Abstract
Objectives
The majority of care for those with Alzheimer’s Disease and other age-related dementias is provided in the home by family members. To date there is no consistently effective intervention for reducing the significant stress burden of many family caregivers. The present pilot randomized controlled trial tested the efficacy of an adapted, 8-week Mindfulness-based Stress Reduction (MBSR) program, relative to a near structurally equivalent, standard Social Support (SS) control condition for reducing caregiver stress and enhancing the care giver-recipient relationship.
Method
Thirty-eight family caregivers were randomized to MBSR or SS, with measures of diurnal salivary cortisol, and perceived stress, mental health, experiential avoidance, caregiver burden, and relationship quality collected pre- and post-intervention and at 3-month follow-up.
Results
MBSR participants reported significantly lower levels of perceived stress and mood disturbance at post-intervention relative to SS participants. At 3-month follow-up, participants in both treatments conditions reported improvements on several psychosocial outcomes. At follow-up there were no condition differences on these outcomes, nor did MBSR and SS participants differ in diurnal cortisol response change over the course of the study.
Conclusion
Both MBSR and SS showed stress reduction effects, and MBSR showed no sustained neuroendocrine and psychosocial advantages over SS. The lack of treatment condition differences could be attributable to active ingredients in both interventions, and to population-specific and design factors.
Keywords: Mindfulness-based Stress Reduction, social support, salivary cortisol, stress
An estimated 4.5 million Americans have Alzheimer’s disease (AD), and most of these individuals reside in the home, where family caregivers provide an estimated 80 percent of the care (Alzheimer's Association, 2012). The caregiving required for a person with AD and other dementias is intense, characterized as requiring a “36-hour day” (Mace & Rabins, 2006) and the median length of in-home caregiving before nursing home placement is 6.5 years (Haley, 1997). AD and other dementia caregiving presents serious cognitive, emotional, relational, and role challenges to family caregivers (e.g., Savla, Roberto, Blieszner, Cox, & Gwazdauskas, 2011), and confers psychological and physical health risks, including higher risks of both psychiatric morbidity and mortality (Capistrant et al., 2012; Joling et al., 2010; Klein et al., 2014; Perkins et al., 2013). Interventions aimed at maintaining caregiver well-being are beneficial in important ways. First, reducing family caregiver stress may successfully delay patient institutionalization by allowing family members to provide home care longer (Gaugler et al., 2000; Mausbach, et al., 2004; Stevens et al., 2004). Second, interventions that contribute to maintaining the mental and physical health of family caregivers can reduce the cost of their own healthcare and contribute to greater quality of life (Mittelman, 2005). Finally, interventions targeting caregiver burden can help to maintain and improve their psychological and physical functioning.
Recognizing this need, researchers have developed various interventions for AD/dementia caregivers, including psycho-educational programs, support groups, behavioral management programs, individual or family counseling, and multicomponent interventions (e.g., Akkerman & Ostwald, 2004; Mittelman et al., 2004; Nichols et al., 2008). Such interventions have been shown to reduce caregiver distress and psychological morbidity, and improve patients’ psychological well-being. However, treatment effect sizes have been relatively small (Sörensen & Conwell, 2011), and numerous reviews have failed to identify a consistently effective method for reducing the distress and burden experienced by AD caregivers (e.g., Bourgeois, Schulz, & Burgio, 1996; Gottlieb & Wolfe, 2002; Schulz, Martire, & Klinger, 2005), suggesting that additional research is needed to determine how best to intervene to benefit caregiver well-being and other key outcomes. There is also a need for interventions that address the needs of families before severe disease progression and more stressful caregiver conditions occur, and before caregivers develop physical and mental health problems that could interfere with their abilities to provide home-based support to relatives with AD/dementia.
The present study examined the efficacy of a mindfulness-based intervention to effectively target AD/dementia caregiver burden. Mindfulness concerns ‘presence of mind’ – a receptive attentiveness to events and experiences occurring in the present moment, in contrast to a state of mind in which occurrences are habitually filtered through appraisals, evaluations, memories, and beliefs about events and experience (Brown, Ryan, & Creswell, 2007). Over the past 25 years, mindfulness-based interventions have been increasingly incorporated into clinical interventions and wellness programs to teach individuals to better manage stress-related thoughts, emotions, and behavior (for reviews see Baer, 2003; Brown et al., 2007; Sedlmeier et al., 2012). A mindfulness-based intervention may successfully target AD/dementia caregiver burden because existing interventions typically teach caregivers specific techniques to manage domain-specific patient-related problems (e.g., caregiver burden, depression; Sörensen & Conwell, 2011), which may become less useful over time as behaviors change. In contrast, mindfulness interventions teach a generic approach to stress management that can be adapted to a variety of stressful situations. Mindfulness interventions have been shown to reduce a variety of psychological symptoms amongst health care providers (Irving, Dobkin, & Park, 2009), and while studies are still few, there is indication that such training can improve interpersonal sensitivity and relationship functioning (Sedlmeier et al., 2012).
To date, several randomized controlled trials (RCTs) have examined the efficacy of mindfulness-based interventions in alleviating AD/dementia caregiver burden and stress (Hou et al., 2014; Oken et al., 2010; Whitebird et al., 2012). The findings, most based on self-reported mental health and care relationship outcomes, provide preliminary support for the utility of mindfulness training in reducing caregiver burden and improving their psychological well-being. While little examined amongst AD/dementia caregivers (but see Oken et al., 2010), there is indication that mindfulness can beneficially impact both diurnal and acute levels of cortisol, a neuroendocrine stress marker (Creswell & Lindsay, 2014), regularly elevated levels of which have predicted physical and mental health problems (McEwen, 1998). Stressors increase health risks via diminished neurobiological resources, and the study of diurnal cortisol responses over time has begun to offer an examination of the effect of caregiver-targeted interventions on this neuroendocrine-mediated stress – health pathway (Klein et al., 2014).
