Abstract
A group of side chain partially saturated tocotrienol analogues, namely tocoflexols, have been previously designed in an effort to improve the pharmacokinetic properties of tocotrienols. (2R,8′S,3′E,11′E)-δ-Tocodienol (1) was predicted to be a high value tocoflexol for further pharmacological evaluation. We now report here an efficient 8-step synthetic route to compound 1 utilizing naturally-occurring δ-tocotrienol as a starting material (24% total yield). The key step in the synthesis is oxidative olefin cleavage of δ-tocotrienol to afford the chroman core of 1 with retention of chirality at the C-2 stereocenter.
Keywords: Tocotrienol, Tocodienol, Oxidative olefin cleavage, C-C coupling, Desulfonylation
Graphical Abstract
1.Introduction
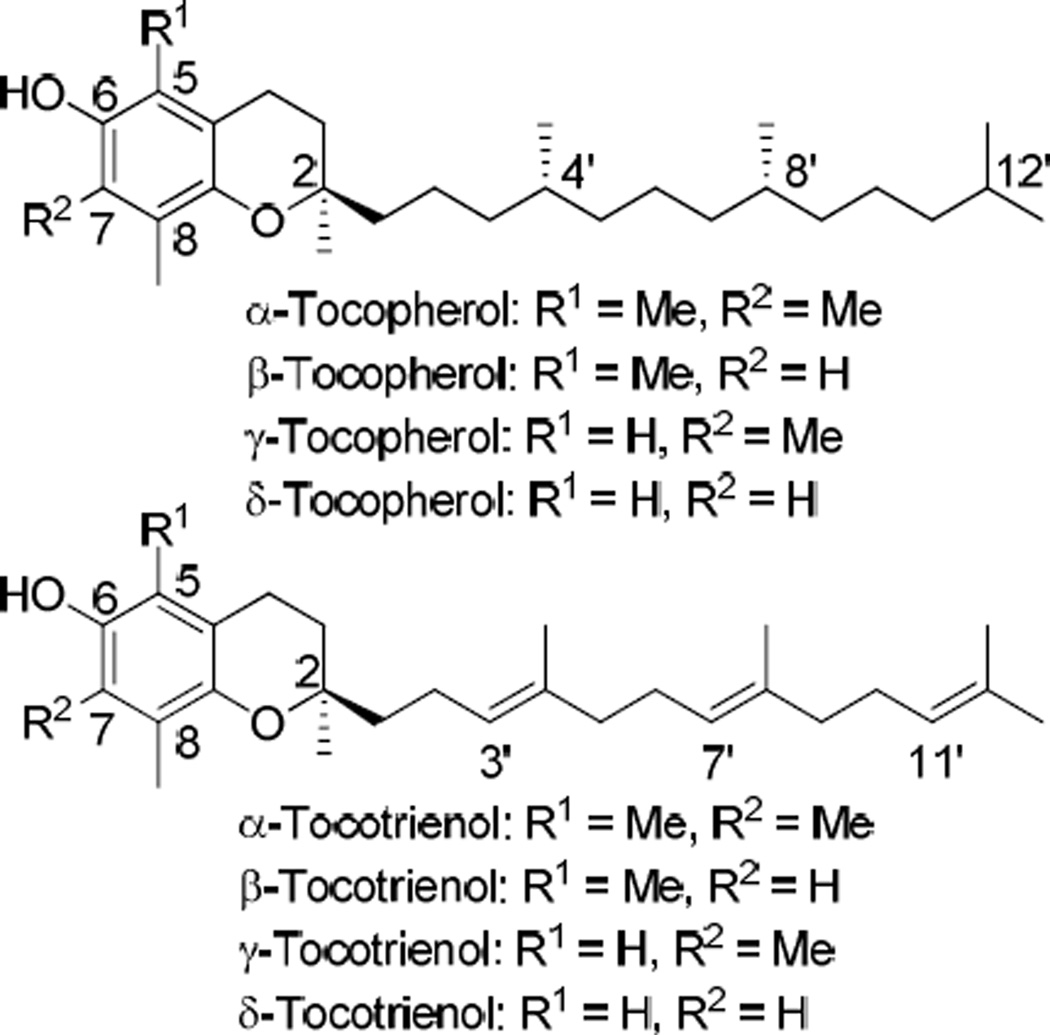
Tocotrienol, and its tocopherol counterpart belong to the vitamin E family. Both tocotrienol and tocopherol have α-, β-, γ-, and δ-homologues and share a common 6-hydroxychroman moiety, with tocotrienol having an unsaturated farnesyl side chain that differs from the saturated phytyl side chain in tocopherol (Figure 1). Tocotrienols have been shown to have many bioactivities that are often not observed with tocopherols.1 Both tocopherols and tocotrienols have been investigated for their use as potential radiation protectors. Among them, γ- and δ-tocotrienol exhibit the most significant radioprotective effects.2 However, both γ- and δ-tocotrienol have low bioavailability and short plasma elimination half-lives3 that limit their exposure in systemic circulation and require that they be administered in large doses.2 The low bioavailability and short half-live of γ- and δ-tocotrienol are, at least in part, caused by their weak binding affinity to α-tocopherol transfer protein (α-TTP). α-TTP is a specific transport protein responsible for transferring the vitamin E compounds out of the liver, where they are subject to metabolism, into the systemic circulation.4 α-Tocopherol is rapidly secreted from the liver into plasma because it has the highest affinity for α-TTP out of all vitamin E homologues.5 In contrast, γ- and δ-tocotrienol are restrained in the liver due to their low affinity for α-TTP. As a result, the estimated apparent elimination half-lives of γ-tocotrienol (t1/2 = 4.3 h) and δ-tocotrienol (t1/2 = 2.3 h) in humans are 4.5- and 8.7-fold shorter, respectively, than α-tocopherol (t1/2 = 20 h).3
Figure 1.
Structures of naturally occurring tocopherols and tocotrienols.
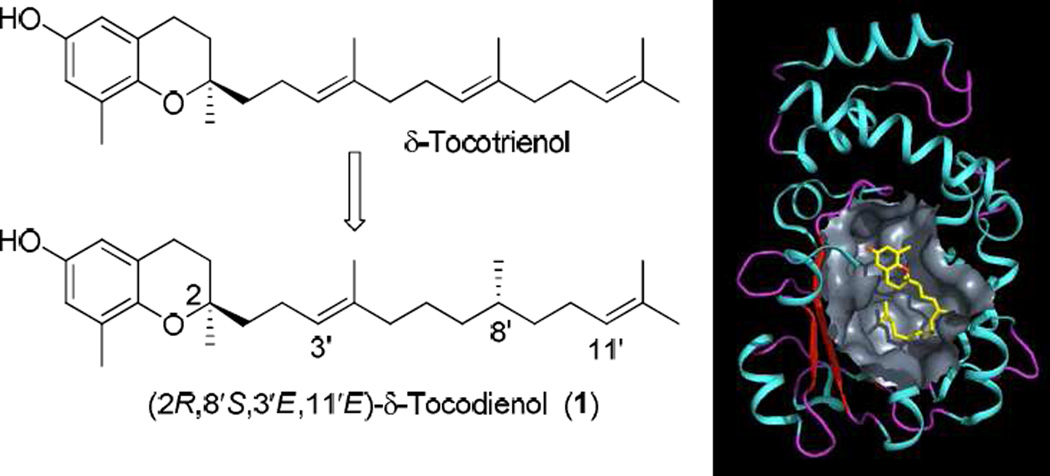
The crystal structures of naturally occurring (2R,4′R,8′R)-α-tocopherol bound to α-TTP reveal that the C4′–C9′ fragment of the saturated phytyl side chain in the α-tocopherol molecule is bent into a U-shape.6 It is difficult for the C4′–C9′ fragment in the farnesyl chain of tocotrienol to take up the same shape because of the relatively high rigidity caused by the presence of the Δ3′ and Δ7′ double bonds, which compromises the incorporation of tocotrienols into the binding pocket of α-TTP. These observations prompted us to design more flexible analogues with enhanced bendability in their side chains by saturating Δ3′ and/or Δ7′ double bonds in the δ-tocotrienol molecule. This group of “flexible” δ-tocotrienol analogues was collectively termed tocoflexols, from which (2R,8′S,3′E,11′E)-δ-tocodienol (1, Figure 2) was identified as a compound with the highest affinity for α-TTP through a vigorous molecular dynamics-based screening program.7 Herein, we describe the synthesis of compound 1 from naturally-occurring δ-tocotrienol.
