronic phase of disease.
TGF-β
TGF-β is a pleiotropic growth factor with significant anti-inflammatory and immunosuppressive properties and plays central roles in homeostasis of immune system.206 TGF-β is produced by different cells, including CD4+ T cells (Tregs), monocytes, neutrophils, and DCs.207–210 TGF-β cytokine and Treg cells are essential for control of immune responses against foreign pathogens, the maintenance of homeostasis, and promoting immune tolerance.170,208,211,212 TGF-β suppresses both adaptive immune response and the innate immune response by inhibiting the function of inflammatory cells and promoting the function of Treg cells.213–215 Also, TGF-β suppresses differentiation of T cells to Th1 and Th2 subsets.216,217 In addition, TGF-β suppresses generation of activated T cells by inhibiting production of IL-2 and IL-1.218,219 TGF-β differentiates CD4+CD25− naïve T cells to iTreg cells (CD4+CD25+ Tregs) in peripheral lymphoid organs and other tissues.220 Although nTregs does not require TGF-β for their differentiation in the thymus, TGF-β is essential for survival and function of nTregs221–223 nTregs and iTregs (Tr1 andTh3 cells) produce high levels of TGF-β.169,170,172,173,175 Therefore, Tregs produce TGF-β and provide a positive feedback loop.
TGF-β is an immunoregulatory cytokine that inhibits Th1 responses against Leishmania parasite by downregulation of IFN-γ, inactivation of macrophages, and inhibition of IL-2R stimulation.224 TGF-β enhances susceptibility to Leishmania infection by suppression of NO, TNF and IFN-γ production.225 This cytokine exacerbates infection due to L. amazonensis and L. braziliensis via stimulation of production of Th2 cytokines such as IL-10. TGF-β modulates differentiation of T cells into Th1 cells via down regulation of T-bet, an independent mechanism of down regulation of IL-12 receptor β2 chain expression leading to decrease in IFN-γ production and inhibits parasite clearance.226 Barral et al.227 reported that the addition of recombinant TGF-β to murine or human macrophages increased the parasite load in vitro.227 Similar studies showed that in vivo immunotherapy with TGF-β for resistant mice changed their immune response and led to overexpression of IL-10 in draining lymph nodes, whereas treatment of susceptible animals with anti-TGF-β mAb led to the decrease in IL-4 expression and increase in IFN-γ expression in draining lymph nodes.228,229 Hence, TGF-β acts as an infection promoting factor in CL.228,229 Another study230 showed that local inoculation of anti-TGF-β mAb into the Leishmania lesion led to decrease in parasite burden and more rapid healing of wound without alteration in IL-4 and IFN-γ production. Immunohistochemical test showed that anti-TGF-β treatment increased NO production within parasitized lesions.230 This study suggested that TGF-β may act as an important regulatory cytokine during chronic stages of CL that inhibits NO production in macrophages.230 In addition, this study expressed that during the lack of TGF-β, even with dominant Th2-type responses, relatively low levels of IFN-γ are sufficient to activate macrophages for parasite killing within parasitized lesions.230 Also, several studies have reported that IL-10 and TGF-β expression increase in long lasting lesions than acute lesions in CL.231–233 TGF-β modulates lymphocyte proliferation and production of inflammatory cytokines as it limits increased inflammatory reactions that are responsible for tissue damage.234,235 Moreover, TGF-β plus IL-6 promote the differentiation of Th17 cells from naïve T cells.236,237 In contrast, increased production of TGF-β and IL-6 restimulates activated Th17 cells that have resulted in IL-10 production to control immunopathology caused by Th17 cells (a self-regulating mechanism).122 TGF-β is a crucial immunoregulatory cytokine that limits inflammatory reactions by downregulating inflammatory cytokines in leishmaniasis.
Conclusion
Cytokines play vital roles in cell propagation and differentiation toward defense against pathogens. However, the balances of pro- and anti-inflammatory cytokines are needed to prevent immunopathological disorders. In leishmaniasis, protective immunity depends predominantly on a Th1 response and production of pro-inflammatory cytokines like TNF-α, IL-12, IFN-γ. A less controlled inflammatory response and contributing cytokines are responsible for immunopathology and tissue damage in CL.30,237 In addition, secreted cytokines of Th17 cells promote the pathogenesis and lesion development in leishmaniasis85,131,132 although recent studies demonstrate that Th17 cytokines induce protection against the disease as well.19,138,139 Th2 cytokines such as IL-13 and IL-4 promote disease progression. On the other hand, IL-4 and IL-13 suppress immunopathology caused by Th17 cells.123,124,127 Regulatory cytokines such as IL-10 and TGF-β counteract the immunopathology caused by pro-inflammatory cytokines but are also responsible for maintenance of parasites in infection site.39 Therefore, the cytokines act like a double-edged sword that may induce protection against leishmaniasis or implicate in the pathogenesis of CL (Figure 2). A balance between immune responses control the infection and prevent inflammatory reactions that leads to wound healing in CL. Overall, immune mechanisms involved in leishmaniasis are complex because the exact role of some cytokines remains unclear until now. Also cytokines act as a network because different cytokines have synergistic or antagonistic effects during an immunological reaction against CL. Comprehensive understanding of the immunity mechanisms can help researchers find new therapeutic strategies and development of effective vaccines.
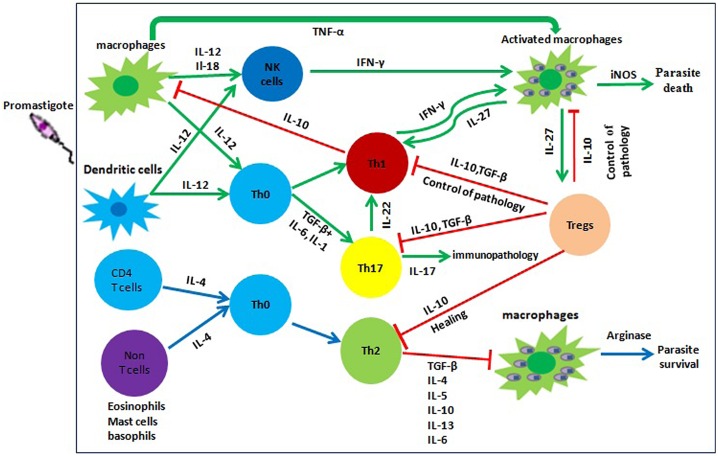
Figure 2.