The present randomized controlled trial was designed to evaluate the efficacy of a mindfulness-based intervention, adapted from the empirically supported Mindfulness-based Stress Reduction program (Kabat-Zinn, 1990), for reducing psychological and neuroendocrine markers of stress, improving psychological morbidity, and caregiver-recipient relationship quality in family caregivers of persons in the early stages of AD and other dementias.
A focus on caregivers of early-stage care recipients, novel in this area of investigation, was intended to build resilience and relationship quality to better meet the challenges presented by ongoing care of progressive dementias. Participants, randomized to mindfulness-based training or near-structurally equivalent standard social support groups, completed subjective measures of perceived stress, experiential avoidance, mental and physical health, caregiver burden, and relationship quality, and collected salivary samples for assay of diurnal cortisol at pre-intervention, post-intervention (8 weeks later), and at a 3-month follow-up point.
Method
Participants
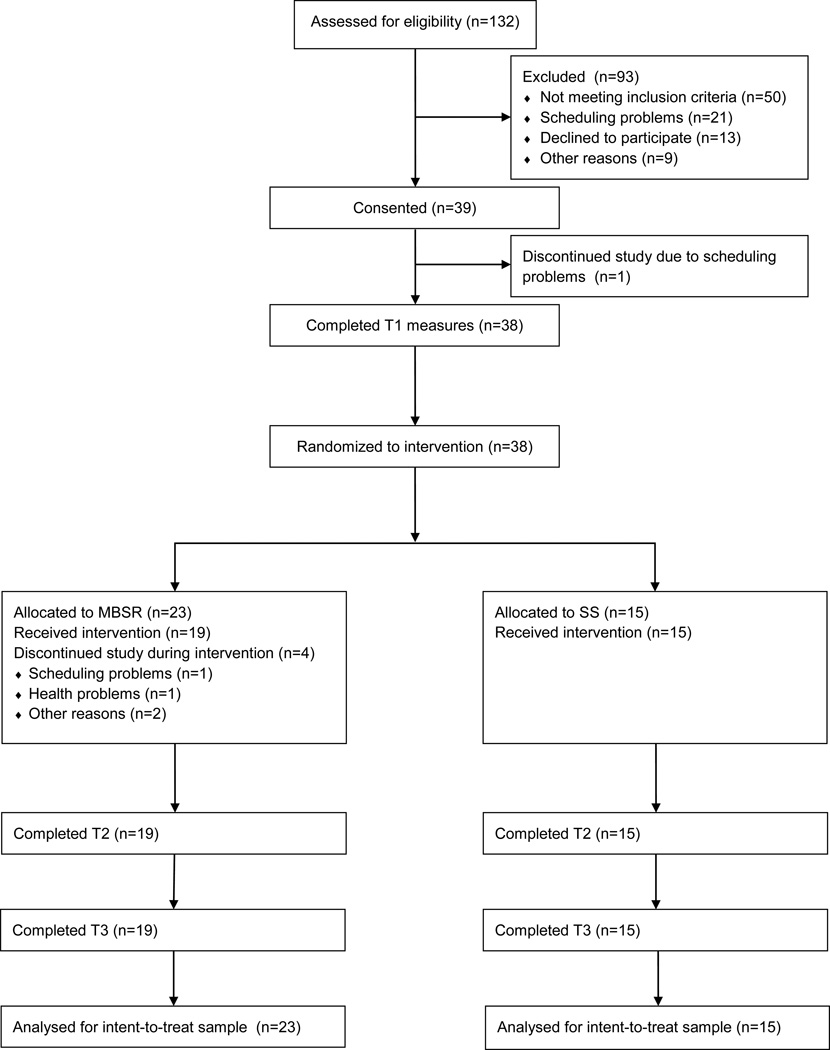
Participants were U.S. Mid-Atlantic region adult caregivers of individuals with AD or other dementias who were blood or by-marriage relatives of care recipients (spouses, siblings, children, grandchildren, etc). If taking antidepressant or anxiolytic medications, their medication regimen was stable for 8 weeks prior to enrollment. All participants were caring for family members with early stage AD or other dementia, as ascertained by telephone interview using the Functional Assessment Staging of Alzheimer’s Disease (FAST; Reisberg, 1988) (stage 5 or lower).1 Individuals self-reporting one or more of the following psychiatric disorders or history thereof were excluded from participation: major depression with psychotic features; psychosis; lifetime history of schizophrenia, bipolar disorder, organic brain syndrome, or mental retardation; and alcohol or substance abuse within the previous year. Individuals with major, uncorrected sensory impairments or cognitive deficits, defined as a score of less than 31 on the Telephone Interview for Cognitive Status (TICS; Brandt, Spencer, & Folstein, 1988) were also excluded. Figure 1 shows the participant flow through the study.
Figure 1.
CONSORT diagram showing participant flow through the study.
Procedure
Prospective participants were recruited through print media, internet, and radio ads, and through posters and flyers disseminated at local Alzheimer’s Association chapter support groups and at other public community locations (e.g., churches, physicians’ offices, retirement communities). After phone screening to assess inclusion and exclusion criteria, potential participants heard a brief description of the study and were invited to attend a study briefing session. At this session, wherein the study methods and purposes were described in more detail, those electing to participate provided written informed consent and completed self-report questionnaires assessing caregiving characteristics (e.g., years or months spent caregiving, weekly hours spent in caregiving activities), and psychological characteristics (see Measures below).
Participants were also provided with salivettes and instructions to collect their saliva at six time points during one day: before rising in the morning, 45 min after awakening, 2.5 h after awakening, 8 h after awakening, 12 h after awakening, and bedtime at each of the 3 study phases (pre- and post-intervention, and 3-month follow-up). Trackcaps (MEMS 6; Aardex, Zug, Switzerland) on participants’ salivary collection tube bottles recorded date/time compliance with instructions. A salivary log was also completed on each of the 3 collection days to record cortisol-relevant behaviors that may influence cortisol levels: time of waking, hours of sleep the night before, food and beverages consumed, physical activity, alcohol intake, smoking, illness symptoms, medications, and for women, menstrual activity and oral contraceptive use. Participants were instructed to store Salivettes collection bottles in the refrigerator and return the materials by FedEx the day following each collection using pre-labeled boxes.
