Abstract
Background
False-negative rates (FNR) of sentinel node biopsy (SNB) after neoadjuvant chemotherapy (NAC) in node-positive (N+) breast cancer patients are < 10% when ≥ 3 negative SNs are obtained. Marking positive nodes has been suggested to reduce FNR. Identification of treatment effect in the nodes post-NAC is an alternative to decrease FNR. We evaluated the frequency of treatment effect in N+ patients after a pathologic complete response (pCR) with NAC.
Methods
Biopsy-proven N+ patients receiving NAC were identified. Patients with nodal pCR after ALND or SNB with dual mapping and ≥ 3 SNs removed were evaluated for treatment effect; ALND and SNB patients were compared.
Results
From 01/09–12/15, 528 N+ patients received NAC. Of these, 204 had a nodal pCR; 135 had an ALND and 69 had SNB. Median age was 49yrs, 15% were hormone receptor positive (HR+)/HER2-, 27% triple negative, and 58% HER2+. The median number of nodes removed in ALND patients was 17 versus 4 in SNB patients. Treatment effect in nodes was identified in 192 (94%) patients, and was more common in ALND versus SNB patients (97% versus 88%, p=0.02). HR+ patients and patients without a breast pCR were less likely to have treatment effect in the nodes (p=0.05). Other characteristics did not differ.
Conclusions
Following NAC, SNs with treatment effect were retrieved in 88% of patients without marking nodes, suggesting that nodal clipping may not be necessary to achieve an acceptable FNR. Longer follow-up is needed to determine regional recurrence rates in the SN-only cohort.
Keywords: neoadjuvant chemotherapy, treatment effect, pathologic complete response, sentinel node biopsy, axillary lymph node dissection
Introduction
Neoadjuvant chemotherapy (NAC), initially used for the treatment of women with locally advanced breast cancer, is also beneficial in women with operable disease. In addition to reducing the size of the primary breast tumor, allowing breast conservation instead of mastectomy, NAC can also eradicate disease in the axillary lymph nodes, reducing the need for axillary dissection. Approximately 40% of node-positive (N+) breast cancer patients achieve a nodal pathologic complete response (pCR) with preoperative therapy1,2, indicating that a substantial number might benefit from this approach.
Three recently published prospective studies have demonstrated that sentinel node biopsy (SNB) after NAC in patients initially presenting with N+ disease is accurate, with false-negative rates of < 10%, provided that ≥ 3 negative sentinel nodes (SNs) are obtained.1,3,4 As the clinical effects of a false-negative event in this population are unknown, some have suggested marking the positive lymph node with a clip at the time of percutaneous biopsy to ensure its removal post-NAC, as a method to decrease false-negative rates, particularly when only 2 negative SNs are obtained.5-7 Among patients enrolled in the American College of Surgeons Oncology Group (ACOSOG) Z1071 trial with cN1 disease and at least 2 SNs removed, the false-negative rate of SNB was 6.8% when the clipped node was one of the SNs identified, compared to 13.4% when no clip was placed.5 However, the benefit of clip placement when 3 or more negative SNs are obtained is uncertain.
Another method to document retrieval of a positive node at presentation after NAC is to identify histologic changes of treatment effect in the node. Removal of lymph nodes with treatment effect would similarly indicate that an initially positive node has been removed, without needing to mark the node. It is not known whether all positive nodes that achieve nodal pCR exhibit histologic evidence of treatment effect after NAC. Therefore, we sought to determine the frequency with which N+ patients demonstrate treatment effect in the lymph nodes after a nodal pCR with NAC, and to compare rates of treatment effect after axillary lymph node dissection (ALND) and SNB performed without nodal marking.
Methods
After institutional review board approval, patients with biopsy-proven N+ breast cancer treated with NAC between January 2009 and December 2015 were identified from a prospectively maintained database. Patients with a nodal pCR after ALND or SNB represented our study cohort; these patients were evaluated for treatment effect in the nodes.
Until 2014, ALND was considered routine for all initially N+ patients after NAC. After 2014, SNB was performed for N+ patients who converted to clinically node negative, as determined by physical exam at the completion of NAC. Clips were not placed in the nodes at the time of histologic confirmation of metastases. All initially N+ patients undergoing SNB after NAC had lymphatic mapping with dual tracer using technetium-99m sulfur colloid and isosulfan blue dye with retrieval of at least 3 SNs. Abnormal nodes palpated at the time of SNB were also considered SNs. ALND in ypN0 patients was indicated for failed SN mapping, retrieval of fewer than 3 SNs, or for patients considered SNB-ineligible, such as those presenting with cT4 or cN2/N3 disease.