Immunological pathways of against cutaneous leishmaniasis. This figure shows different immunological pathways in CL that all depend on the differentiation of CD4+ T cell subsets into Th1, Th2, Treg, and Th17. Following parasite entry, APCs (macrophages and dendritic cells) are stimulated to produce pro-inflammatory cytokines such as IL-12. These cytokines promote Th1 differentiation and IFN-γ production lead to activation of macrophages and parasite killing by NO production. Conversely, anti-inflammatory cytokines promote differentiation of Th0 toward Th2 that inhibit macrophage activity and lead to parasite survival. Overproduction of inflammatory cytokines results in severe immunopathology and non-healing infection. TGF-β and IL-27 cytokines secreted by macrophages or DCs stimulate Treg cells to produce IL-10 that act back on the macrophages and DCs to reduce the release of inflammatory mediators, forming a negative feedback loop and the balance of pro- and anti-inflammatory cytokines controls pathology and tissue destruction.
Conflict of interest
Authors disclose that there is no conflict of interest.
Acknowledgments
The authors would like to thank the contributions made by two anonymous referees for their valuable criticisms and suggestions which were helpful in improving the paper. The authors apologize to researchers whose works were not adequately cited in this article because of space limitations.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5):e35671. 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimkhani C, Wanga V, Coffeng LE, Naghavi P, Dellavalle RP, Naghavi M. Global burden of cutaneous leishmaniasis: a cross-sectional analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016; 16(5): 584–91. [DOI] [PubMed] [Google Scholar]
- 3.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9(8):604–15. 10.1038/nrmicro2608 [DOI] [PubMed] [Google Scholar]
- 4.Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol. 2001;55:453–83. 10.1146/annurev.micro.55.1.453 [DOI] [PubMed] [Google Scholar]
- 5.Scott P, Novais FO. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol. 2016; 16(9): 581–92. [DOI] [PubMed] [Google Scholar]
- 6.Bailey MS, Lockwood DN. Cutaneous leishmaniasis. Clin Dermatol. 2007;25(2):203–11. 10.1016/j.clindermatol.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 7.von Stebut E, Ehrchen JM, Belkaid Y, Kostka SL, Mölle K, Knop J, et al. Interleukin 1α promotes Th(1) differentiation and inhibits disease progression in Leishmania major – susceptible BALB/c mice. J Exp Med. 2003;198(2):191–99. 10.1084/jem.20030159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2(11):845–58. 10.1038/nri933 [DOI] [PubMed] [Google Scholar]
- 9.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. 10.1038/nri978 [DOI] [PubMed] [Google Scholar]
- 10.Kropf P, Fuentes JM, Fahnrich E, Arpa L, Herath S, Weber V, et al. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. Faseb J. 2005;19(8):1000–02. [DOI] [PubMed] [Google Scholar]
- 11.Nylén S, Eidsmo L. Tissue damage and immunity in cutaneous leishmaniasis. Parasite Immunol. 2012;34(12):551–61. 10.1111/pim.12007 [DOI] [PubMed] [Google Scholar]
- 12.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trend Cell Biol. 2005;15(11):599–607. 10.1016/j.tcb.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 13.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–25. 10.1038/sj.jid.5700701 [DOI] [PubMed] [Google Scholar]
- 14.Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. BBA-Mol Basis Dis. 2013;1832(7):1049–60. 10.1016/j.bbadis.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14(5):289–301. 10.1038/nri3646 [DOI] [PubMed] [Google Scholar]
- 16.Allen JE, Wynn TA. Evolution of Th2 Immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathogens. 2011;7(5):e1002003. 10.1371/journal.ppat.1002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13(8):607–14. 10.1038/nri3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–58. 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- 19.Wu W, Huang L, Mendez S. A live Leishmania major vaccine containing CpG motifs induces the de novo generation of Th17 cells in C57BL/6 mice. Eur J Immunol. 2010;40(9):2517–27. 10.1002/eji.201040484 [DOI] [PubMed] [Google Scholar]
- 20.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. 10.1016/S0065-2776(07)96002-2 [DOI] [PubMed] [Google Scholar]
- 21.Sharma U, Singh S. Immunobiology of leishmaniasis. Indian J Exp Biol. 2009;47(6):412–23. [PubMed] [Google Scholar]
- 22.Alexander J, Satoskar AR, Russell DG. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112(18):2993–3002. [DOI] [PubMed] [Google Scholar]
- 23.Pinheiro RO, Rossi-Bergmann B. Interferon-gamma is required for the late but not early control of Leishmania amazonensis infection in C57Bl/6 mice. Mem Inst Oswaldo Cruz. 2007;102(1):79–82. 10.1590/S0074-02762007000100013 [DOI] [PubMed] [Google Scholar]
- 24.Liew FY, Wei XQ, Proudfoot L. Cytokines and nitric oxide as effector molecules against parasitic infections. Philos Trans R Soc Lond B Biol Sci. 1997;352(1359):1311–15. 10.1098/rstb.1997.0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilhelm P, Ritter U, Labbow S, Donhauser N, Rollinghoff M, Bogdan C, et al. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking tnf. J Immunol. 2001;166(6):4012–19. 10.4049/jimmunol.166.6.4012 [DOI] [PubMed] [Google Scholar]
- 26.Theodos CM, Povinelli L, Molina R, Sherry B, Titus RG. Role of tumor necrosis factor in macrophage leishmanicidal activity in vitro and resistance to cutaneous leishmaniasis in vivo. Infect Immune. 1991;59(8):2839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia I, Miyazaki Y, Araki K, Araki M, Lucas R, Grau GE, et al. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol. 1995;25(8):2401–07. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 28.Liew FY, Li Y, Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145(12):4306–10. [PubMed] [Google Scholar]
- 29.Gaafar A, Veress B, Permin H, Kharazmi A, Theander TG, El Hassan AM. Characterization of the local and systemic immune responses in patients with cutaneous leishmaniasis due to Leishmania major. Clin Immunol. 1999;91(3):314–20. 10.1006/clim.1999.4705 [DOI] [PubMed] [Google Scholar]