After these instructions, participants were randomly assigned to the 8-week MBSR intervention or to an 8-week social support (SS) control group for AD/dementia caregivers, both involving attendance at weekly 1.5–2 hour classes with 10–20 other participants all referred and screened in a similar fashion. Two Master’s degree-level, trained facilitators with over 10 years of experience offering their respective interventions led each group. The MBSR program also included a day-long intensive mindfulness practice and discussion session. The MBSR program was slightly adapted for this study population from the canonical MBSR program (Kabat-Zinn, 1990), particularly in its frequent focus of class discussion of caregiving and in minor adjustments to some mindfulness exercises to accommodate physical limitations common amongst older individuals. The MBSR manualized curriculum was followed. The program has been shown adaptable to a variety of healthy and clinical populations (Brown, Creswell & Ryan, 2015). Participants learned a range of skills aimed at increasing awareness of experiences and sensations related to physical symptoms, emotions, thoughts, and behaviors during interactions with care recipients. Special emphasis was placed on mindful movement, meditation, and mindful communication.
Participants randomly assigned to the Alzheimers’ Association-sponsored SS control condition engaged in leader-facilitated discussion of group-generated topics related to caring for their AD/dementia care recipient. At the end of the 8-week interventions, and again at the 3-month follow-up point, participants completed the same self-report questionnaires and saliva collection as done at baseline. They were also asked to refrain from continuing to meet with their respective groups in the interval between the end of the interventions and the 3-month follow-up point to help ensure standardized and equivalent treatment exposure across conditions. Participants were paid $100 for completing the study.
Measures
Self-report scales
The pretreatment questionnaire measures were completed in a private space at a large mid-Atlantic university; subsequent measures were completed at participants’ homes and returned by courier along with saliva samples. The following measures of psychological, physical, and relationship functioning were completed at each assessment:
Perceived stress
The 10-item version of the Perceived Stress Scale (PSS; Cohen, Kamarck & Mermelstein, 1983) assessed the extent to which life situations are appraised as stressful on a 5-point scale (never to very often) (α = .75).
Experiential avoidance
The 10-item Acceptance and Action Questionnaire II (AAQ II; Bond et al., 2011) measured the tendency to avoid unwanted experiences and conversely, to accept unwanted experiences using a 1–7 scale (never true to always true). Items include: ‘My painful experiences and memories make it difficult for me to live a life that I would value.’ The sample α was .86.
Mood
The Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971) assessed mental health-relevant mood states on 6 subscales relevant to the study population: tension (α = .90), depressive symptoms (α = .96), anger (α = .94), vitality (α = .90), fatigue (α = .92), and confusion (α = .83). Items are adjectives describing psychological symptoms such as sad, helpless, gloomy, restless, panicky, and terrified, rated on a 5-point Likert scale (not at all to extremely).
Mental and physical functioning
The Medical Outcomes Study Short-Form Health Survey (MOS-SF-36; McHorney, Ware, Lu, & Sherbourne, 1994) is a generic measure of functional physical health and mental health with 8 subscales; results for composite mental health and physical health indexes (both αs = .81) are reported herein.
Caregiver burden
The 22-item version of the Zarit Burden Interview (ZBI; Zarit, Reever, & Back-Peterson, 1980) measured perceived burden among dementia caregivers, and particularly, burden associated with functional impairments and the home care situation (α = .89). Statements are scored on a 5-point scale (never to nearly always).
Caregiver-recipient relationship quality
This was measured with the 15-item Mutuality scale of the Family Care Inventory (FCI-MS; Archbold et al., 1990), which concerns the caregiver’s perceived quality of relationship between the caregiver and care recipient (α = .94). Items include “How attached are you to him or her?” and “To what extent do you enjoy the time the two of you spend together?” Responses were scored on a 5-point scale (not at all to a great deal).
Salivary cortisol
Saliva samples were collected 6 times during each day of collection (see Procedure for schedule). Samples were collected via 2-min sublingual placement of synthetic Salivettes (Salimetrics, State College, PA). Returned samples were immediately freezer-stored at −20 °C until entire-sample assay. Samples were then thawed and centrifuged for 15 min at 1500 × g at 10 °C. Cortisol was assayed using the Salimetrics competitive immunoassay method. Inter-assay coefficient of variation (CV) was 6.69—6.88%, intra-assay CV was 3.88—7.12%, and the sensitivity was < 0.007 ug/dL.
Statistical Analyses
Psychosocial outcomes
Analyses of the full repeated psychosocial outcomes were conducted using restricted maximum likelihood mixed models (REML; e.g., Bryk & Raudenbush, 1992). This approach is well suited to hierarchically nested data structures in which a lower level unit of analysis (level 1; e.g., repeated measures outcome) is nested within a higher level of analysis (level 2; e.g., treatment condition). SAS MIXED was used to estimate these models (SAS Institute, 2008), in which primary interest was in changes in the outcome measures over assessment phase (pre-intervention, post-intervention, and 3-month follow up), and condition differences in these changes over phase (the latter modeled as linear and curvilinear slopes). To permit investigation of the intent-to-treat (ITT) sample, all available participant data were included in the analyses. To examine the magnitude of statistically significant effects independent of sample size, Cohen’s d estimates (Cohen, 1988) were used where appropriate.
Before beginning the REML analyses of psychosocial outcomes, all continuous variables were checked for normality and corrected where necessary. Specifically, several outlying values in POMS depression, anger, and confusion at post-treatment and follow-up were winsorized (Tabachnick & Fidell, 2007). A compound symmetry covariance structure was found to best fit all outcome data. Participant age was covaried in in all models.