SNs were serially sectioned at 2 mm intervals along the major axis of the lymph node at the time of gross examination. A single hematoxylin and eosin (H&E) stained section was prepared from each block. Immunohistochemistry was not routinely performed for lymph node evaluation post-NAC.
Information regarding treatment effect in ALND and SNB patients was obtained from the original pathology report. Identification of treatment effect was not assessed intra-operatively, but was included on the final pathology report. Cases were re-reviewed by a dedicated breast pathologist (M.E.) if there was no mention of treatment effect (n = 16) or if treatment effect was not identified in the nodes (n = 11) on the original pathology report. In SNB patients, the absence of treatment effect in the SNs was not an indication for ALND.
Histologic evidence of treatment effect was defined as either “classic” or “subtle”.8 Lymph nodes with classic treatment effect had fibrosis, a foamy histiocytic infiltrate, hemosiderin laden macrophages, and increased vascularity (FIG. 1a). Nodes with subtle treatment effect had focal fibrosis and a thickened lymph node capsule with associated increased vascularity (FIG. 1b).
Fig. 1.
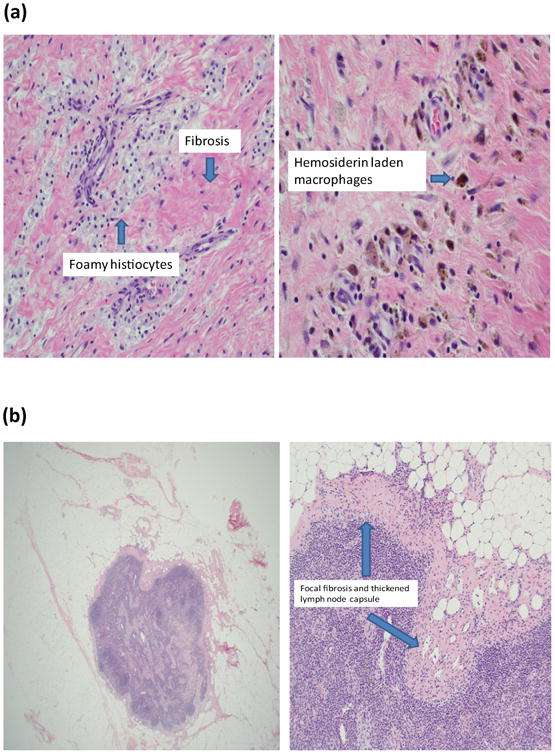
(a) Classic treatment effect and (b) subtle treatment effect.
Patient and treatment characteristics were summarized by treatment effect group (yes versus no, and classic versus subtle versus no) using median and range (continuous variables), and frequency and percent (categorical variables); differences were tested with Kruskal Wallis tests and Fisher's exact tests. All statistical analysis was done in R 3.1.1 (R Foundation, Vienna, Austria).
Results
Clinical Characteristics
From January 2009 to December 2015, 528 biopsy-proven N+ patients received NAC followed by axillary surgery. Of these, 204 were ypN0 after NAC and represent our study cohort; 135 had an ALND and 69 had an SNB. Clinical characteristics of the study cohort are depicted in Table 1. Invasive ductal histology was present in 99% of patients, 58% were HER2+, and 83% presented with cN1 disease. Breast pCR, defined as the absence of invasive disease in the breast (in situ allowed), occurred in 70% of the 202 ypN0 patients who had breast surgery. When breast pCR was defined as the absence of invasive and in situ disease, 96 patients (48%) had a breast pCR.
Table 1.