- 30.Pompeu MM, Brodskyn C, Teixeira MJ, Clarencio J, Van Weyenberg J, Coelho IC, et al. Differences in gamma interferon production in vitro predict the pace of the in vivo response to Leishmania amazonensis in healthy volunteers. Infect Immun. 2001;69(12):7453–60. 10.1128/IAI.69.12.7453-7460.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonelli LRV, Dutra WO, Almeida RP, Bacellar O, Gollob KJ. Antigen specific correlations of cellular immune responses in human leishmaniasis suggests mechanisms for immunoregulation. Clin Exp Immunol. 2004;136(2):341–8. 10.1111/cei.2004.136.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira F, Bafica A, Rosato AB, Favali CB, Costa JM, Cafe V, et al. Lesion size correlates with Leishmania antigen-stimulated TNF-levels in human cutaneous leishmaniasis. Am J Trop Med Hyg. 2011;85(1):70–3. 10.4269/ajtmh.2011.10-0680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Oliveira Junior A Jr., Machado P, Bacellar O, Cheng LH, Almeida RP, Carvalho EM. Evaluation of IFN-gamma and TNF-alpha as immunological markers of clinical outcome in cutaneous leishmaniasis. Rev Soc Bras Med Trop. 2002;35(1):7–10. 10.1590/S0037-86822002000100002 [DOI] [PubMed] [Google Scholar]
- 34.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, et al. Up-Regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70(12):6734–40. 10.1128/IAI.70.12.6734-6740.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101(2):226–30. 10.1016/j.imlet.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 36.Ivonise F, Cibele A, Olívia B, Clarissa BA, Lucas PC, Roque PA, et al. Epidemiologic and Immunologic Findings for the Subclinical Form of Leishmania braziliensis Infection. Clin Infect Dis. 2002;34(11):e54–e8. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S, et al. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985;135(6):4144–48. [PubMed] [Google Scholar]
- 38.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31(1):143–8. 10.1590/S0100-879X1998000100020 [DOI] [PubMed] [Google Scholar]
- 39.Oliveira WN, Ribeiro LE, Schrieffer A, Machado P, Carvalho EM, Bacellar O. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of human tegumentary leishmaniasis. Cytokine. 2014;66(2):127–32. 10.1016/j.cyto.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Güler ML, Gorham JD, Hsieh C-S, Mackey AJ, Steen RG, Dietrich WF, et al. Genetic susceptibility to leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996;271(5251):984–7. 10.1126/science.271.5251.984 [DOI] [PubMed] [Google Scholar]
- 41.Constantinescu CS, Hondowicz BD, Elloso MM, Wysocka M, Trinchieri G, Scott P. The role of IL-12 in the maintenance of an established Th1 immune response in experimental leishmaniasis. Eur J Immunol. 1998;28(7):2227–33. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 42.Sypek JP, Chung CL, Mayor SE, Subramanyam JM, Goldman SJ, Sieburth DS, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177(6):1797–1802. 10.1084/jem.177.6.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer EM, Farrokh-Siar L, Maguire van Seventer J, Van Seventer GA. IL-12 Decreases activation-induced cell death in human naive Th Cells costimulated by intercellular adhesion molecule-1. I. IL-12 alters caspase processing and inhibits enzyme function. J Immunol. 2001;167(2):749–58. 10.4049/jimmunol.167.2.749 [DOI] [PubMed] [Google Scholar]
- 44.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292(5523):1907–10. 10.1126/science.1059835 [DOI] [PubMed] [Google Scholar]
- 45.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. 10.1146/annurev.immunol.16.1.495 [DOI] [PubMed] [Google Scholar]
- 46.Mal X, Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol. 2001;79:55–92. 10.1016/S0065-2776(01)79002-5 [DOI] [PubMed] [Google Scholar]
- 47.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13(8):722–8. 10.1038/ni.2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8(1):40–52. 10.2174/187152809787582507 [DOI] [PubMed] [Google Scholar]
- 49.Park AY, Hondowicz B, Kopf M, Scott P. The role of IL-12 in maintaining resistance to Leishmania major. J Immunol. 2002;168(11):5771–77. 10.4049/jimmunol.168.11.5771 [DOI] [PubMed] [Google Scholar]
- 50.Mattner F, Magram J, Ferrante J, Launois P, Padova K, Behin R, et al. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26(7):1553–59. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 51.Teng MWL, Bowman EP, McElwee JJ, Smyth MJ, Casanova J-L, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719–29. 10.1038/nm.3895 [DOI] [PubMed] [Google Scholar]
- 52.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the Heterodimeric cytokine IL-23 Is Composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168(11):5699–708. 10.4049/jimmunol.168.11.5699 [DOI] [PubMed] [Google Scholar]
- 53.Afonso L, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263(5144):235–7. 10.1126/science.7904381 [DOI] [PubMed] [Google Scholar]
- 54.Kenney RT, Sacks DL, Sypek JP, Vilela L, Gam AA, Evans-Davis K. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J Immunol. 1999;163(8):4481–8. [PubMed] [Google Scholar]
- 55.Maspi N, Ghaffarifar F, Sharifi Z, Dalimi A. Co-delivery of DNA vaccination encoding LeIF gene and IL-12 increases protection against Leishmania major infection in BALB/c mice. Parasit Immunol. 2016;38(4):228–35. [DOI] [PubMed] [Google Scholar]
- 56.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177(5):1505–09. 10.1084/jem.177.5.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. 10.1146/annurev.immunol.26.021607.090357 [DOI] [PubMed] [Google Scholar]
- 58.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201(5):723–35. 10.1084/jem.20041982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–90. [DOI] [PubMed] [Google Scholar]
- 60.Gaffen SL, Liu KD. Overview of interleukin-2 function, production and clinical applications. Cytokine. 2004;28(3):109–23. 10.1016/j.cyto.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 61.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240(4856):1169–76. 10.1126/science.3131876 [DOI] [PubMed] [Google Scholar]
- 62.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172(7):3983–8. 10.4049/jimmunol.172.7.3983 [DOI] [PubMed] [Google Scholar]
- 63.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice: implications for the nonredundant function of IL-2. Immunity. 2002;17(2):167–78. 10.1016/S1074-7613(02)00367-9 [DOI] [PubMed] [Google Scholar]
- 64.Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol. 2000;164(6):2905–14. 10.4049/jimmunol.164.6.2905 [DOI] [PubMed] [Google Scholar]
