Abstract
Background
Delivery of monomeric Amphotericin B (AmB), i.e. deaggregated AmB, has been a major tactic in the reduction of renal toxicity at a membrane level, taking advantage of the selectivity of monomeric AmB for binding ergosterol over cholesterol.
Objective
The aim of this study was to characterize the pharmacokinetic (PK) and renal toxicity of monomeric AmB in rats following a multiple dose regimen.
Method
AmB existed primarily in a monomeric state in poly(ethylene glycol)-block-poly(N-hexyl stearate L-aspartamide) (PEG-b-PHSA) micelles (mAmB) at 2:1 ratio (mol:mol), whereas AmB as its standard formulation, Fungizone®, was highly self-aggregated based on absorption spectroscopy.
Results
After single intravenous injection, mAmB significantly (p < 0.001) increased the area under the plasma drug concentration-time curve (AUC) and reduced the volume of distribution (Vd) and total systemic clearance (CL) relative to Fungizone®. After daily intravenous injections at dose of 1.0 mg/kg for 7 days, PK parameters of mAmB and Fungizone® were similar to day 1. The treatment of Fungizone® also significantly (p < 0.05) increased levels of urinary enzymes, N-acetyl-β-D-glucosaminidase (NAG) and kidney injury molecule-1 (KIM-1) by 3.1- and 3.0 fold, respectively, whereas levels of NAG and KIM-1 were unchanged for mAmB, consistent with hematoxylin and eosin (H&E) staining of excised kidneys.
Conclusion
In summary, mAmB has less renal toxicity than AmB as Fungizone® in rats after a multiple dose regimen, validating the aggregation state hypothesis of AmB in vivo.
Keywords: aggregation state, amphotericin B, antifungal, polymeric micelle, nephrotoxicity, pharmacokinetics, systemic fungal disease
1. INTRODUCTION
Amphotericin B (AmB) deoxycholate (Fungizone®; Amphotericin B for Injection USP) is an effective antifungal agent for life-threatening systemic fungal diseases, e.g. candidiasis and aspergillosis [1]. AmB binds ergosterol in fungal cell membranes, leading to membrane pore formation and monovalent ion leakage. However, use of AmB is hampered by renal toxicity: Renal tubular acidosis, nephrocalcinosis, azotemia, and hyposthenuria [2]. These side effects are mediated in part by renal vasoconstriction, which leads to decreased renal blood flow and glomerular filtration rate. AmB causes tubular injury by damaging cellular membranes, noting similarity of structure and composition between ergosterol and cholesterol [3, 4].
Two tactics to lower the renal toxicity of AmB involve liposomes and delivery of monomeric AmB, i.e. aggregation state hypothesis. Amphocil® (colloidal dispersion), Abelcet® (lipid complex), and Ambisome® (liposomal formulation) lower the incidence of AmB renal impairment by a reduction in distribution to kidneys [5]. However, higher doses (ca. 4 to 10 times) are required than Fungizone®, and they are expensive. Further, renal toxicity still causes discontinuation of antifungal treatment.
As an alternative strategy, the aggregation state hypothesis states that monomeric AmB selectively binds ergosterol over cholesterol, leading to pore formation in fungal cell membranes, whereas soluble self-aggregates of AmB form pores in both fungal and mammalian cell membranes. Thus, delivery of monomeric AmB has the potential to reduce kidney damage and yet retain antifungal efficacy. In primarily in vitro studies, organic solvents, cyclodextrins, micelles, and polymeric micelles have been shown to deaggregate AmB and reduce toxicity to mammalian cells while maintaining antifungal activity [6–8]. More recently, evidence that monomeric AmB causes less kidney impairment than Fungizone® has emerged in rats, validating the aggregation state hypothesis in vivo. In this report, PK and renal toxicity of mAmB has been evaluated in rats following a multiple dose regimen, more closely mirroring the clinical dosing schedule of AmB. The results point to higher systemic exposure of AmB relative to Fungizone® (i.e. increased AUC) and yet reduced renal toxicity upon repeat dosing. These in vivo results strongly support continued pre-clinical and clinical development of mAmB for the treatment of systemic fungal diseases.