Clinical characteristics of the study cohort
| Participant Characteristics | Total (n = 204) | ||
|---|---|---|---|
|
| |||
| Median | Range | ||
|
|
|||
| Age (years) | 49 | 25–85 | |
|
| |||
| n | % | ||
|
| |||
| Histology | Ductal | 203 | 99 |
|
| |||
| Tumor subtype | HR+/HER2- | 30 | 15 |
| Triple negative | 55 | 27 | |
| HER2+ | 119 | 58 | |
|
| |||
| cT | T0*/1 | 24 | 12 |
| T2/3 | 142 | 70 | |
| T4 | 38 | 18 | |
|
| |||
| cN | N1 | 170 | 83 |
| N2 | 27 | 13 | |
| N3 | 7 | 3 | |
|
| |||
| Axillary surgery | ALND† | 135 | 66 |
| SNB | 69 | 34 | |
3 patients presented with an occult primary breast cancer and were classified as cT0
5 ALND patients had attempted SNB; 1 failed to map and 4 had <3 SNs retrieved
HR, hormone receptor; cT, clinical tumor stage; cN, clinical nodal stage; ALND, axillary lymph node dissection; SNB, sentinel node biopsy
Frequency of Treatment Effect in N+ Patients with Nodal pCR
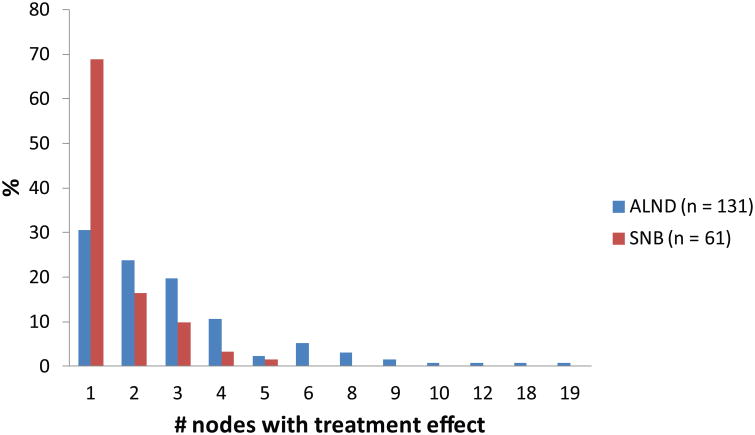
The median number of nodes removed in the ALND patients was 17 (range, 5–46) compared to 4 (range, 3–13) in SNB patients (p < 0.001). Overall, treatment effect was identified in the lymph nodes in 192 patients, or 94% of the total cohort; 185 patients had classic and 7 had subtle treatment changes. Treatment effect in the nodes was more frequently identified in patients undergoing ALND (97%) compared to SNB (88%) (p = 0.02). FIG. 2 demonstrates the distribution of nodes with treatment effect among ALND patients compared to SNB patients (median nodes with treatment effect: 2 versus 1, respectively; p < 0.001). Treatment effect in 4 or more nodes was an uncommon finding, seen in 19% of those with treatment effect, and patients with ≥ 4 nodes with treatment effect were more likely to present with clinical stage 3 versus stage 2 disease (p = 0.005).
Fig. 2.
Distribution of number of nodes with treatment effect in axillary lymph node dissection and sentinel lymph node biopsy patients with treatment effect (n = 192).
ALND, axillary lymph node dissection; SNB, sentinel node biopsy
Predictors of Treatment Effect
We examined whether the absence of treatment effect within surgical subgroups was associated with removal of fewer nodes. A median of 26.5 nodes was removed in ALND patients without treatment effect (n = 4) compared to 17 nodes in patients with treatment effect (n = 131) (p = 0.03). There was no difference in the number of nodes removed in SNB patients with (n = 61) and without (n = 8) treatment effect (median: 4 versus 3.5, p = 0.34), suggesting that the absence of treatment effect within surgical subgroups was not related to retrieval of a smaller number of nodes. Predictors of treatment effect for the entire cohort (n = 204) are outlined in Table 2. Patients with hormone receptor positive (HR+) breast cancers were less likely to have treatment effect in the nodes compared to patients with triple-negative or HER2+ breast cancers (83% versus 96% versus 96%, respectively; p = 0.05). Treatment effect was also less common in patients without a breast pCR (89%) compared to patients with a breast pCR (97%) (p = 0.05). Age (p = 0.16), clinical T stage (p = 0.81), and clinical N stage (p = 0.39) were not predictors of treatment effect in the nodes. In order to minimize any potential effect of false-negative SN biopsy on the lack of treatment effect, we looked at predictors of treatment effect in the subset of patients treated with ALND (Table 3). Findings were similar to those observed in the entire cohort; HR+ patients were significantly less likely to have treatment effect in the nodes (p = 0.016). Patients without a breast pCR were also less likely to have treatment effect, although this association did not reach statistical significance in the ALND cohort (p = 0.08); these findings suggest that the absence of treatment effect in these patients is likely related to a true biologic difference in nodal response to NAC.