- 65.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74(6):961–5. 10.1189/jlb.0603272 [DOI] [PubMed] [Google Scholar]
- 66.Wang X, Mosmann T. In vivo priming of CD4 T cells that produce interleukin (IL)-2 but not IL-4 or interferon (IFN)-gamma, and can subsequently differentiate into IL-4- or IFN-gamma-secreting cells. J Exp Med. 2001;194(8):1069–80. 10.1084/jem.194.8.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mashayekhi Goyonlo V, Elnour H, Nordlind K. Interleukin-2 expression in lupoid and usual types of old world cutaneous leishmaniasis. Iran Red Crescent Med J. 2014;16(11):e5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oliveira PR, Dessein H, Romano A, Cabantous S, de Brito ME, Santoro F, et al. IL2RA genetic variants reduce IL-2-dependent responses and aggravate human cutaneous leishmaniasis. J Immunol. 2015;194(6):2664–72. 10.4049/jimmunol.1402047 [DOI] [PubMed] [Google Scholar]
- 69.Espir TT, de Paula Figueira L, de Farias Naiff M, da Costa AG, Ramalho-Ortigão M, Malheiro A, et al. The role of inflammatory, anti-inflammatory, and regulatory cytokines in patients infected with cutaneous leishmaniasis in amazonas state, Brazil. J Immunol Res. 2014;2014:10. doi: 10.1155/2014/481750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Divanovic S, Trompette A, Ashworth JI, Rao MB, Karp CL. Therapeutic enhancement of protective immunity during experimental leishmaniasis. PLoS Negl Trop Dis. 2011;5(9). doi: 10.1371/journal.pntd.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman F, Paul W. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172(3):921–9. 10.1084/jem.172.3.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kondo M, Takeshita T, Ishii N, Nakamura M, Watanabe S, Arai K, et al. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993;262(5141):1874–77. 10.1126/science.8266076 [DOI] [PubMed] [Google Scholar]
- 73.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993;262(5141):1880–3. 10.1126/science.8266078 [DOI] [PubMed] [Google Scholar]
- 74.Heinzel FP, Rerko RM, Hatam F, Locksley RM. IL-2 is necessary for the progression of leishmaniasis in susceptible murine hosts. J Immunol. 1993;150(9):3924–31. [PubMed] [Google Scholar]
- 75.Apte RN, Voronov E. Is interleukin-1 a good or bad ‘guy’ in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222(1):222–41. 10.1111/j.1600-065X.2008.00615.x [DOI] [PubMed] [Google Scholar]
- 76.Auron PE. The interleukin 1 receptor: ligand interactions and signal transduction. Cytokine Growth Factor Rev. 1998;9(3–4):221–37. 10.1016/S1359-6101(98)00018-5 [DOI] [PubMed] [Google Scholar]
- 77.Mantovani A, Muzio M, Ghezzi P, Colotta C, Introna M. Regulation of inhibitory pathways of the interleukin-1 system. Ann N Y Acad Sci. 1998;840:338–51. 10.1111/nyas.1998.840.issue-1 [DOI] [PubMed] [Google Scholar]
- 78.Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075–79. 10.1016/S1357-2725(98)00081-8 [DOI] [PubMed] [Google Scholar]
- 79.Charmoy M, Hurrell BP, Romano A, Lee SH, Ribeiro-Gomes F, Riteau N, et al. The Nlrp3 inflammasome, IL-1beta, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur J Immunol. 2016;46(4):897–911. 10.1002/eji.201546015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early Th17 cell differentiation by IL-1 signaling. Immunity. 2009;30(4):576–87. 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva AL, Mineo TW, et al. Inflammasome-derived IL-1beta production induces nitric oxide-mediated resistance to Leishmania. Nat Med. 2013;19(7):909–15. 10.1038/nm.3221 [DOI] [PubMed] [Google Scholar]
- 82.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159(2):591–8. [PubMed] [Google Scholar]
- 83.Kostka SL, Knop J, Konur A, Udey MC, von Stebut E. Distinct roles for IL-1 receptor type I Signaling in early versus established Leishmania major infections. J Invest Dermatol. 2006;126(7):1582–9. 10.1038/sj.jid.5700309 [DOI] [PubMed] [Google Scholar]
- 84.Gurung P, Karki R, Vogel P, Watanabe M, Bix M, Lamkanfi M, et al. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest. 2015;125(3):1329–38. 10.1172/JCI79526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonzalez-Lombana C, Gimblet C, Bacellar O, Oliveira WW, Passos S, Carvalho LP, et al. IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection. PLoS Pathog. 2013;9(3):e1003243. 10.1371/journal.ppat.1003243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Voronov E, Dotan S, Gayvoronsky L, White RM, Cohen I, Krelin Y, et al. IL-1-induced inflammation promotes development of leishmaniasis in susceptible BALB/c mice. Int Immunol. 2010;22(4):245–57. 10.1093/intimm/dxq006 [DOI] [PubMed] [Google Scholar]
- 87.Kautz-Neu K, Kostka SL, Dinges S, Iwakura Y, Udey MC, von Stebut E. IL-1 signalling is dispensable for protective immunity in Leishmania-resistant mice. Exp Dermatol. 2011;20(1):76–8. 10.1111/j.1600-0625.2010.01172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ikejima T, Okusawa S, Ghezzi P, Van der Meer JW, Dinarello CA. Interleukin-1 induces tumor necrosis factor (TNF) in human peripheral blood mononuclear cells in vitro and a circulating TNF-like activity in rabbits. J Infect Dis. 1990;162(1):215–23. 10.1093/infdis/162.1.215 [DOI] [PubMed] [Google Scholar]
- 89.Tsutsui H, Adachi K, Seki E, Nakanishi K. Cytokine-induced inflammatory liver injuries. Curr Mol Med. 2003;3(6):545–59. 10.2174/1566524033479618 [DOI] [PubMed] [Google Scholar]
- 90.Dinarello CA, Fantuzzi G. Interleukin‐18 and host defense against infection. J Infect Dis. 2003;187(s2):S370–84. 10.1086/jid.2003.187.issue-s2 [DOI] [PubMed] [Google Scholar]
- 91.Wei XQ, Leung BP, Niedbala W, Piedrafita D, Feng GJ, Sweet M, et al. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J Immunol. 1999;163(5):2821–8. [PubMed] [Google Scholar]
- 92.Monteforte GM, Takeda K, Rodriguez-Sosa M, Akira S, David JR, Satoskar AR. Genetically resistant mice lacking IL-18 gene develop Th1 response and control cutaneous Leishmania major infection. J Immunol. 2000;164(11):5890–3. 10.4049/jimmunol.164.11.5890 [DOI] [PubMed] [Google Scholar]
- 93.Xu D, Trajkovic V, Hunter D, Leung BP, Schulz K, Gracie JA, et al. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol. 2000;30(11):3147–56. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 94.Gurung P, Kanneganti T-D. Novel role for inflammasome-dependent IL-18 in promoting Th2 responses during Leishmania infections (IRC7P.420). J Immunol. 2015;194(1 Supplement):128. [Google Scholar]