2. MATERIALS AND METHODS
Materials
AmB and AmB for Injection, USP (Fungizone®) were purchased from X-GEN Pharmaceuticals Inc. (Horseheads, NY). HPLC-grade acetonitrile, methanol, glacial acetic acid, formic acid, and chloroform were obtained from Themo Fisher Scientific Inc. (Waltham, MA). Sterile 5% Dextrose Injection, USP (D5W) and 0.9% sodium chloride injection, USP were purchased from Baxter Healthcare (Deerfield, IL). 10% buffered formalin solution were obtained from Fisher Chemical Co (Fairlawn, NJ). All other chemicals or reagents were purchased from Sigma-Aldrich Inc. (St. Louis, MO).
Preparation of mAmB
PEG-b-PHSA was synthesized with some modifications as described by Lavasanifar et al. [9, 10]. PEG-b-PHSA had a PEG block at 10,000 g/mole, a PHSA block with a degree of polymerization of 12, and a degree of stearic acid substitution at 50%, based on 1H NMR spectroscopy. AmB was incorporated in PEG-b-PHSA micelles (2:1 molar ratio) using a thin film-hydration method as described previously [11]. Briefly, AmB dissolved in 6 mL of methanol (0.3 mg/mL) was added to PEG-b-PHSA dissolved in 10 mL of chloroform (6 mg/mL) in a round bottom flask. Organic solvent was removed at 65 °C under vacuum using a rotary evaporator. The film was purged with argon gas to remove residual organic solvent and hydrated with sterile D5W while rotating the flask for 10 min at 65 °C. The aqueous solution was sonicated for 30 sec and filtered through a sterile 0.22 µm nylon syringe filter. The hydrodynamic diameter and size distribution of mAmB were determined by dynamic light scattering (DLS) using Zetasizer Nano ZS (Malvern Instruments Inc., UK) employing a He-Ne laser at 633 nm as a light source with a detection angle at 173°. mAmB diluted with 3 mL of double-distilled water (final concentration of 0.45 mg/ml) was equilibrated for 2 min at 25 °C for measurement. The mean hydrodynamic size and polydispersity index (PDI) were calculated by cumulant analysis. Measurements were performed in triplicate, and data evaluated by volume-weighted analysis were represented as mean ± SD. Absorption spectra of mAmB and Fungizone® were determined over the range of 300–450 nm by CARY® 50 BIO UV-Vis Spectrophotometers (Varian, Palo Alto, CA) using a quartz cuvette (10 mm path length; Starna Cells Inc., Atascadero, CA). HPLC analytical method for AmB was adapted from a method reported previously [11].
Plasma pharmacokinetics of AmB mAmB and Fungizone®
Jugular vein cannulated Sprague-Dawley rats (male, 200–220 g) were purchased from Charles River (Chicago, IL). Cannulae were flushed with 0.9% sodium chloride solution 5–7 days after surgery as directed by provider. Fungizone® (1.0 mg/kg) or mAmB (1.0 mg/kg) were administered to rats through the lateral tail vein every day for 7 days. Blood samples of 100 to 200 µL were collected via a jugular vein cannula at 0, 5, 15, 30 min, 1, 2, 4, 8, 24 hr after dosing at day 1 and day 7, and were centrifuged at 1,200 g for 10 min at 4 °C to collect plasma. Samples were snap-frozen in liquid nitrogen, and kept at −80 °C until analysis by HPLC-UV, as described above. Animals were kept in a light- and temperature-controlled room while they were housed individually to avoid dislocation of cannula by other animals placed in the same cage. Standard rat chow diet and water were provided ad libitum. All procedures related to animal handling and care complied with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin-Madison in accordance with the US Public Health Service (PHS) Policy on Human Care and Use of Laboratory Animals.