Figure 2.
Structures of δ-tocotrienol and (2R,8′S,3′E,11′E)-δ-tocodienol (1) (left); and simulated docking of 1 in the binding cavity of α-TTP (right).
2.Results and discussion
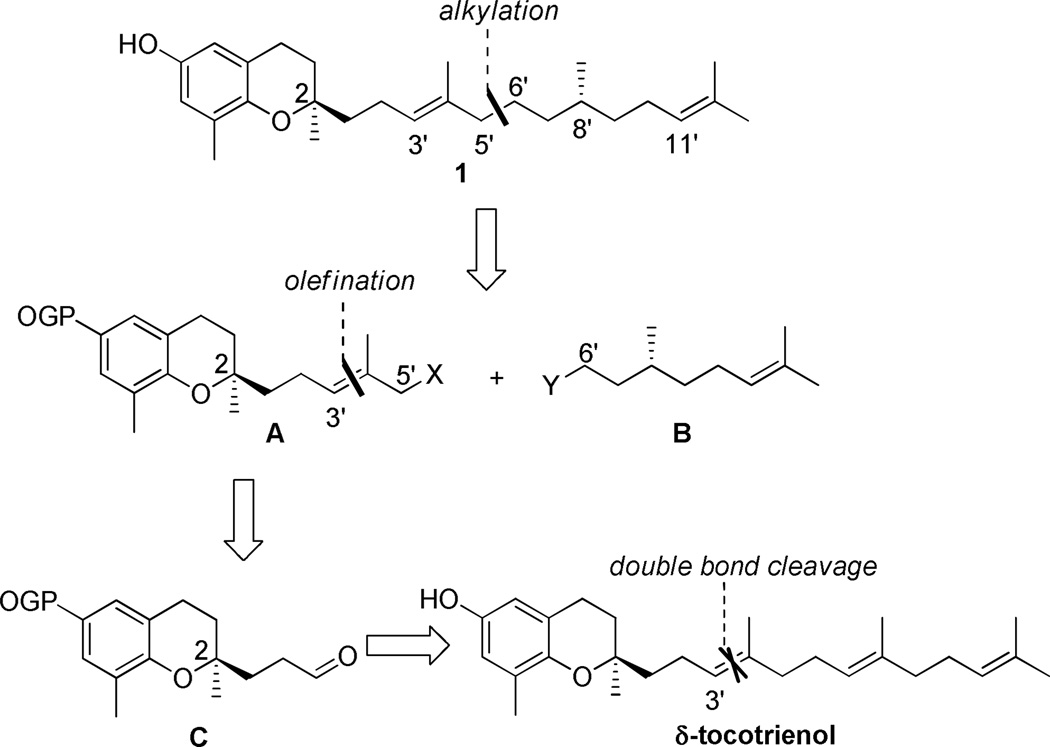
Our retrosynthetic strategy is outlined in Scheme 1. A disconnection at the C5′–C6′ bond of (2R,8′S,3′E,11′E)-δ-tocodienol (1) would lead to segments A and B, which can be coupled through various alkylation methods. Segment B can be derived from commercially available R-(+)-citronellol, while segment A can be derived from aldehyde C via a Horner-Wadsworth-Emmons (HWE) olefination reaction to form the E-double bond.
Scheme 1.
Retrosynthetic approach to (2R,8′S,3′E,11′E)-δ-tocodienol (1).
The syntheses of aldehyde C (3-chromanylpropanals) type building blocks, including the corresponding 3-chromanylpropanols and 3-chromanylpropanoic acids, in optically pure forms have been reported.8 There are also a variety of reported enantioselective approaches to the synthesis of the chiral chroman core of vitamin E, i.e. chromanylmethanols,9 which could serve as a precursor for the synthesis of aldehyde C. However, most of these syntheses are low-yielding, multistep procedures. Given the potential utility of C as a key intermediate for the synthesis of δ-tocotrienol analogues with modifications on the side chain, we decided to search for an alternative but simpler and more efficient method to this compound. As δ-tocotrienol is readily available from natural sources, we envisioned that C could be synthesized by oxidative cleavage of the double bonds of naturally occurring optical pure δ-tocotrienol.