Cortisol outcomes
Before analysis, compliance with the saliva collection schedule was checked. Specifically, clock times of cortisol measurements were visually inspected to remove first cortisol measurements taken before 4:00 A.M. and last cortisol measurements taken later than 5:00 A.M. the day after collection had begun. Also, cortisol measurements with elapsed times more than 3 standard deviations above or below the mean for their time point were removed. A small number of recording times were missing; these were imputed by averaging the non-missing times for the appropriate sample. Cortisol measurements > 5 ug/dL were also excluded from analysis as outliers. Elapsed time since the first sample of each day was computed for each study participant at each assessment phase (baseline, post-intervention, and follow-up). In this, we followed several recent studies showing that “cortisol rhythms are driven by time elapsed since awakening and less by clock time” (Karlamangla, Friedman, Seeman, Stawksi, & Almeida, 2013; see also Hastie, 1992; Ranjit et al., 2009). As in these previous studies, cortisol measures were log transformed to normalize the distributions.
Using the nlme and splines packages (Hastie, 1992; Pinheiro & Bates, 2000; Pinheiro, Bates, DebRoy, & Sarkar, 2014; R Core Team, 2014; Smith, 1979) in R statistical software (R Core Team, 2014), mixed effects regression models were estimated to examine changes in average level of cortisol throughout the day (the trajectory), and to estimate any differences in this trajectory between study assessment phases and treatment groups. A cubic spline was incorporated in the models; this spline yields a continuous, smooth curve such that the steepness of the curve at any point during the day, and the times of day when the curvature changes, are determined by the data. An AR(1) (order-1 autocorrelated) covariance structure was specified for all cortisol models. Details of the spline modeling approach to diurnal cortisol analysis are discussed in Wegelin and Brown (in preparation).
In the mixed effects models, a likelihood ratio test was performed to compare a 6-trajectory model, in which each level of the condition × phase interaction determined a distinct trajectory (39 degrees of freedom [df]), against a model in which a single trajectory characterized the entire sample (9 df). This comparison permitted a test of the main effects of treatment condition and study phase, as well as the test of primary interest here, the effect of treatment condition on cortisol trajectories over study phase. Given the pilot nature of the study, an alpha level of .05 was used to determine the statistical significance of the results.
Results
Sample Characteristics
As a whole, the sample of 38 participants (MBSR n = 23; SS n = 15) was predominantly female (84.2%) and most were Caucasian (75.7%); the remainder were African American (21.6%) or Hispanic/Latino(a) (2.7%). The average age of participants was 61.14 years of age (SD = 10.41, range = 39 to 88 years). Most individuals were married (65.8%); the remainder (34.2%) were widowed, separated, divorced or never married. The sample was generally well-educated, with most having attended college (32.4%) or earned a college degree (35.1%). An additional 29.7% had graduate school training. Only one (2.7%) had just a high school education. Among those who reported household income (n = 9 missing) the annual median was $60,000 (range $19,000 – $120,000). The MBSR and SS groups did not significantly differ on these demographic characteristics (gender, age, ethnicity, civil status, income), all ps > .21.
Table 1 shows caregiving characteristics according to treatment condition. Most care recipients were either a spouse or parent; several others (n=3) were a sibling, in-law, or another relative. In both conditions, the participants were the primary caregivers, although this proportion was higher in the MBSR condition than in the SS condition (95.7% versus 66.7%, p = .017). In both conditions, care had been provided for approximately 4 years. Participants in the MBSR and SS conditions estimated that care recipients had shown memory deficits for approximately 5 years and 6.5 years, respectively. Using a 1 (not at all) to 5 (extremely) scale, participants on average stated that care recipients were somewhat dependent on them for the activities of daily living, and using the same 1–5 scale, on average indicated that they were somewhat burdened by caregiving. Except as noted above, these characteristics did not significantly differ by treatment condition.
Table 1.
Caregiving Characteristics According to Treatment Condition.
| MBSR (n = 23) | SS (n = 15) | ||||
|---|---|---|---|---|---|
| Variable | N | % | N | % | pdiff |
| Care recipient relation | .11 | ||||
| Spouse | 12 | 52.2 | 4 | 26.7 | |
| Parent | 11 | 47.8 | 8 | 53.3 | |
| Other | 0 | 0.0 | 3 | 20.0 | |
| Main care provider | 22 | 95.7 | 10 | 66.7 | .02 |
| Care provided, months (M ± SD) | 48.22 ± 35.10 | 44.24 ± 27.60 | .71 | ||
| Daily living dependence (M ± SD) | 3.35 ± 1.34 | 3.49 ± 1.24 | .74 | ||
| Memory deficits, months (M ± SD) | 60.02 ± 27.68 | 76.13 ± 50.55 | .27 | ||
| Caregiving amount (M ± SD) | 3.30 ± 0.80 | 2.91 ± 1.38 | .40 | ||
| Caregiving burden (M ± SD) | 3.31 ± 1.18 | 2.88 ± 1.41 | .31 | ||
Notes. N = 38. The pdiff column shows significance levels based on t and χ2 tests of condition differences. MBSR = Mindfulness-based Stress Reduction; SS = Social Support.
After randomization and before interventions were begun, participants were asked to rate, on a 1 (not at all) to 10 (extremely) scale the extent to which they expected their assigned course to benefit them. MBSR and SS participants did not differ on expected benefit (MBSR M = 8.0, SD = 1.95; SS M = 8.6, SD = 1.40, p = .32).