Plasma sample preparation
For sample preparation, 50 µL of plasma sample was mixed with ice-cold acetonitrile (250 µL) and methanol (100 µL) and vortexed to precipitate plasma proteins. This mixture was centrifuged at 1,200 g for 10 min after adding internal standard (IS) solution (20 µL) diluted in mobile phase. Deproteinized supernatant was transferred to a 1.5 mL centrifuge tube and dried using a SpeedVac Concentrator (SVC200H, Savant Instruments, Inc., Holbrook, NY) with Universal Vacuum System (UVS400). Dried residue was reconstituted with mobile phase of 70 µL, and vortex-mixed followed by centrifugation at 1,200 g for 10 min. Supernatants were transferred into low volume glass vial inserts for HPLC-UV analysis.
Pharmacokinetic analysis
PK parameters such as apparent volume of distribution (Vd), total systemic clearance (CL), half-life (t1/2) and area under the plasma drug concentration-time curve (AUC) were calculated using a non-compartmental analysis using Phoenix/WinNonlin (64-bit version 6.4.0.768, Pharsight, Certara, Princeton, NJ). Parameter estimates for each groups were compared using a one-way ANOVA followed by post hoc Tukey’s honestly significant difference using Prism (Graph Pad, San Diego, CA). A p < 0.05 was considered as statistically significant.
Renal toxicity study
For urinary enzyme activity, Sprague Dawley (SD) rats were housed in individual metabolic cages (Techniplast, West Chester, PA) to collect urine separately from feces at day −1 (as control) and day 7. The collection tube was kept on the ice, and the collected urine was stored at −80 °C until analysis. The activity of urinary enzymes such as NAG and KIM-1 were measured using NAG assay kit (Diazyme Laboratory, Poway, CA) and rat KIM-1 ELISA (Alpco, Salem, NH), respectively. To minimize variation in urine concentration, the level of NAG was corrected by the urine creatinine level, which was determined by a creatinine colorimetric assay (Cayman Chemical, Ann Arbor, MI). Absorbance values were measured using a microplate reader (Synergy HT Multi-Mode Microplate Reader; BioTek Instruments Inc., Winooski, VT) at 505 nm and 450 nm for NAG and KIM-1, respectively.
For histological evaluation, rats were anesthetized using isoflurane and sacrificed by cervical dislocation. Kidneys were harvested rapidly, and stored in 10% neutral buffered formalin solution at 4 °C for 24 hr. After being bisected, samples were embedded in paraffin, followed by staining with hematoxylin and eosin (H&E). The stained sections of each samples were examined using an Olympus BX41 microscope equipped with an Olympus DP26 digital camera. Evidence of renal toxicity was assessed on H&E stained renal sections by a pathologist (Dr. Weixiong Zhong) who was blinded to the sample identity.