Table 2. Predictors of treatment effect for the entire cohort (n = 204).
| Characteristic | n | Treatment effect | No treatment effect | P-value | |
|---|---|---|---|---|---|
| Age, years (median, range) | 204 | 48.5 (25, 85) | 55.5 (28, 83) | 0.16 | |
| cT* | T1 | 21 | 91% | 9% | 0.81 |
| T2 | 89 | 93% | 7% | ||
| T3 | 53 | 96% | 4% | ||
| T4 | 38 | 95% | 5% | ||
| cN | N1 | 170 | 95% | 5% | 0.39 |
| N2 | 27 | 89% | 11% | ||
| N3 | 7 | 100% | 0% | ||
| Tumor subtype | HR+/HER2- | 30 | 83% | 17% | 0.05 |
| Triple negative | 55 | 96% | 4% | ||
| HER2+ | 119 | 96% | 4% | ||
| Breast pCR§ | Yes | 141 | 97% | 3% | 0.05 |
| No | 61 | 89% | 11% | ||
cT: 3 patients presented with occult primary breast cancer (cT0) and are not included in the table
Breast pCR: 2 patients did not have primary breast surgery and were excluded from the analysis
cT, clinical tumor stage; cN, clinical nodal stage; pCR, pathologic complete response
Table 3. Predictors of treatment effect in axillary lymph node dissection patients (n = 135).
| Characteristic | n | Treatment effect | No treatment effect | P-value | |
|---|---|---|---|---|---|
| Age, years (median, range) | 135 | 49 (25, 85) | 40 (28, 83) | 0.37 | |
| cT* | T1 | 10 | 100% | 0% | 1 |
| T2 | 53 | 96% | 4% | ||
| T3 | 35 | 97% | 3% | ||
| T4 | 34 | 97% | 3% | ||
| cN | N1 | 103 | 98% | 2% | 0.33 |
| N2 | 25 | 92% | 8% | ||
| N3 | 7 | 100% | 0% | ||
| Tumor subtype | HR+/HER2- | 21 | 86% | 14% | 0.016 |
| Triple negative | 35 | 100% | 0% | ||
| HER2+ | 79 | 99% | 1% | ||
| Breast pCR§ | Yes | 93 | 99% | 1% | 0.08 |
| No | 42 | 93% | 7% | ||
cT: 3 patients presented with occult primary breast cancer (cT0) and are not included in the table
Breast pCR: 2 patients did not have primary breast surgery and were excluded from the analysis
cT, clinical tumor stage; cN, clinical nodal stage; pCR, pathologic complete response
Classic Versus Subtle Treatment Effect
Of the 192 patients with treatment effect in the nodes, 7 (4%) patients had subtle changes. Despite a small population size, triple negative patients were more likely to have subtle treatment effect (9%) compared to HER2+ (1%) or HR+ (3.3%) patients (p = 0.006). In addition, subtle treatment effect was only seen in SNB patients compared to ALND patients (p < 0.01). In all 7 patients with subtle treatment effect in the nodes, similar concordant subtle findings were also seen in the tumor bed of the breast.
Discussion
Our study demonstrates that more than 90% of patients with biopsy-proven nodal metastases pre- NAC who achieve a nodal pCR after chemotherapy have histologic changes indicative of treatment effect which are identifiable with routine histologic examination. Although treatment effect was more common in ALND patients compared to SNB patients (97% versus 88%, respectively; p = 0.02), the vast majority of SNB patients had treatment effect identified in the nodes. The differential identification of treatment effect between ALND and SNB patients has several possible explanations. ALND patients had more nodes removed than SNB patients (17 versus 4, p < 0.001), raising the possibility that the lack of treatment effect in SNB patients may represent a false-negative SN. Even when ≥ 3 SNs were removed, the false-negative rate of SN biopsy after NAC was 7.3 and 9.1 % in the SENTinel NeoAdjuvant (SENTINA) and ACOSOG Z1071 trials, respectively.3,4 Furthermore, Brown et al demonstrated that the absence of histologic findings in negative SNs after preoperative chemotherapy correlated with a higher false-negative rate. However, in Brown's study, the median number of SNs removed was 2, and treatment effect was identified in only 50% of the 34 patients with negative SNs.8 In our study, among patients with at least 3 negative SNs removed, the observed difference in treatment effect between ALND and SNB patients was only 9%, and comparable to the known false-negative rate of SNB in the adjuvant setting, reported as 7.3% in a recent meta-analysis.9
However, it is unlikely that the false-negative rate of SNB is the sole explanation for the failure to identify treatment effect in all cases. Even among patients undergoing ALND, no treatment effect was seen in 3%. Patients undergoing ALND who did not have treatment effect identified had a similar number of nodes retrieved as patients with treatment effect; this was also true in the SNB group. These findings suggest that once a minimum threshold of nodes is removed to minimize false-negative rates, factors beyond the extent of nodal surgery likely influence the detection of treatment effect in the nodes. In our study, patients with hormone receptor + breast cancers and patients without a breast pCR were less likely to have treatment effect in the nodes after NAC. The association between tumor subtype and, to a lesser degree, breast pCR, with treatment effect was also seen in ALND patients, a group in which the likelihood of inadequate nodal sampling is negligible. Different tumor subtypes are known to have different response rates to neoadjuvant chemotherapy in both the breast and the lymph nodes as demonstrated in ACOSOG Z1071, with triple negative and HER2+ patients having the highest nodal pCR rates (49.4% and 64.7%, respectively), and hormone receptor + patients having lower pCR rates in the nodes (21.1%) (p < 0.0001).3 It is plausible that histologic changes in the lymph nodes after NAC would also be influenced by tumor subtype and may account for the difference in detection of treatment effect by subtype in our study.