Outcome Characteristics
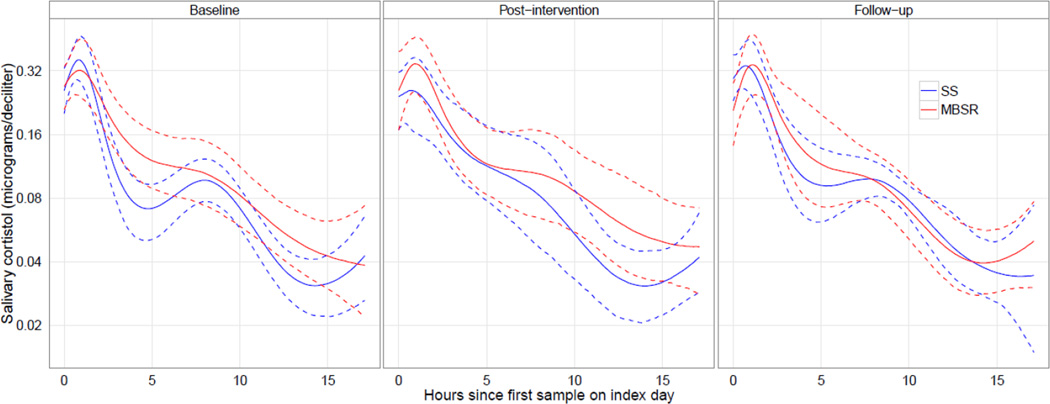
Table 2 shows that on average participants in both conditions showed score declines on the psychosocial outcomes, and markedly so for MBSR participants between pre- and post-treatment; however their scores increased somewhat from post-treatment to follow-up. Of 636 cortisol measurements, 13 (2.04%) came from noncompliant sample times and 9 (1.42%) had missing cortisol values or values greater than 5 ug/dL, leaving 614 (96.5%) samples for analysis. For 3 (0.49%) of these measurements, a missing clock time was imputed. Figure 2 displays the six-trajectory spline model of average cortisol trajectories for MBSR and SS treatment conditions in each study phase, with 95% confidence intervals obtained by bootstrapping (Harrell, 2001).
Table 2.
Mean (and SD) Psychosocial Outcome Values in MBSR and SS Conditions at Pre-Treatment, Post-Treatment, and Follow-Up Phases for Intent-to-Treat Sample.
| MBSR | SS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Pre-treat | Post-treat | Follow-up | d | Pre-treat | Post-treat | Follow-up | d | pinter |
| PSS | 21.52 ± 5.31 | 15.84 ± 5.26 | 18.11 ± 5.50 | .63 | 19.93 ± 5.70 | 18.93 ± 6.80 | 17.80 ± 6.03 | .36 | .003 |
| AAQ | 49.35 ± 8.29 | 54.21 ± 7.55 | 53.79 ± 7.61 | −.58 | 49.13 ± 14.04 | 53.67 ± 9.66 | 53.40 ± 9.78 | −.35 | .821 |
| POMS | |||||||||
| Tension | 2.68 ± 0.73 | 2.04 ± 0.72 | 2.17 ± 0.75 | .69 | 2.73 ± 1.06 | 2.62 ± 0.96 | 2.29 ± 0.82 | .46 | .024 |
| Depression | 1.97 ± 0.58 | 1.37 ± 0.35 | 1.67 ± 0.63 | .50 | 2.18 ± 1.24 | 1.80 ± 0.76 | 1.83 ± 0.96 | .32 | .196 |
| Anger | 2.00 ± 0.78 | 1.43 ± 0.39 | 1.72 ± 0.71 | .38 | 2.18 ± 1.08 | 2.06 ± 0.89 | 1.84 ± 0.89 | .34 | .016 |
| Confusion | 2.41 ± 0.67 | 1.92 ± 0.61 | 2.09 ± 0.60 | .50 | 2.30 ± 0.97 | 2.18 ± 0.87 | 2.04 ± 1.06 | .26 | .090 |
| Fatigue | 2.79 ± 1.02 | 2.14 ± 1.00 | 2.25 ± 0.96 | .55 | 2.82 ± 1.04 | 2.53 ± 1.18 | 2.35 ± 0.95 | .47 | .180 |
| Vitality | 2.69 ± 0.68 | 3.24 ± 0.88 | 2.94 ± 0.85 | −.33 | 2.70 ± 0.91 | 3.02 ± 0.66 | 3.09 ± 0.80 | −.46 | .213 |
| SF-36 | |||||||||
| Mental health | 46.74 ± 10.36 | 53.81 ± 9.55 | 49.09 ± 7.62 | −.26 | 48.81 ± 13.72 | 51.66 ± 11.65 | 49.83 ± 11.55 | −.08 | .233 |
| Physical health | 32.16 ± 7.56 | 29.75 ± 6.24 | 35.12 ± 6.40 | −.42 | 32.87 ± 6.83 | 32.18 ± 8.40 | 34.23 ± 5.57 | −.22 | .281 |
| ZBI | 48.17 ± 12.34 | 38.58 ± 11.93 | 43.05 ± 11.35 | .43 | 40.60 ± 16.71 | 37.80 ± 21.41 | 36.43 ± 20.89 | .22 | .046 |
| FCI-MS | 41.52 ± 10.29 | 40.53 ± 10.81 | 40.42 ± 10.32 | .11 | 41.07 ± 9.79 | 42.53 ± 11.03 | 41.36 ± 10.92 | −.03 | .487 |
Notes. N = 38 at baseline. MBSR = Mindfulness-based Stress Reduction; SS = Social Support. PSS = Perceived Stress Scale; AAQ = Acceptance and Action Questionnaire II; POMS = Profile of Mood States; SF-36 = Medical Outcomes Study Short-Form Health Survey; ZBI = Zarit Burden Interview; FCI-MS = Family Care Inventory - Mutuality Scale. The d column shows Cohen’s d effect sizes based on unadjusted pre-test and follow-up means within each condition. The pinter column shows REML mixed model treatment condition × study phase interaction significance levels.
Figure 2.
Average diurnal salivary cortisol for MBSR and SS conditions in each study phase, modeled as a continuous function of time since first sample of each collection day, with 95% pointwise confidence interval, based on the six-trajectory spline model.
Treatment Effects on Psychosocial Outcomes
Preliminary t-tests revealed no differences between the MBSR and SS conditions in the self-reported outcome variables at pre-intervention, all ps > .10. Preliminary REML models showed that participant age did not predict any of these outcomes (ps > .13) and so this variable was not further considered. Primary REML models showed treatment condition × curvilinear study phase effects on perceived stress (p = .003), POMS tension (p = .02) and anger (p = .016), and caregiver burden (p = .046). All of these changes favored MBSR except for the last, which favored SS. However, t-tests showed no condition differences on these outcomes at the 3-month follow-up point (ps > .09), thus the condition differences noted here were limited to pre-post treatment changes. Magnitude of treatment effects that is independent of sample size is provided by Cohen’s d (Cohen, 1988) effect size estimates, based on pre-test to follow-up changes in group means. As Table 2 shows, the effect sizes associated with MBSR participation on the four statistically significant mental health outcomes noted already were moderate to large in this ITT sample. However, at the follow-up time point, and paralleling the statistical results reported earlier, effect size estimates of condition differences on these four outcomes were generally small (Md = .18; ranged = −.15 to .39).