Commercially available DeltaGold®, a tocopherol free vitamin E supplement obtained in an enriched form from annatto oil, contains 67% δ-tocotrienol and 7.5% γ- tocotrienol, and was used as the source of δ-tocotrienol. The isolation of δ-tocotrienol from DeltaGold® using silica gel based flash column chromatography was straightforward and afforded 3.5 g of δ-tocotrienol in a pure form from 6.0 g of the mixture.10
The 6-OH group of δ-tocotrienol was then protected as a silyl ether moiety and the resulting TBS-protected product 2 was submitted to olefin oxidative cleavage (Table 1). Compound 2 was treated with OsO4 (5 mol%) and NaIO4 (9 equiv) in THF/H2O at room temperature; the consumption of the starting material and intermediates was monitored by GC-MS and TLC. Initial screening of reaction conditions revealed that the ratio of the two solvents, THF and H2O, was an important factor for the progress of the reaction, and a 3:1 ratio of THF/H2O afforded the best result (Table 1, entry 1). A relatively low reaction concentration (0.05–0.1 M) of 2 was necessary for the progress of the reaction, likely due to the low solubility of both 2 and NaIO4 in the solvent mixture. Disappointingly, only low yields of aldehyde 3 (10–17% yield) were obtained from these reactions.11 No improvement was seen in the yield of 3 using various solvents (1,4-dioxane, diethyl ether, acetone, and t-butanol were examined) or by adding 2,6-lutidine.12
Table 1.
Screening of reaction conditions for the oxidative cleavage reaction
| entry | conditions | time (h) | yield |
|---|---|---|---|
| 1 | OsO4 (5 mol%), NaIO4 (9 equiv), THF/H2O (3:1) | 24 | 10–17%a |
| 2 | OsO4 (5 mol%), NaIO4 (9 equiv), THF/H2O (1:1) | 48 | n.d.b |
| 3 | OsO4 (5 mol%), NaIO4 (9 equiv), THF/H2O (10:1) | 24 | tracec |
| 4 | i) OsO4 (5 mol%), NMO (4 equiv), THF/H2O (10:1); ii) NaIO4 (4 equiv), THF/H2O (1:1)d |
24 12 |
38%a |
| 5 | i) OsO4 (5 mol%), NMO (4 equiv), 2,6-lutidine (6 equiv), acetone/H2O (10:1); ii) PhI(OAc)2 (4 equiv), acetone/H2O (10:1)e |
15 2 |
62%f |
Yields were based on the corresponding alcohol.
n.d. = not determined.
The major product was olefin dihydroxylation product containing three pairs of 1,2-diols.
One-pot reaction, NaIO4 and H2O were added directly to the reaction mixture.
One-pot reaction, PhI(OAc)2 was added directly to the reaction mixture.
Yield of purified 3.