Statistical differences of data were determined using a two-way ANOVA followed by post hoc Tukey’s honestly significant difference using Prism (Graph Pad, San Diego, CA). Differences with a p < 0.05 were considered as statistically significant.
3. RESULTS AND DISCUSSION
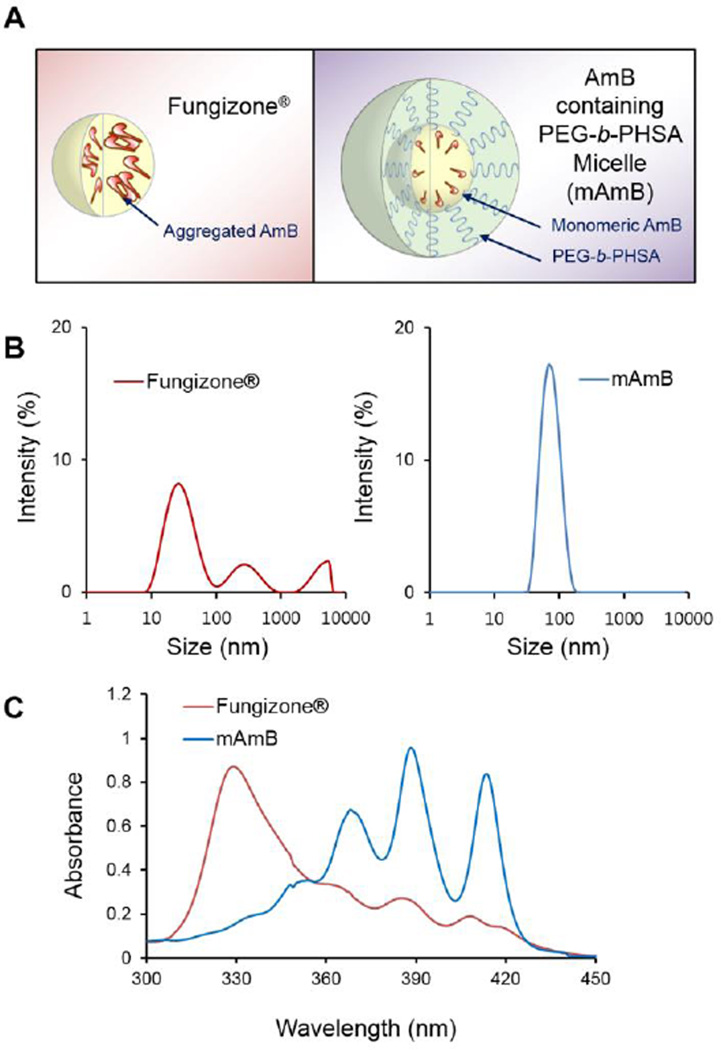
Physicochemical properties of mAmB versus Fungizone® are presented in Table 1. At a 2:1 mole ratio, the level of AmB attained in water by loading in PEG-b-PHSA micelles was ca. 0.50 mg/mL versus 0.10 mg/mL for Fungizone®. The level of AmB in water by itself is ca. 1 µg/mL. mAmB was physically stable against precipitation over 24 hours during storage at 4 °C (data not shown). mAmB had a z-average hydrodynamic diameter of 70.4 nm and a polydispersity index of 0.132 (Table 1 and Figure 1). By contrast, Fungizone® formed smaller colloidal aggregates with a z-average hydrodynamic diameter of 41.0 nm and a higher polydispersity index of 0.393 (Table 1 and Figure 1). Critically, mAmB exists predominately in a monomeric state based on its absorption spectrum, which is characterized by absorbance at ca. 412 nm (monomeric peak) and an absence of absorbance at 328 nm (aggregated peak) (Figure 1). By contrast, AmB as Fungizone® has strong absorbance at 328 nm, characteristic of AmB in a highly aggregated state (Figure 1). The absorbance ratio at 328 and 412 nm of reflects the degree of self-aggregation of AmB and has a value of 0.267 and 3.80 for mAmB and Fungizone®, respectively. In summary, while AmB is well solubilized in water both as mAmB and Fungizone® and has nanoscopic dimensions, AmB exists predominately as a monomeric species in PEG-b-PHSA micelles, presumably as a result of intermolecular interaction with stearic acid side chains as opposed to self-aggregation, whereas AmB as Fungizone® exists in a highly aggregated state, indicating that AmB interacts with both sodium deoxycholate and itself in water as a colloidal dispersion (Figure 1).
Table 1.
Characterization of Fungizone® and mAmB.
| Formulation | Fungizone® | mAmB |
|---|---|---|
| Excipient:AmB ratio (mol/mol) |
2:1 | 2:1 |
| Final level of AmB (mg/mL) |
0.1 | 0.53 ± 0.04 |
| z-average particle diameter (nm) |
41.0 ± 0.4 | 70.4 ± 0.2 |
| Polydispersity index (PDI) |
0.393 ± 0.029 | 0.132 ± 0.017 |
| Peak ratio at 340 and 412 nm (I340/I412) |
3.80 ± 0.56 | 0.267 ± 0.013 |
Fig. 1.
(A) Schematic illustration of Fungizone® and mAmB. (B) Size distribution of Fungizone® and mAmB. PEG-b-PHSA reduces particle size and PDI of AmB. (C) Spectra of Fungizone® and mAmB. AmB becomes more deaggregated in the presence of PEG-b-PHSA.