- 95.Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev. 2002;13(6):429–39. 10.1016/S1359-6101(02)00029-1 [DOI] [PubMed] [Google Scholar]
- 96.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–8. 10.1126/science.8178155 [DOI] [PubMed] [Google Scholar]
- 97.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. 10.1182/blood.V97.1.14 [DOI] [PubMed] [Google Scholar]
- 98.Jullien D, Sieling PA, Uyemura K, Mar ND, Rea TH, Modlin RL. IL-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158(2):800–06. [PubMed] [Google Scholar]
- 99.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, et al. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest. 1995;96(6):2578–82. 10.1172/JCI118321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. 10.1146/annurev.immunol.17.1.19 [DOI] [PubMed] [Google Scholar]
- 101.Lauwerys BR, Garot N, Renauld J-C, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165(4):1847–53. 10.4049/jimmunol.165.4.1847 [DOI] [PubMed] [Google Scholar]
- 102.Loza MJ, Zamai L, Azzoni L, Rosati E, Perussia B. Expression of type 1 (interferon gamma) and type 2 (interleukin-13, interleukin-5) cytokines at distinct stages of natural killer cell differentiation from progenitor cells. Blood. 2002;99(4):1273–81. 10.1182/blood.V99.4.1273 [DOI] [PubMed] [Google Scholar]
- 103.D’Agostino P, Milano S, Arcoleo F, Di Bella G, La Rosa M, Ferlazzo V, et al. Interleukin-15, as interferon-gamma, induces the killing of leishmania infantum in phorbol-myristate-acetate-activated macrophages increasing interleukin-12. Scand J Immunol. 2004;60(6):609–14. 10.1111/sji.2004.60.issue-6 [DOI] [PubMed] [Google Scholar]
- 104.Milano S, Bella G, D’agostino P, Barbera C, Caruso R, Rosa M, et al. IL-15 in human visceral leishmaniasis caused by Leishmania infantum. Clin Exp Immunol. 2002;127(2):360–5. 10.1046/j.1365-2249.2002.01749.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hammond ME, Lapointe GR, Feucht PH, Hilt S, Gallegos CA, Gordon CA, et al. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J Immunol. 1995;155(3):1428–33. [PubMed] [Google Scholar]
- 106.Badolato R, Sacks DL, Savoia D, Musso T. Leishmania major:infection of human monocytes induces expression of IL-8 and MCAF. Exp Parasitol. 1996;82(1):21–6. 10.1006/expr.1996.0003 [DOI] [PubMed] [Google Scholar]
- 107.Müller K, Zandbergen G, Hansen B, Laufs H, Jahnke N, Solbach W, et al. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med Microbiol Immunol. 2001;190(1–2):73–6. 10.1007/s004300100084 [DOI] [PubMed] [Google Scholar]
- 108.Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceicao-Silva F, et al. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci USA. 2009;106(16):6748–53. 10.1073/pnas.0900226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rochael NC, Guimarães-Costa AB, Nascimento MT, DeSouza-Vieira TS, Oliveira MP, Garcia e Souza LF, et al. Classical ROS-dependent and early/rapid ROS-independent release of Neutrophil Extracellular Traps triggered by Leishmania parasites. Sci Rep. 2015;5:18302. 10.1038/srep18302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Novais FO, Santiago RC, Bafica A, Khouri R, Afonso L, Borges VM, et al. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J Immunol. 2009;183(12):8088–98. 10.4049/jimmunol.0803720 [DOI] [PubMed] [Google Scholar]
- 111.Carmo ÉVdS, Katz S, Barbiéri CL. Neutrophils reduce the parasite burden in Leishmania (Leishmania) amazonensis-infected macrophages. PLoS ONE. 2010;5(11):e13815. 10.1371/journal.pone.0013815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, et al. In vivo imaging reveals an essential role for neutrophils in Leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–4. 10.1126/science.1159194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged ‘silent’ phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165(2):969–77. 10.4049/jimmunol.165.2.969 [DOI] [PubMed] [Google Scholar]
- 114.Laufs H, Muller K, Fleischer J, Reiling N, Jahnke N, Jensenius JC, et al. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect Immun. 2002;70(2):826–35. 10.1128/IAI.70.2.826-835.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. 10.1034/j.1600-065X.2000.17706.x [DOI] [PubMed] [Google Scholar]
- 116.Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, et al. Neutrophil-Derived CCL3 Is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010;6(2):e1000755. 10.1371/journal.ppat.1000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ribeiro-Gomes FL, Sacks D. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front Cell Infect Microbiol. 2012;2:59. doi: 10.3389/fcimb.2012.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ribeiro-Gomes FL, Romano A, Lee S, Roffê E, Peters NC, Debrabant A, et al. Apoptotic cell clearance of Leishmania major-infected neutrophils by dendritic cells inhibits CD8(+) T-cell priming in vitro by Mer tyrosine kinase-dependent signaling. Cell Death Dis. 2015;6:e2018. 10.1038/cddis.2015.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, et al. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173(11):6521–5. 10.4049/jimmunol.173.11.6521 [DOI] [PubMed] [Google Scholar]
- 120.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–76. 10.1016/j.immuni.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 121.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28(4):445–53. 10.1016/j.immuni.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 122.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–7. 10.1038/ni1539 [DOI] [PubMed] [Google Scholar]
- 123.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41. 10.1038/ni1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. 10.1038/ni1254 [DOI] [PubMed] [Google Scholar]
- 125.Sutton C, Brereton C, Keogh B, Mills KHG, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–91. 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–61. 10.1084/jem.20070663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GK, Kolls JK, et al. A functional IL-13 receptor Is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009;182(9):5317–21. 10.4049/jimmunol.0803868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–36. 10.1038/ni1375 [DOI] [PubMed] [Google Scholar]
- 129.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–45. 10.1038/ni1376 [DOI] [PubMed] [Google Scholar]