Participants in both conditions reported significant improvements across study phase in AAQ experiential avoidance (p = .0003), POMS depressive symptoms (p = .0005), vitality (p = .025), fatigue (p = .01), and confusion (p = .028), as well as SF-36-reported mental and physical functioning (ps = .006 and .02, respectively). There were no main or interaction effects on the FCI-MS caregiver–care recipient relationship (ps > .43).
Treatment Effects on Diurnal Cortisol Responses
The likelihood ratio test of the simpler (df = 9) versus the more complex model (df = 39) of cortisol trajectories yielded χ2 (30) = 24.1, p = 0.77. Thus, a single trajectory was sufficient to characterize the diurnal cortisol pattern, indicating no evidence for main effects of treatment condition and study phase, nor a condition × phase interaction effect on the cortisol trajectories. Thus, no evidence of a treatment effect on cortisol responses from pre- to post-treatment to follow-up was found.
Discussion
Family caregivers of those with dementias are under considerable stress and the present randomized trial was designed to examine whether a mindfulness-based psychosocial intervention (MBSR), novel for a population of early stage dementia caregivers, would provide stress relief relative to standard social support (SS). This pilot study found that caregiver participants in MBSR reported levels of perceived stress, tension and anger that were significantly lower at post-intervention relative to SS participants. On one outcome, caregiver burden, SS showed a comparative advantage over the same time period. In a discussion of the REACH study interventions, Gitlin et al. (2003) noted that interventions focused on stress reduction (as MBSR also does) did not impact caregiver burden. In contrast, the SS intervention tested here highlighted an understanding and acceptance of AD/dementia behaviors, which may have helped to reduce perceived burden. At a 3-month follow-up point, however, there were no treatment condition differences on these or other psychosocial outcomes. Similarly, there were no treatment condition differences in diurnal cortisol response change over phase of the study. In fact, neither condition showed changes in the diurnal cortisol response curve from the pre-intervention to post-intervention to follow-up time points.
MBSR and other secular mindfulness-based interventions have shown considerable success in enhancing mental health and well-being in a variety of stressed healthy and clinical populations (Brown et al., 2015). The present study represents one of the first RCTs to apply MBSR to the treatment of family dementia caregiver stress relative to a structurally equivalent, active control treatment. The sample, while small, was representative in terms of the age and gender of caregivers of those with dementia, and relationship to the care recipient, according to recent survey statistics (Richardson et al., 2013). The present results are consistent with recent RCTs examining the effects of MBSR versus active control interventions for dementia caregivers, in finding treatment advantages for MBSR on several mental health outcomes (Hou et al., 2014; Whitebird et al., 2012), similar improvements in outcomes for both MBSR and control social support or education interventions (Hou et al., 2014; Oken et al., 2010), and null results for cortisol change (Oken et al., 2010).
The lack of sustained differences between treatment conditions in the neuroendocrine and psychosocial outcomes could be attributable to one or more of several factors. First, it may be that social support is an active ingredient in both treatments, which would eliminate any stress reduction advantage of MBSR over SS over time. In fact, both conditions showed salutary changes in several mental health and functional outcomes, including depressive symptoms, vitality, and general mental and physical functioning. Second, and mutually inclusive with this, the short-term (pre-post-intervention) psychosocial benefits of MBSR on perceived stress and mood may have been due to the daily practice of mindfulness prescribed in MBSR – practice that may have been curtailed or abandoned after course completion, resulting in null differences on these outcomes at the follow-up point. Recent research indicates that ongoing practice of mindfulness is related to mental health outcomes up to a year following course completion (Morgan et al., 2014). It is also possible that the study did not assess specific psychosocial or neurobiological outcomes that would have shown treatment condition differences. The study did not comprehensively measure stress-related or caregiver-recipient relationship outcomes, and further research may disclose key outcomes favoring MBSR over SS. Finally, while participants in each group were requested to refrain from meeting together in the interval been the intervention close and the follow-up point, information on mindfulness-based, social support, or other group attendance in this interval was not collected, and such attendance may have altered condition differences at follow-up.
Lack of anticipated change over study phase in the diurnal cortisol profiles of MBSR participants may likewise have had several contributing causes. It is possible that participants in the current trial did not experience such high levels of stress as to show elevated cortisol levels at study entry – that is, levels sufficiently high as to permit an examination of declines in this stress hormone over the duration of the study. Future research may focus on caregivers with high stress loads and low social support to determine whether neuroendocrine and psychological stress profiles are more responsive to the group-based MBSR or SS interventions. Also possible is that the MBSR program was insufficient to produce changes in diurnal cortisol response over the course of the study for some, chronically stressed caregivers. Finally, a single day of saliva collection at each assessment point may have been insufficient to detect changes in cortisol levels over the study interval. Because of day-to-day changes in waking time, duration of sleep, and the intensity of stressors, it has been recommended that diurnal cortisol be measured over multiple days to best assess long-term changes in cortisol rhythms (Hellhammer et al., 2007; Karlamangla et al, 2013; Matousek, Dobkin, & Pruessner, 2010).
In sum, the present pilot study indicated that both MBSR and SS are well-tolerated by family caregivers and can have beneficial effects on several mental health and resilience outcomes among dementia caregivers. This study joins several other recent investigations showing that MBSR does not necessarily offer treatment advantages when compared to an active, structurally equivalent control treatment (e.g., MacCoon, MacLean, Davidson, Saron, & Lutz, 2014). Yet a number of factors related to sample size, study design and procedures, and treatment delivery preclude definitive conclusions about the relative stress-reducing efficacy of MBSR and SS for this population. These factors represent questions for future research to address in this still new and important area of investigation.