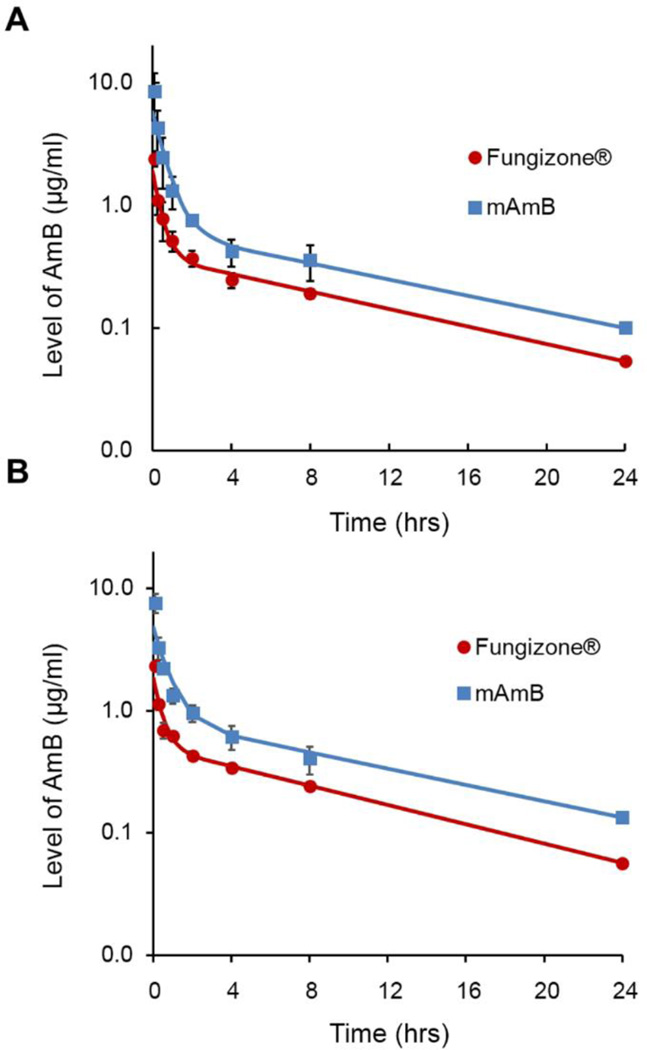
PK properties and renal toxicity of AmB were characterized after a multiple dosing regimen to mimic the clinical dosing schedule used for antifungal therapy, where Fungizone® is infused IV daily for several days to weeks, depending on the type of fungal infection. As shown in Figure 2, plasma level-time profiles of mAmB and Fungizone® were characterized by a rapid distribution phase and a slow elimination phase. mAmB produced higher plasma levels of AmB versus Fungizone® at all time points in this rat study. Plasma level-time profiles on days 1 and 7 were statistically similar, indicating an absence of AmB accumulation in plasma upon repeat dosing over one week for both mAmB and Fungizone®.
Fig. 2.
Plasma concentration-time profiles of AmB at day 1 (A) and day 7 (B) after multiple injection of Fungizone® and mAmB for 7 days.