When the ratio of THF/H2O was changed to 1:1, a significant amount of unreacted starting material (2) still remained (TLC and GC-MS analyses) after 48 hours, likely due to the low solubility of 2 in the solvent mixture (Table 1, entry 2). A decrease in the proportion of H2O in the solvent mixture by changing the ratio of THF/H2O to 10:1 resulted in a relatively rapid disappearance of 2. However, only trace amounts of 3 were detected and the major product was the olefin dihydroxylation product (with three pairs of 1,2-diols) due to the low solubility of NaIO4 in the reaction mixture (entry 3). We reasoned that the sluggish reaction was caused by the very different solubilities of 2 and NaIO4 in the reaction media, and the low yields were probably caused by the long reaction time and the relatively unstable nature of aldehyde 3 in the reaction mixture. The dihydroxylation step was completed in 24 hrs when the reaction was conducted in a 10:1 ratio of THF/H2O (entry 3). Olefin dihydroxylation of 2 afforded three pairs of 1,2-diols, which should provide sufficient solubility in a polar solvent system that can dissolve NaIO4. We envisaged that such homogeneous or near homogeneous reaction mixture would result in rapid cleavage of the 1,2-diol products. Thus, we tried the reaction in a two-step one-pot fashion by initial treatment of 2 with OsO4 (5 mol%) and N-methylmorpholine-N-oxide (NMO, 4 equiv) in a 10:1 ratio mixture of THF/H2O to form the tris-dihydroxylation product, followed by addition of H2O (to a final 1:1 ratio of THF/H2O) and NaIO4 to the reaction mixture. Under these conditions, compound 3 was obtained in a significantly improved conversion (38% after conversion to the alcohol) (entry 4). Further investigation of the reaction revealed that a procedure developed by Nicolaou et al.,13 involving PhI(OAc)2 as an oxidative cleaving agent of 1,2-diols, afforded the best results. Aldehyde 3 was isolated in 62% yield (entry 5) and the enantiopurity at C-2 was retained with >95% ee based on chiral HPLC analysis.14
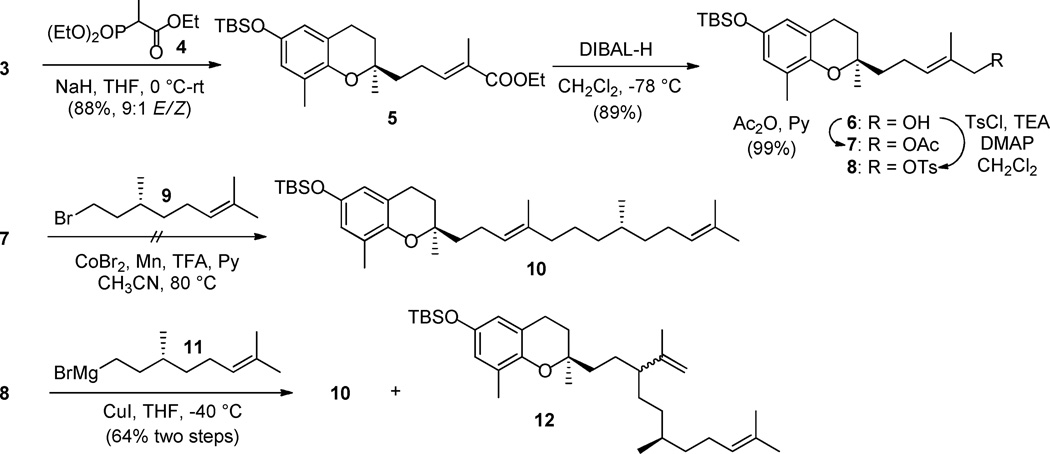
Aldehyde 3 was then converted into 5 through a HWE olefination procedure using triethyl 2-phosphonopropionate (4) under the treatment of sodium hydride (88% yield, 9:1 E/Z selectivity based on GC-MS analysis) (Scheme 2). DIBAL-H reduction of ester 5 gave the corresponding alcohol 6 (89% yield), which was converted into acetate 7 in quantitative yield. Coupling of compound 7 with (R)-(−)-citronellyl bromide (9) was attempted under conditions reported by Gosmini et al.15 Surprisingly, 7 remained unchanged during the course of the reaction, while a model coupling reaction between 2-methylallyl acetate and 9 worked as expected. Alternatively, a coupling reaction between tosylate 8 and Grignard reagent 11, derived from 6 and 9, respectively, proceeded smoothly to the desired product 10 with good regioselectivity (α:γ 92:8 based on GC-MS analysis). Unfortunately, removal of the γ-alkylation product 12 from the product mixture was unsuccessful.
Scheme 2.
Synthesis of intermediates 7/8 and attempts for the formation of 10.