- 130.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204(1):161–70. 10.1084/jem.20061738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lopez Kostka S, Dinges S, Griewank K, Iwakura Y, Udey MC, Von Stebut E. IL-17 promotes progression of cutaneous Leishmaniasis in susceptible mice. J Immunol. 2009;182(5):3039–46. 10.4049/jimmunol.0713598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bacellar O, Faria D, Nascimento M, Cardoso TM, Gollob KJ, Dutra WO, et al. Interleukin 17 production among patients with American cutaneous Leishmaniasis. J Infect Dis. 2009;200(1):75–8. 10.1086/599173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peters NC, Bertholet S, Lawyer PG, Charmoy M, Romano A, Ribeiro-Gomes FL, et al. Evaluation of recombinant Leishmania polyprotein plus glucopyranosyl lipid a stable emulsion vaccines against sand fly-transmitted Leishmania major in C57BL/6 mice. J Immunol. 2012;189(10):4832–41. 10.4049/jimmunol.1201676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banerjee A, Bhattacharya P, Joshi AB, Ismail N, Dey R, Nakhasi HL. Role of pro-inflammatory cytokine IL-17 in Leishmania pathogenesis and in protective immunity by Leishmania vaccines. Cell Immunol. 2016. doi: 10.1016/j.cellimm.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 135.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13(1):21–38. [DOI] [PubMed] [Google Scholar]
- 136.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252(1):116–32. 10.1111/imr.12027 [DOI] [PubMed] [Google Scholar]
- 137.Gimblet C, Loesche MA, Carvalho L, Carvalho EM, Grice EA, Artis D, et al. IL-22 protects against tissue damage during cutaneous Leishmaniasis. PLOS ONE. 2015;10(8):e0134698. 10.1371/journal.pone.0134698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hezarjaribi HZ, Ghaffarifar F, Dalimi A, Sharifi Z. Evaluation of protective effect of IL-22 and IL-12 on cutaneous leishmaniasis in BALB/c mice. Asian Pacific J Trop Med. 2014;7(12):940–5. 10.1016/S1995-7645(14)60166-8 [DOI] [PubMed] [Google Scholar]
- 139.Hezarjaribi HZ, Ghaffarifar F, Dalimi A, Sharifi Z, Jorjani O. Effect of IL-22 on DNA vaccine encoding LACK gene of Leishmania major in BALB/c mice. Exp Parasitol. 2013;134(3):341–8. 10.1016/j.exppara.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 140.Brosch S, Dietze-Schwonberg K, Lopez Kostka S, Lorenz B, Haak S, Becher B, et al. Disease control in cutaneous Leishmaniasis is independent of IL-22. J Invest Dermatol. 2015;135(1):308–11. 10.1038/jid.2014.282 [DOI] [PubMed] [Google Scholar]
- 141.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA). Mol Cell Res. 2011;1813(5):878–88. [DOI] [PubMed] [Google Scholar]
- 142.Titus RG, DeKrey GK, Morris RV, Soares MBP. Interleukin-6 deficiency influences cytokine expression in susceptible BALB mice infected with Leishmania major but does not alter the outcome of disease. Infect Immune. 2001;69(8):5189–92. 10.1128/IAI.69.8.5189-5192.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saha B, Saini A, Germond R, Perrin PJ, Harlan DM, Davis TA. Susceptibility or resistance to Leishmania infection is dictated by the macrophages evolved under the influence of IL-3 or GM-CSF. Eur J Immunol. 1999;29(7):2319–29. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 144.Moskowitz NH, Brown DR, Reiner SL. Efficient immunity against Leishmania major in the absence of interleukin-6. Infect Immun. 1997;65(6):2448–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hatzigeorgiou DE, He S, Sobel J, Grabstein KH, Hafner A, Ho J. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J Immunol. 1993;151(7):3682–92. [PubMed] [Google Scholar]
- 146.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. 10.1146/annurev.immunol.22.012703.104758 [DOI] [PubMed] [Google Scholar]
- 147.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev. 2004;202(1):106–14. 10.1111/imr.2004.202.issue-1 [DOI] [PubMed] [Google Scholar]
- 148.Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol. 2004;173(2):715–20. 10.4049/jimmunol.173.2.715 [DOI] [PubMed] [Google Scholar]
- 149.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, et al. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177(8):5377–85. [DOI] [PubMed] [Google Scholar]
- 150.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183(4):2435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, et al. WSX-1 Is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15(4):569–78. 10.1016/S1074-7613(01)00206-0 [DOI] [PubMed] [Google Scholar]
- 152.Artis D, Johnson LM, Joyce K, Saris C, Villarino A, Hunter CA, et al. Cutting edge: early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J Immunol. 2004;172(8):4672–5. 10.4049/jimmunol.172.8.4672 [DOI] [PubMed] [Google Scholar]
- 153.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420(6915):502–7. 10.1038/nature01152 [DOI] [PubMed] [Google Scholar]
- 154.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- 155.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–81. 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- 156.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2(9):816–22. 10.1038/ni0901-816 [DOI] [PubMed] [Google Scholar]
- 157.O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7(6):425–8. 10.1038/nri2097 [DOI] [PubMed] [Google Scholar]
- 158.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204(2):239–243. 10.1084/jem.20070104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O’Garra A, Vieira P. TH1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7(6):425–8. 10.1038/nri2097 [DOI] [PubMed] [Google Scholar]
- 160.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of Anti–IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194(10):1497–1506. 10.1084/jem.194.10.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Anderson CF, Mendez S, Sacks DL. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J Immunol. 2005;174(5):2934–41. 10.4049/jimmunol.174.5.2934 [DOI] [PubMed] [Google Scholar]
- 162.Castellano LR, Filho DC, Argiro L, Dessein H, Prata A, Dessein A, et al. Th1/Th2 immune responses are associated with active cutaneous leishmaniasis and clinical cure is associated with strong interferon-gamma production. Hum Immunol. 2009;70(6):383–390. 10.1016/j.humimm.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 163.Salhi A, Rodrigues V Jr, Santoro F, Dessein H, Romano A, Castellano LR, et al. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol. 2008;180(9):6139–48. 10.4049/jimmunol.180.9.6139 [DOI] [PubMed] [Google Scholar]