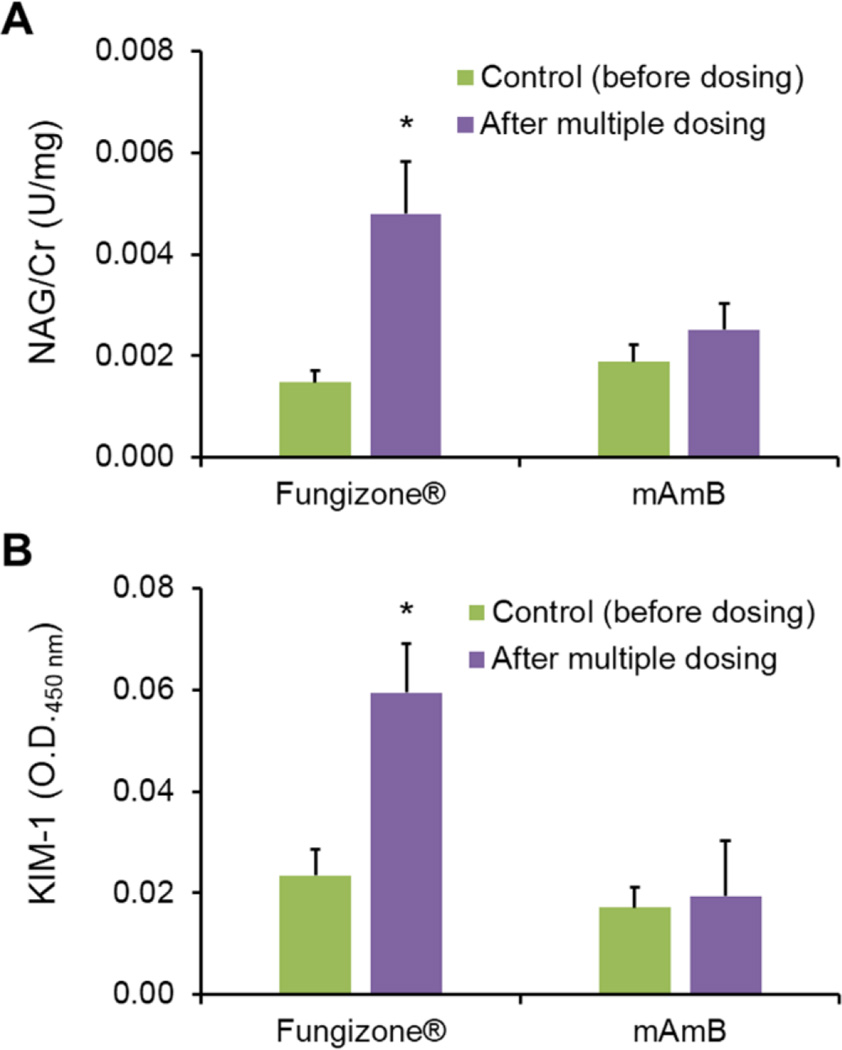
PK parameters of mAmB and Fungizone® were calculated by non-compartmental analysis (Table 2). Notably, PK properties of AmB were altered significantly by PEG-b-PHSA micelles versus Fungizone®: AUC of AmB increased 2.7-fold in animals dosed with mAmB, indicating an enhanced systemic exposure of mAmB over Fungizone®. Vd of mAmB was 0.521 L/kg versus 1.17 L/kg for Fungizone®, indicating lower distribution into tissue. CL of mAmB was reduced relative Fungizone®: 0.0741 and 0.200 L/hr/kg, respectively. Differences in PK parameters between mAmB and Fungizone® may be explained by the stability of PEG-b-PHSA micelles in blood that results in an evident nanocarrier effect by gradual release of AmB. This hypothesis requires validation by a PK study on PEG-b-PHSA micelles, requiring radiolabeling of PEG-b-PHSA. Support for this hypothesis, however, comes from an earlier single dose study on mAmB and Fungizone®, which did not reveal differences in PK profiles in rats after IV injection. In this earlier study, PEG-b-PHSA had a slightly different polymer composition, resulting in reduced stability in blood and faster CL. To assess the renal toxicity of mAmB, urinary enzymes, NAG and KIM-1, were measured prior to the start of treatment (day −1) and after 7 consecutive days of IV injection of mAmB or Fungizone®. NAG is a proximal tubular lysosomal enzyme known to be a highly sensitive and reliable biomarker for detecting tubular injury. KIM-1 is a transmembrane glycol-protein expressed in kidney epithelial cells also known to be a diagnostic marker for proximal tubular damage. It has been shown that an ectodomain of KIM-1 is cleaved from glycoprotein into the urine, and its expression is upregulated after kidney injury [12]. A central finding in this study was the absence of renal toxicity for mAmB after 7 consecutive days of IV injection. As shown in Figure 3, levels of urinary enzymes, NAG and KIM-1 were unchanged by mAmB. By contrast, Fungizone® increased urinary levels of NAG and KIM-1 by 3.1- and 3.0-fold, respectively (Figure 3).
Table 2.