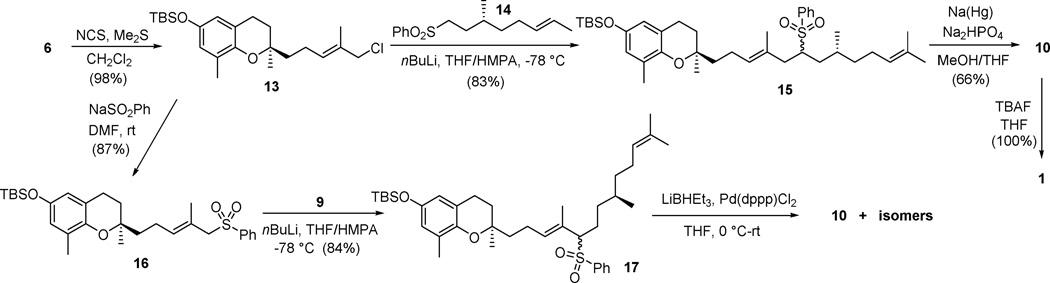
To avoid the formation of 12, we turned our effort to a sulfone based C-C coupling method (Scheme 3).16 Corey-Kim chlorination17 of 6 afforded 13 in 98% yield. Reacting of 13 with the anion obtained on treatment of sulfone 14 with nBuLi in THF/HMPA furnished 15 in 83% yield as a diastereoisomers. Under these conditions, no γ-alkylation product was observed. It is noteworthy that no coupling product was formed in the absence of HMPA. HMPA is known to facilitate carbanion formation and to strongly coordinate lithium cation, thereby increase the reactivity of the carbanion.18 The subsequent reductive cleavage of the sulfone group of 15 using sodium amalgam to form 10 occurred in 66% yield. Removal of the TBS-group with TBAF/THF led to the final product 1 in quantitative yield.
Scheme 3.
Sulfone based coupling reaction for synthesis of compound 1.
It has been reported that Kotake’s Pd-mediated desulfonylation procedure usually affords high yields.16d,16e,19 However, the sulfone group required to be at the allylic position. Accordingly, chloride 13 was converted to sulfone 16 (87% yield), which was then coupled with bromide 9 to furnish 17 in 84% yield (Scheme 3). The sulfone group of 17 was removed smoothly by treating with super-hydride (LiBHEt3) in the presence of catalytic amount of Pd(dppp)Cl2. However, the desulfonylation reaction afforded an inseparable mixture of 10 and its three isomers, presumably 3'Z-isomer of 10 and 4'E/Z mixture of double isomerized product.20
3.Conclusions
In summary, we have developed an efficient synthesis of (2R,8′S,3′E,11′E)-δ-tocodienol (1), a δ-tocotrienol analogue designed to have improved pharmacokinetic properties. A novel strategy, involving an oxidative olefin cleavage of naturally-occurring δ-tocotrienol, was employed for the generation of the chroman core of 1. The aldehyde intermediate from the olefin cleavage reaction is a key building block for the synthesis of tocotrienol analogues, which are of great interest in the search for new radiation protective agents. Preliminary studies showed that 1 was equally potent as an antioxidant when compared to its parent compound, δ-tocotrienol (see Supporting Information). Further investigations on the biological properties of 1 and its potential to be considered as a clinical candidate are currently ongoing in our laboratory.
4.Experimental section
DeltaGold® was obtained from American River Nutrition, Inc. Dry THF, CH2Cl2, and DMF were obtained via a solvent purification system by filtering through two column packed with activated alumina and 4 Å molecular sieve, respectivelye. All other reagents and solvents obtained from commercial sources were used without further purification. If dry and air-free conditions were required, reactions were performed in oven-dried glassware (130 °C) under a positive pressure of argon. Flash chromatography was performed using silica gel (230–400 mesh) as the stationary phase. Reaction progress was monitored by thin layer chromatography (silica-coated glass plates) and visualized under UV light and after exposure to iodine vapor, and by GC-MS. NMR spectra were recorded in CDCl3 at 400 MHz for 1H, and 100 MHz for 13C NMR. Chemical shifts δ are given in ppm using tetramethylsilane as an internal standard. Multiplicities of NMR signals are designated as singlet (s), broad singlet (br s), doublet (d), doublet of doublets (dd), triplet (t), quartet (q), and multiplet (m). HRMS were recorded on an Agilent LCTOF spectrometry unit run in the ESI/APCI mode (Mass Spectrometry Facility, University of California Riverside).