- 164.Buxbaum LU, Scott P. Interleukin 10- and Fcγ receptor-deficient mice resolve Leishmania mexicana lesions. Infect Immune. 2005;73(4):2101–08. 10.1128/IAI.73.4.2101-2108.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J Immunol. 2005;175(4):2517–24. 10.4049/jimmunol.175.4.2517 [DOI] [PubMed] [Google Scholar]
- 166.Darrah PA, Hegde ST, Patel DT, Lindsay RW, Chen L, Roederer M, et al. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med. 2010;207(7):1421–33. 10.1084/jem.20092532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gomes-Silva A, De Cássia Bittar R, Dos Santos Nogueira R, Amato VS, Da Silva Mattos M, Oliveira-Neto MP, et al. Can interferon-γ and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol. 2007;149(3):440–4. 10.1111/j.1365-2249.2007.03436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. 10.1146/annurev.immunol.21.120601.141122 [DOI] [PubMed] [Google Scholar]
- 169.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194(5):629–44. 10.1084/jem.194.5.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wan YY, Flavell RA. ‘Yin-Yang’ functions of TGF-β and Tregs in immune regulation. Immunol Rev. 2007;220:199–213. 10.1111/imr.2007.220.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2(6):389–400. [DOI] [PubMed] [Google Scholar]
- 172.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172(10):5986–93. 10.4049/jimmunol.172.10.5986 [DOI] [PubMed] [Google Scholar]
- 173.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. 10.1034/j.1600-065X.2001.1820105.x [DOI] [PubMed] [Google Scholar]
- 174.Stassen M, Fondel S, Bopp T, Richter C, Müller C, Kubach J, et al. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34(5):1303–11. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 175.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. 10.1034/j.1600-065X.2001.1820117.x [DOI] [PubMed] [Google Scholar]
- 176.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204(2):285–97. 10.1084/jem.20061886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Castellano LR, Argiro L, Dessein H, Dessein A, da Silva MV, Correia D, et al. Potential use of interleukin-10 blockade as a therapeutic strategy in human cutaneous Leishmaniasis. J Immunol Res. 2015;2015:5, Article ID 152741. doi: 10.1155/2015/152741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kopf M, Gros G, Bachmann M, Lamers M, Bluethmann H, Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362(6417):245–8. 10.1038/362245a0 [DOI] [PubMed] [Google Scholar]
- 179.Zamorano J, Rivas M, Perez G. Interleukin-4: a multifunctional cytokine. Immunologia. 2003;22(2):215–24. [Google Scholar]
- 180.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77(9):1859–70. [PubMed] [Google Scholar]
- 181.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. 10.1038/nri978 [DOI] [PubMed] [Google Scholar]
- 182.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185(5):817–24. 10.1084/jem.185.5.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lazarski CA, Ford J, Katzman SD, Rosenberg AF, Fowell DJ. IL-4 attenuates Th1-associated chemokine expression and Th1 trafficking to inflamed tissues and limits pathogen clearance. PLoS ONE. 2013;8(8):e71949. 10.1371/journal.pone.0071949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. 10.1016/j.immuni.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 185.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160(11):5347–54. [PubMed] [Google Scholar]
- 186.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163(7):3771–7. [PubMed] [Google Scholar]
- 187.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by Type 1/Type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167(11):6533–44. 10.4049/jimmunol.167.11.6533 [DOI] [PubMed] [Google Scholar]
- 188.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann KH, Lefrang K, et al. IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med. 1996;184(3):1127–36. 10.1084/jem.184.3.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Radwanska M, Cutler AJ, Hoving JC, Magez S, Holscher C, Bohms A, et al. Deletion of IL-4ralpha on CD4 T cells renders BALB/c mice resistant to Leishmania major infection. PLoS Pathog. 2007;3(5):e68. 10.1371/journal.ppat.0030068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;171(1):115–27. 10.1084/jem.171.1.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169(1):59–72. 10.1084/jem.169.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Noben-Trauth N, Paul WE, Sacks DL. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J Immunol. 1999;162(10):6132–6140. [PubMed] [Google Scholar]
- 193.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major Infection in Interleukin-4-deficient mice. Science. 1996;271(5251):987–90. 10.1126/science.271.5251.987 [DOI] [PubMed] [Google Scholar]
- 194.Alexander J, Brombacher F, McGachy HA, McKenzie AN, Walker W, Carter KC. An essential role for IL-13 in maintaining a non-healing response following Leishmania mexicana infection. Eur J Immunol. 2002;32(10):2923–33. [DOI] [PubMed] [Google Scholar]
- 195.Scott P, Eaton A, Gause WC, di Zhou X, Hondowicz B. Early IL-4 production does not predict susceptibility to Leishmania major. Exp Parasitol. 1996;84(2):178–87. 10.1006/expr.1996.0103 [DOI] [PubMed] [Google Scholar]
- 196.Kropf P, Herath S, Weber V, Modolell M, Muller I. Factors influencing Leishmania major infection in IL-4-deficient BALB/c mice. Parasite Immunol. 2003;25(8–9):439–47. 10.1111/pim.2003.25.issue-8-9 [DOI] [PubMed] [Google Scholar]
- 197.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17(1–4):1–52. 10.3109/08830189809084486 [DOI] [PubMed] [Google Scholar]
- 198.de Waal Malefyt R, Figdor CG, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151(11):6370–81. [PubMed] [Google Scholar]
- 199.Doherty TM, Kastelein R, Menon S, Andrade S, Coffman RL. Modulation of murine macrophage function by IL-13. J Immunol. 1993;151(12):7151–60. [PubMed] [Google Scholar]
- 200.Oswald IP, Gazzinelli RT, Sher A, James SL. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992;148(11):3578–82. [PubMed] [Google Scholar]
- 201.Doherty TM, Kastelein R, Menon S, Andrade S, Coffman RL. Modulation of murine macrophage function by IL-13. J Immunol. 1993;151(12):7151–60. [PubMed] [Google Scholar]
- 202.Matthews DJ, Emson CL, McKenzie GJ, Jolin HE, Blackwell JM, McKenzie AN. IL-13 Is a susceptibility factor for Leishmania major infection. J Immunol. 2000;164(3):1458–62. 10.4049/jimmunol.164.3.1458 [DOI] [PubMed] [Google Scholar]