Pharmacokinetic parameters for AmB after multiple dosing of Fungizone® and mAmB.
| Day | Formulation | AUC0−∞ (µg· hr/mL) |
CL (L/hr/kg) |
t1/2 (hr) |
Vd (L/kg) |
|---|---|---|---|---|---|
| 1 | Fungizone® (CV %) |
4.99 (4.04) |
0.200 (4.03) |
5.06 (3.59) |
1.17 (6.64) |
| mAmB (CV %) |
13.5 (2.94)** |
0.0741 (2.94)** |
7.38 (5.90) |
0.521 (5.47)* |
|
| 7 | Fungizone® (CV %) |
5.99 (3.65) |
0.167 (3.64) |
4.82 (4.01) |
1.03 (4.64) |
| mAmB (CV %) |
16.1 (2.31)** |
0.0623 (2.31)** |
8.02 (5.26) |
0.542 (4.21)* |
p<0.001 and
p<0.05 vs. Fungizone® group
Fig. 3.
Average NAG (A) and KIM-1 (B) levels in rat urine before dosing (control; green bar) and after multiple dosing of Fungizone® and mAmB (purple bar). *p ≤ 0.05 vs. control. The urinary levels of NAG and KIM-1 after treatment with Fungizone® for 7 days were significantly higher than that of mAmB. All data were presented as means ± SD (n=3)
Histological examination of excised kidneys treated with mAmB or Fungizone® were consistent with the NAG and KIM-1 results. As shown in Figure 4, kidney tissue sections of rats treated with mAmB (n=4) showed a normal morphology with typically scant interstitium, closely spaced tubules, and normal appearance of glomeruli and blood vessels similar to the control group. By contrast, renal toxicity after treatment with Fungizone® was clearly observed by histological examination: Several focal tubular damage and focal interstitial inflammation were observed in kidney tissue sections. Fungizone® is known to induce nephrocalcinosis, deposition of calcium salts in the kidneys, and it was observed in the Fungizone® group, but not the mAmB group.
Fig. 4.
Histological examination of kidney tissues stained with H&E (50×, scale bars = 50 µm). The sections of Fungizone®-treated groups (A) showed evidence of tubular damage such as epithelial thinning, focal interstitial inflammation or calcium phosphate deposits, whereas no evidence of tubular damage was found in the section of groups treated with mAmB (B) (n=3 for Fungizone®; n=4 for mAmB).
These toxicological results strongly suggest the mAmB, i.e. monomeric AmB, is dramatically less renal toxic than Fungizone®, i.e. aggregated AmB after a one week course of treatment in a rat model, and they are consistent with an earlier single dose study in rats comparing mAmB and Fungizone®, where the same conclusion was drawn [11]. In this earlier study, a change in kidney distribution of AmB as a factor in reduced renal toxicity was ruled out, showing no statistical difference in kidney distribution between mAmB and Fungizone®. The results of this multiple dose study are significant given that AmB is dosed daily over several days or weeks, depending on the fungal infection, as opposed to a single IV injection. Furthermore, while there was a substantial reduction in renal toxicity for mAmB versus Fungizone®, there was not change in the minimal inhibitory concentration against Candida albicans for mAmB versus Fungizone® (data not shown). This is consistent with the aggregation state hypothesis and many in vitro studies that have shown that monomeric AmB is selectively toxic for fungal cells, whereas aggregated species of AmB form damaging pores in fungal and mammalian cell membranes [6, 13].
4. CONCLUSION
We demonstrated that a PEG-b-PHSA micelle formulation of AmB exhibits less renal toxicity than a standard formulation, Fungizone, after a multiple dosing regimen without altering its in vivo pharmacokinetics. These effects are believed to be related to its deaggregated state (monomeric AmB) in the presence of PEG-b-PHSA micelles. Future studies are needed to determine the antifungal effect and optimal exposure-response profile of mAmB in a murine model of candidiasis as well as tissue distribution after a multiple dosing regimen. Nevertheless, this study showed delivery of monomeric AmB by PEG-b-PHSA micelles represents a promising strategy to reduce the dose-limiting renal toxicity of AmB.