4.1. tert-Butyl{[2,8-dimethyl-2(R)-(4,8,12-trimethyltrideca-3E,7E,11-trien-yl)chroman-6-yl]oxy}dimethylsilane (2)
To a solution of δ-tocotrienol (400 mg, 1.0 mmol) in CH2Cl2 (10 mL) at 0 °C was added imidazole (172 mg, 2.5 mmol) and TBSCl (183 mg, 1.2 mmol) and the resulting mixture was stirred at room temperature for 5 h. The reaction mixture was partitioned between ethyl acetate (30 mL) and water (30 mL). The aqueous layer was extracted with ethyl acetate (2 × 10 mL), and the combined organic layers were washed with water (20 mL) and brine (20 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 30:1) to afford compound 2 (470 mg, 92%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 6.47 (d, J = 2 Hz, 1H), 6.37 (d, J = 2 Hz, 1H), 5.13 (m, 3H), 2.70 (t, J = 6.4 Hz, 2H), 2.07–2.16 (m, 9H), 1.98 (m, 4H), 1.73–1.81 (m, 2H), 1.69 (s, 3H), 1.54–1.66 (m, 11H), 1.27 (s, 3H), 0.98 (s, 9H), 0.17 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 147.7, 146.5, 135.2, 135.1, 131.4, 127.0, 124.5, 124.4, 124.3, 120.9, 120.2, 117.3, 75.4, 39.9, 39.8, 31.6, 26.9, 26.7, 25.9, 25.8, 24.2, 22.6, 22.3, 18.3, 17.8, 16.2, 16.2, 16.0, −4.3; MS (EI) m/z 510.5 (M+); HRMS (ESI/APCI) m/z calcd for C33H55O2Si [M+H]+ 511.3966, found 511.3957.
4.2. 3-[2,8-Dimethyl-6-tert-butyldimethylsiloxychroman-2(S)-yl]propanal (3)
To a solution of compound 2 (170 mg, 0.33 mmol) in acetone/water (4 mL/0.19 mL) was added N-methylmorpholine-N-oxide (NMO) (155 mg, 1.32 mmol), 2,6-lutidine (181 mg, 2 mmol), and OsO4 (2% aqueous solution, 0.21 mL, 0.016 mmol). The final acetone/water ratio was 10:1. After stirring at room temperature for 15 h, (diacetoxyiodo)benzene (425 mg, 1.50 mmol) was added and the resulting mixture was allowed to stir at room temperature for 2 h before being quenched with saturated aqueous Na2SO3 (5 mL). The mixture was extracted with ethyl acetate (3 × 10 mL) and the combined organic layers were washed with brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 20:1) to afford compound 3 (71 mg, 62%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 9.80 (s, 1H), 6.46 (d, J = 2.4 Hz, 1H), 6.37 (d, J = 2.4 Hz, 1H), 2.69–2.75 (m, 2H), 2.60–2.64 (m, 2H), 2.00–2.11 (m, 4H), 1.86–1.92 (m, 1H), 1.72–1.81 (m, 2H), 1.23 (s, 3H), 0.97 (s, 9H), 0.15 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 202.6, 148.0, 145.9, 127.0, 120.7, 120.4, 117.4, 74.5, 38.7, 32.4, 31.7, 25.9, 23.7, 22.4, 18.2, 16.3, −4.3. MS (EI) m/z 348.2 (M+); HRMS (ESI/APCI) m/z calcd for C20H33O3Si [M+H]+ 349.2194, found 349.2178.