Acknowledgments
We gratefully acknowledge the research assistance of Jessica Hellerstein, Shari Cordon, and Suzette Chopin. We also thank the Alzheimer’s Association (Greater Richmond, VA chapter) for facilitating participant recruitment. This project was supported by grant 90AI0017/01 from the U.S. Administration on Aging, U.S. Department of Health and Human Services, to K.W.B. and C.L.C., and by grant UL1TR000058 from the National Center for Advancing Translational Sciences to J.W. Grantees undertaking projects under government sponsorship are encouraged to express their findings and conclusions freely. Points of view or opinions do not, therefore, necessarily represent official Administration on Aging policy, nor the policies of the National Center for Advancing Translational Sciences and the National Institutes of Health. A report on this project prepared for the Commonwealth of Virginia Department for the Aging and the US Department of Health and Human Services, Administration on Aging, may be found at www.sahp.vcu.edu/vcoa/program/reports/pdfs/aoa_reporting.pdf.
Footnotes
This cut-off score for ‘early stage’ is slightly different from the FAST cut-off.
Contributor Information
Kirk Warren Brown, Email: kwbrown@vcu.edu, Department of Psychology, Virginia Commonwealth University 806 West Franklin Street, Richmond, VA 23284-2018.
Constance L. Coogle, Email: ccoogle@vcu.edu, Virginia Center on Aging, Virginia Commonwealth University 730 East Broad St., 2nd floor, Richmond, VA 23219.
Jacob Wegelin, Email: jwegelin@vcu.edu, Department of Biostatistics, Virginia Commonwealth University Medical Center One Capitol Square, Room 732, 830 East Main St., Richmond, VA 23219.
References
- Akkerman RL, Ostwald SK. Reducing anxiety in Alzheimer’s disease family caregivers: The effectiveness of a nine-week cognitive–behavioral intervention. American Journal of Alzheimer’s Disease and Other Dementias. 2004;19:117–123. doi: 10.1177/153331750401900202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. [Retrieved April 30, 2015];2012 Alzheimer’s disease facts and figures. 2012 from http://www.alz.org/downloads/Facts_Figures_2012.pdf. [Google Scholar]
- Archbold PG, Stewart BJ, Greenlick MR, Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Research in Nursing and Health. 1990;13(6):375–384. doi: 10.1002/nur.4770130605. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–143. [Google Scholar]
- Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Zettle RD. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: A Revised measure of psychological inflexibility and experiential avoidance. Behavior Therapy. 2011;42(4):676–688. doi: 10.1016/j.beth.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Bourgeois MS, Schulz R, Burgio L. Interventions for caregivers of patients with Alzheimer’s disease: A review and analysis of content, process, and outcomes. International Journal of Aging and Human Development. 1996;43:35–92. doi: 10.2190/AN6L-6QBQ-76G0-0N9A. [DOI] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- Brown KW, Creswell JD, Ryan RM, editors. Handbook of mindfulness: Theory, research, and practice. New York: Guilford; 2015. [Google Scholar]
- Brown KW, Ryan RM, Creswell JD. Mindfulness: Theoretical foundations and evidence for its salutary effects. Psychological Inquiry. 2007;18(4):211–237. [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Capistrant BD, Moon JR, Berkman LF, Glymour MM. Current and long- term spousal caregiving and onset of cardiovascular disease. Journal of Epidemiology and Community Health. 2012;66(10) doi: 10.1136/jech-2011-200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences, 2e. Mahwah, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Creswell JD, Lindsay EK. How does mindfulness training affect health? A mindfulness stress buffering account. Current Directions in Psychological Science. 2014;23(6):401–407. [Google Scholar]
- Gaugler JE, Edwards AB, Femia EE, Zarit SH, Stephens MP, Townsend A, et al. Predictors of institutionalization of cognitively impaired elders: Family help and the timing of placement. Journal of Gerontology. 2000;55B:247–255. doi: 10.1093/geronb/55.4.p247. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Belle SH, Burgio LD, Czaja SJ, Mahoney D, Gallagher-Thompson D, Ory MG. Effect of multicomponent interventions on caregiver burden and Depression: the REACH multisite initiative at 6-month follow-up. Psychology and Aging. 2003;18(3):361–374. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb BH, Wolfe J. Coping with family caregiving to persons with dementia: A critical review. Aging and Mental Health. 2002;6:325–342. doi: 10.1080/1360786021000006947. [DOI] [PubMed] [Google Scholar]
- Haley WE. The family caregiver's role in Alzheimer's disease. Neurology. 1997;48:S25–S29. doi: 10.1212/wnl.48.5_suppl_6.25s. [DOI] [PubMed] [Google Scholar]
- Harrell FE., Jr . Regression modeling strategies. Springer series in statistics. New York: Springer; 2001. [Google Scholar]
- Hastie TJ. Parametric additive models: bs() and ns() In: Chambers JM, Hastie TJ, editors. Statistical models in S. New York: Chapman & Hall/CRC; 1992. [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state-and trait components. Psychoneuroendocrinology. 2007;32(1):80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hou RJ, Wong SS, Yip BK, Hung AT, Lo HM, Chan PH, Ma SH. The effects of mindfulness-based stress reduction program on the mental health of family caregivers: A randomized controlled trial. Psychotherapy and Psychosomatics. 2013;83(1):45–53. doi: 10.1159/000353278. [DOI] [PubMed] [Google Scholar]