An additional explanation for the failure to observe treatment effect in some cases is the method of nodal processing. In this study, nodes were sectioned at 2 mm intervals and a single slide prepared from each block. It is possible that more extensive sampling might have identified treatment changes in some of these cases just as the use of serial sectioning of the SN identifies more tumor than bisecting the node and making a slide from each face.10,11
Methods to decrease false-negative rates in node-positive patients after NAC are all meant to reduce the likelihood of leaving behind residual disease in the nodes in a patient population where the effects of residual nodal burden of chemotherapy-resistant cells after NAC are unknown. Studies documenting resection of the clipped node report low false-negative rates5,6; none of the studies examining nodal clipping have reported its utility in patients undergoing SNB with dual mapping and retrieval of ≥ 3 SNs. In the study by Caudle et al evaluating the benefit of selective evaluation of clipped nodes after NAC, only 55% of patients had dual mapping, and the median number of SLNs retrieved was 2, suggesting that the SLN procedure was not optimized.6 In the patient subset mapped with dual tracer and having ≥ 3 SNs identified, with documented false-negative rates of < 10%, it is unknown whether nodal clipping will lower false-negative rates enough to result in a clinically meaningful difference in regional recurrence rates. Retrieval of at least 3 negative SNs at the time of SNB, with careful pathologic analysis of the node to identify histologic evidence of treatment effect, may be sufficient to minimize false-negative rates without the necessity of marking the node.
At this time, the significance of the absence of treatment effect in the nodes in node-positive patients undergoing SNB after NAC is not known. Due to other factors which may influence the identification of treatment effect in node-positive patients after NAC, the absence of treatment effect in the SNs is not currently an indication for ALND at our institution.
In conclusion, identification of treatment effect in negative nodes post-NAC in patients presenting with nodal metastases is another method of confirming that a positive node has been retrieved, similar to clipping the node. The use of dual tracer mapping and retrieval of ≥ 3 more SNs in N+ patients post-NAC resulted in identification of treatment effect in 88% of SNB patients without nodal marking, suggesting that nodal clipping may not be necessary to achieve an acceptable false-negative rate.
Synopsis.
Following neoadjuvant chemotherapy, sentinel nodes with treatment effect were identified in 88% of initially node-positive patients who achieved a nodal pathologic complete response without marking the nodes, suggesting an acceptable false-negative rate may be achieved without nodal clipping.
Acknowledgments
Disclosures: This study was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 and presented in podium format at the 69th Society of Surgical Oncology Annual Cancer Symposium, Boston, MA, March 2-5, 2016.
Footnotes
The authors have no conflict of interest disclosures to report, and the findings presented in this manuscript have not been published elsewhere.
References
- 1.Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 2.Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608–14. doi: 10.1097/SLA.0000000000000924. discussion 14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. Jama. 2013;310(14):1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 5.Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance) Ann Surg. 2016;263(4):802–7. doi: 10.1097/SLA.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittendorf EA, Caudle AS, Yang W, Krishnamurthy S, Shaitelman S, Chavez-MacGregor M, et al. Implementation of the american college of surgeons oncology group z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014;21(8):2468–73. doi: 10.1245/s10434-014-3775-6. [DOI] [PubMed] [Google Scholar]
- 8.Brown AS, Hunt KK, Shen J, Huo L, Babiera GV, Ross MI, et al. Histologic changes associated with false-negative sentinel lymph nodes after preoperative chemotherapy in patients with confirmed lymph node-positive breast cancer before treatment. Cancer. 2010;116(12):2878–83. doi: 10.1002/cncr.25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106(1):4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AE, Dale PS, Turner RR, Morton DL, Evans SW, Krasne DL. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222(3):394–9. doi: 10.1097/00000658-199509000-00016. discussion 9-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tvedskov TF, Jensen MB, Balslev E, Ejlertsen B, Kroman N. Stage migration after introduction of sentinel lymph node dissection in breast cancer treatment in Denmark: a nationwide study. Eur J Cancer. 2011;47(6):872–8. doi: 10.1016/j.ejca.2010.11.022. [DOI] [PubMed] [Google Scholar]