- 203.Saura M, Martinez-Dalmau R, Minty A, Perez-Sala D, Lamas S. Interleukin-13 inhibits inducible nitric oxide synthase expression in human mesangial cells. Biochem J. 1996;313(2):641–6. 10.1042/bj3130641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bourreau E, Prévot G, Pradinaud R, Launois P. Interleukin (IL)–13 Is the predominant Th2 cytokine in localized cutaneous Leishmaniasis lesions and renders specific CD4+ T cells unresponsive to IL-12. J Infect Dis. 2001;183(6):953–9. 10.1086/jid.2001.183.issue-6 [DOI] [PubMed] [Google Scholar]
- 205.Sosa MR, Rosas LE, McKenzie AN, Satoskar AR. IL-13 gene-deficient mice are susceptible to cutaneous L. mexicana infection. Eur J Immunol. 2001;31(11):3255–60. 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 206.Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, et al. CD4+ T helper cells engineered to produce latent TGF-β1 reverse allergen-induced airway hyperreactivity and inflammation. J Clin Invest. 2000; 105(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Grotendorst GR, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol. 1989;140(2):396–402. 10.1002/(ISSN)1097-4652 [DOI] [PubMed] [Google Scholar]
- 208.Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3+ regulatory T cells. J Cell Mol Biol. 2012; 4(1): 29–37. [DOI] [PubMed] [Google Scholar]
- 209.Gorelik L, Flavell RA. Transforming growth factor-[beta] in T-cell biology. Nat Rev Immunol. 2002;2(1):46–53. 10.1038/nri704 [DOI] [PubMed] [Google Scholar]
- 210.Speck S, Lim J, Shelake S, Matka M, Stoddard J, Farr A, et al. TGF-β signaling initiated in dendritic cells instructs suppressive effects on Th17 differentiation at the site of neuroinflammation. PLoS ONE. 2014;9(7):e102390. 10.1371/journal.pone.0102390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Huber S, Schramm C. TGF-beta and CD4+CD25+ regulatory T cells. Front Biosci. 2006;11:1014–23. 10.2741/1859 [DOI] [PubMed] [Google Scholar]
- 212.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 – precursors. Am J Transplant. 2004;4(10):1614–27. 10.1111/ajt.2004.4.issue-10 [DOI] [PubMed] [Google Scholar]
- 213.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6(6):600–07. 10.1038/ni1197 [DOI] [PubMed] [Google Scholar]
- 214.Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor beta II receptor. J Exp Med. 2000;191(7):1187–96. 10.1084/jem.191.7.1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, Ra C, et al. Blockade of transforming growth factor beta/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000;192(2):151–8. 10.1084/jem.192.2.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000;165(9):4773–7. 10.4049/jimmunol.165.9.4773 [DOI] [PubMed] [Google Scholar]
- 217.Sad S, Mosmann TR. Single IL-2-secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J Immunol. 1994;153(8):3514–22. [PubMed] [Google Scholar]
- 218.Wahl SM, Hunt DA, Wong HL, Dougherty S, McCartney-Francis N, Wahl LM, et al. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988;140(9):3026–32. [PubMed] [Google Scholar]
- 219.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163(5):1037–50. 10.1084/jem.163.5.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86. 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–9. 10.1038/359693a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25(3):455–71. 10.1016/j.immuni.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 223.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–7. 10.1084/jem.20042276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Barral A, Teixeira M, Reis P, Vinhas V, Costa J, Lessa H, et al. Transforming growth factor-beta in human cutaneous leishmaniasis. Am J Path. 1995;147(4):947–54. [PMC free article] [PubMed] [Google Scholar]
- 225.Kedzierski L, Evans KJ. Immune responses during cutaneous and visceral leishmaniasis. Parasitology. 2014;141(12):1–19. [DOI] [PubMed] [Google Scholar]
- 226.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195(11):1499–505. 10.1084/jem.20012076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Barral A, Teixeira M, Reis P, Vinhas V, Costa J, Lessa H, et al. Transforming growth factor-beta in human cutaneous leishmaniasis. Am J Pathol. 1995;147(4):947–54. [PMC free article] [PubMed] [Google Scholar]
- 228.Barral-Netto M, Barral A. Transforming growth factor-beta in tegumentary leishmaniasis. Braz J Med Biol Res. 1994;27(1):1–9. [PubMed] [Google Scholar]
- 229.Barral A, Barral-Netto M, Yong EC, Brownell CE, Twardzik DR, Reed SG. Transforming growth factor beta as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci U S A. 1993;90(8):3442–6. 10.1073/pnas.90.8.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Li J, Hunter CA, Farrell JP. Anti-TGF-beta treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J Immunol. 1999;162(2):974–9. [PubMed] [Google Scholar]
- 231.Melby PC, Andrade-Narvaez FJ, Darnell BJ, Valencia-Pacheco G, Tryon VV, Palomo-Cetina A. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect. Immun. 1994;62(3):837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Diaz NL, Zerpa O, Ponce LV, Convit J, Rondon AJ, Tapia FJ. Intermediate or chronic cutaneous leishmaniasis: leukocyte immunophenotypes and cytokine characterisation of the lesion. Exp Dermatol. 2002;11(1):34–41. 10.1034/j.1600-0625.2002.110104.x [DOI] [PubMed] [Google Scholar]
- 233.Hejazi S, Hoseini S, Javanmard S, Zarkesh S, Khamesipour A. Interleukin-10 and transforming growth factor-beta in early and late lesions of patients with Leishmania major induced cutaneous Leishmaniasis. Iran J Parasitol. 2012;7(2):53–60. [PMC free article] [PubMed] [Google Scholar]
- 234.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334(6179):260–2. 10.1038/334260a0 [DOI] [PubMed] [Google Scholar]
- 235.Takaki H, Minoda Y, Koga K, Takaesu G, Yoshimura A, Kobayashi T. TGF-β1 suppresses IFN-γ-induced NO production in macrophages by suppressing STAT1 activation and accelerating iNOS protein degradation. Gene Cell. 2006;11(8):871–82. 10.1111/gtc.2006.11.issue-8 [DOI] [PubMed] [Google Scholar]
- 236.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, et al. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat Immunol. 2008;9(6):650–7. 10.1038/ni.1613 [DOI] [PubMed] [Google Scholar]
- 237.Nylén S, Eidsmo L. Tissue damage and immunity in cutaneous leishmaniasis. Parasit Immunol. 2012;34(12):551–61. 10.1111/pim.12007 [DOI] [PubMed] [Google Scholar]