Acknowledgments
This research was funded by NIH R01 AI101157.
LIST OF ABBREVIATIONS
- AmB
amphotericin B
- AUC
area under the plasma drug concentration-time curve
- CL
total systemic clearance
- CV
coefficient of variation
- D5W
sterile 5% dextrose Injection, USP
- DLS
dynamic light scattering
- ELISA
enzyme-linked immunosorbent assay
- H&E
hematoxylin and eosin
- IV
intravenous
- KIM-1
kidney injury molecule-1
- mAmB
amphotericin B micelle
- NAG
N-acetyl-β-D-glucosaminidase
- PDI
polydispersity index
- PEG
poly(ethylene glycol)
- PEG-b-PHSA
polyethylene glycol-block-poly(N-hexyl stearate L-aspartamide)
- PK
pharmacokinetics
- SD
standard deviation
- t1/2
half-life
- Vd
apparent volume of distribution
Biography

Glen S. Kwon
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Saliba F, Dupont B. Renal impairment and amphotericin B formulations in patients with invasive fungal infections. Med Mycol. 2008;46(2):97–112. doi: 10.1080/13693780701730469. [DOI] [PubMed] [Google Scholar]
- 2.McCurdy DK, Frederic M, Elkinton JR. Renal tubular acidosis due to amphotericin B. N Engl J Med. 1968;278(3):124–130. doi: 10.1056/NEJM196801182780302. [DOI] [PubMed] [Google Scholar]
- 3.Baginski M, Czub J. Amphotericin B and its new derivatives - mode of action. Curr Drug Metab. 2009;10(5):459–469. doi: 10.2174/138920009788898019. [DOI] [PubMed] [Google Scholar]
- 4.Laniado-Laborin R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26(4):223–227. doi: 10.1016/j.riam.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Andes D, Safdar N, Marchillo K, et al. Pharmacokinetic-pharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob Agents Chemother. 2006;50(2):674–684. doi: 10.1128/AAC.50.2.674-684.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legrand P, Romero EA, Cohen BE, et al. Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob Agents Chemother. 1992;36(11):2518–2522. doi: 10.1128/aac.36.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kajtar M, Vikmon M, Morlin E, et al. Aggregation of amphotericin B in the presence of gamma-cyclodextrin. Biopolymers. 1989;28(9):1585–1596. doi: 10.1002/bip.360280908. [DOI] [PubMed] [Google Scholar]
- 8.Kwon GS. Polymeric micelles for delivery of poorly water-soluble compounds. Crit Rev Ther Drug Carrier Syst. 2003;20(5):357–403. doi: 10.1615/critrevtherdrugcarriersyst.v20.i5.20. [DOI] [PubMed] [Google Scholar]
- 9.Lavasanifar A, Samuel J, Kwon GS. Micelles of poly(ethylene oxide)-block-poly(N-alkyl stearate L-aspartamide): synthetic analogues of lipoproteins for drug delivery. J Biomed Mater Res. 2000;52(4):831–835. doi: 10.1002/1097-4636(20001215)52:4<831::aid-jbm29>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 10.Lavasanifar A, Samuel J, Kwon GS. The effect of fatty acid substitution on the in vitro release of amphotericin B from micelles composed of poly(ethylene oxide)-block-poly(N-hexyl stearate-L-aspartamide) J Control Release. 2002;79(1–3):165–172. doi: 10.1016/s0168-3659(01)00537-5. [DOI] [PubMed] [Google Scholar]
- 11.Diezi TA, Takemoto JK, Davies NM, et al. Pharmacokinetics and nephrotoxicity of amphotericin B-incorporated poly(ethylene glycol)-block-poly(N-hexyl stearate l-aspartamide) micelles. J Pharm Sci. 2011;100(6):2064–2070. doi: 10.1002/jps.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann A, Baginski M, Winczewski S, et al. The effect of sterols on amphotericin B self-aggregation in a lipid bilayer as revealed by free energy simulations. Biophys J. 2013;104(7):1485–1494. doi: 10.1016/j.bpj.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]