- Irving JA, Dobkin PL, Park J. Cultivating mindfulness in health care professionals: A review of empirical studies of mindfulness-based stress reduction (MBSR) Complementary Therapies in Clinical Practice. 2009;15(2):61–66. doi: 10.1016/j.ctcp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Joling KJ, Hout HPJ van, Schellevis FG, Horst HE van der, Scheltens P, Marwijk HWJ van. Incidence of depression and anxiety in the spouses of patients with dementia: A naturalistic cohort study of recorded morbidity with a 6-year follow-up. American Journal of Geriatric Psychiatry. 2010;18(2):146–153. doi: 10.1097/JGP.0b013e3181bf9f0f. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Delacorte Press; 1990. [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: Demographic and socioeconomic differences: Findings from the national study of daily experiences. Psychoneuroendocrinology. 2013;38:2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LC, Kim K, Almeida DM, Femia EE, Rovine MJ, Zarit SH. Anticipating an easier day: Effects of adult day services on daily cortisol and stress. The Gerontologist. 2014 doi: 10.1093/geront/gnu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoon DG, MacLean KA, Davidson RJ, Saron CD, Lutz A. No sustained attention differences in a longitudinal randomized trial comparing Mindfulness Based Stress Reduction versus active control. PloS ONE. 2014;9:e97551. doi: 10.1371/journal.pone.0097551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace NL, Rabins PV. The 36-Hour day: A family guide to caring for persons with Alzheimer disease, related dementing illnesses, and memory loss in later life. 4th. Baltimore, MD: Johns Hopkins University Press; 2010. [Google Scholar]
- Matousek RH, Dobkin PL, Pruessner J. Cortisol as a marker for improvement in mindfulness-based stress reduction. Complementary Therapies in Clinical Practice. 2010;16:13–19. doi: 10.1016/j.ctcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Coon DW, Depp C, Rabinowitz YG, Wilson-Arias E, Kraemer HC, et al. Ethnicity and time to institutionalization of dementia patients: A comparison of Latina and Caucasian female family caregivers. Journal of the American Geriatrics Society. 2004;52(7):1077–1084. doi: 10.1111/j.1532-5415.2004.52306.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care. 1994;32:40–65. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of mood states. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mittelman M. Taking care of the caregivers. Current Opinion in Psychiatry. 2005;18:633–639. doi: 10.1097/01.yco.0000184416.21458.40. [DOI] [PubMed] [Google Scholar]
- Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer’s disease. American Journal of Psychiatry. 2004;161:850–856. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- Morgan LP, Graham JR, Hayes-Skelton SA, Orsillo SM, Roemer L. Relationships between amount of post-intervention mindfulness practice and follow-up outcome variables in an acceptance-based behavior therapy for Generalized Anxiety Disorder: The importance of informal practice. Journal of Contextual Behavioral Science. 2014;3(3):173–178. doi: 10.1016/j.jcbs.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols LO, Chang C, Lummus A, Burns R, Martindale-Adams J, Graney MJ, et al. The cost-effectiveness of a behavior intervention with caregivers of patients with Alzheimer's disease. Journal of the American Geriatrics Society. 2008;56(3):413–420. doi: 10.1111/j.1532-5415.2007.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Fonareva I, Haas M, Wahbeh H, Lane JB, Zajdel D, Amen A. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. Journal of Alternative and Complementary Medicine. 2010;16(10):1031–1038. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M, Howard VJ, Wadley VG, Crowe M, Safford MM, Haley WE, Roth DL. Caregiving strain and all-cause mortality: Evidence from the REGARDS study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2012 doi: 10.1093/geronb/gbs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro P, Bates DM. Mixed-effects models in S and S-PLUS. Statistics and Computing. New York: Springer; 2000. [Google Scholar]
- Pinheiro P, Bates DM, DebRoy S, Sarkar D. nlme: Linear and nonlinear mixed effects models. R package version 3.1–117. 2014 [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Ranjit N, Diez-Roux AV, Sanchez B, Seeman T, Shea S, Shrager S, Watson K. Association of salivary cortisol circadian pattern with cynical hostility: The multi-ethnic study of atherosclerosis. Psychosom Med. 2009;71:748–755. doi: 10.1097/PSY.0b013e3181ad23e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B. Functional Assessment Staging (FAST) Psychopharmacology Bulletin. 1988;24:653–659. [PubMed] [Google Scholar]
- Richardson TJ, Lee SJ, Berg-Weger M, Grossberg GT. Caregiver health: Health of caregivers of Alzheimer’s and other dementia patients. Current Psychiatry Reports. 2013;15(7):1–7. doi: 10.1007/s11920-013-0367-2. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS MIXED. Cary, NC: SAS; 2008. [Google Scholar]
- Savla J, Roberto KA, Blieszner R, Cox M, Gwazdauskas F. Effects of daily stressors on the psychological and biological well-being of spouses of persons with mild cognitive impairment. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66(6):653–664. doi: 10.1093/geronb/gbr041. [DOI] [PubMed] [Google Scholar]
- Schulz R, Martire LM, Klinger JN. Evidence-based caregiver interventions in geriatric psychiatry. Psychiatric Clinics of North America. 2005;28:1007–1038. doi: 10.1016/j.psc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Sedlmeier P, Eberth J, Schwarz M, Zimmermann D, Haarig F, Jaeger S, Kunze S. The psychological effects of meditation: a meta-analysis. Psychological Bulletin. 2012;138:1139–1171. doi: 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- Smith PL. Splines as a useful and convenient statistical tool. The American Statistician. 1979;33:57–62. [Google Scholar]
- Sörensen S, Conwell Y. Issues in dementia caregiving: effects on mental and physical health, intervention strategies, and research needs. American Journal of Geriatric Psychiatry. 2011;19(6):491–496. doi: 10.1097/JGP.0b013e31821c0e6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, Owen J, Roth D, Clay O, Bartolucci A, Haley W. Predictors of time to nursing home placement in White and African American individuals with dementia. Journal of Aging and Health. 2004;16(3):375–397. doi: 10.1177/0898264304264206. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics, 5e. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- Wegelin J, Brown KW. Spline models for cortisol circadian rhythms. Richmond, VA: Virginia Commonwealth University; (in preparation) Unpublished manuscript. [Google Scholar]
- Whitebird RR, Kreitzer M, Crain AL, Lewis BA, Hanson LR, Enstad CJ. Mindfulness-based stress reduction for family caregivers: a randomized controlled trial. The Gerontologist. 2013;53(4):676–686. doi: 10.1093/geront/gns126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit SH, Reever KE, Back-Peterson J. Relatives of the impaired elderly: Correlates of feelings of burden. The Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]