4.3. Ethyl 5-[2,8-dimethyl-6-tert-butyldimethylsiloxychroman-2(R)-yl]-2-methylpent-2(E)-enoate (5)
To a suspension of NaH (60%, 83 mg, 2.1 mmol) in THF (1.5 mL) was added triethyl 2-phosphonopropionate (0.27 mL, 1.26 mmol) dropwise at 0 °C. After stirring at 0 °C for 30 min, a solution of compound 3 (400 mg, 1.15 mmol) in THF (2 mL) was added dropwise. The resulting mixture was allowed to stir at the same temperature for 1 h before being quenched with saturated aqueous NH4Cl (5 mL). The mixture was extracted with ethyl acetate (3 × 10 mL) and the combined organic layers were washed with water (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 15:1) to afford compound 5 (352 mg, 88%, double configuration assigned by NOESY) as a colorless oil: 1H NMR(400 MHz, CDCl3) δ 6.78 (t, J = 7.2 Hz, 1H), 6.46 (s, 1H), 6.37 (s, 1H), 4.18 (q, J = 7.2 Hz, 2H), 2.68–2.73 (m, 2H), 2.33 (q, J = 7.6 Hz, 2H), 2.11 (s, 3H), 1.82 (s, 3H), 1.72–1.88 (m, 3H), 1.62–1.67 (m, 1H), 1.27–1.30 (m, 6H), 0.97 (s, 9H), 0.16 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 168.3, 147.8, 146.2, 142.2, 127.9, 127.0, 120.8, 120.3, 117.4, 75.0, 60.5, 38.5, 31.7, 25.9, 24.0, 23.1, 22.5, 18.3, 16.2, 14.4, 12.3, −4.3; MS (EI) m/z 432.4 (M+); HRMS (ESI/APCI) m/z calcd for C25H40O4NaSi [M+Na]+ 455.2588, found 455.2606.
4.4. 5-[2,8-Dimethyl-6-tert-butyldimethylsiloxychroman-2(R)yl]-2-methylpent-2(E)-en-1-ol (6)
To a solution of compound 5 (260 mg, 0.6 mmol) in CH2Cl2 (3 mL) at −40 °C was added DIBAL-H (1.2 M in toluene, 1.25 mL, 1.5 mmol) dropwise. After stirring at the same temperature for 1.5 h, the reaction was quenched with three drops of MeOH and diluted with CH2Cl2 (10 mL), then poured into saturated Rochelle salt solution (10 mL). The resulting mixture was stirred at room temperature overnight. The aqueous phase was extracted with ethyl acetate (2 × 10 mL) and the combined organic layers were washed with brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 5:1) to afford compound 6 (235 mg, 89%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 6.46 (d, J = 2 Hz, 1H), 6.37 (d, J = 2 Hz, 1H), 5.42 (t, J = 2.8 Hz, 1H), 3.98 (s, 2H), 2.67–2.72 (m, 2H), 2.14–2.21 (m, 2H), 2.12 (s, 3H), 1.65–1.82 (m, 6H), 1.54–1.60 (m, 1H), 1.46 (br s, 1H), 1.27 (s, 3H), 0.97 (s, 9H), 0.16 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 147.7, 146.4, 134.9, 126.9, 126.4, 120.9, 120.3, 117.4, 75.2, 69.1, 39.4, 31.6, 25.9, 24.2, 22.6, 21.9, 18.3, 16.2, 13.6, −4.3; MS (EI) m/z 390.3 (M+); HRMS (ESI/APCI) m/z calcd for C23H38O3NaSi [M+Na]+ 413.2482, found 413.2491.
4.5. 1-Chloro-5-[2,8-dimethyl-6-tert-butyldimethylsiloxychroman-2(R)-yl]-2-methyl-2(E)-pentene (13)
To a solution of N-chlorosuccinimide (60 mg, 0.9 mmol) in CH2Cl2 (3 mL) at 0 °C was added dimethyl sulfide (66 µL, 0.9 mmol) dropwise. The resulting white suspension was stirred at 0 °C for 30 min then a solution of compound 6 (117 mg, 0.3 mmol) in CH2Cl2 (0.5 mL) was added dropwise. The resulting mixture was allowed to stir at 0 °C for 30 min before being quenched with water. The aqueous phase was extracted with CH2Cl2 (2 × 5 mL) and the combined organic layers were washed with water (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 10:1) to afford compound 13 (120 mg, 98%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 6.46 (d, J = 2 Hz, 1H), 6.37 (d, J = 2 Hz, 1H), 5.55 (t, J = 7.2 Hz, 1H), 4.00 (s, 2H), 2.65–2.75 (m, 2H), 2.16–2.22 (m, 2H), 2.11 (s, 3H), 1.64–1.81 (m, 6H), 1.52–1.60 (m, 1H), 1.26 (s, 3H), 0.97 (s, 9H), 0.16 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 147.8, 146.3, 131.8, 131.0, 126.9, 120.9, 120.3, 117.4, 75.1, 52.6, 39.1, 31.7, 25.9, 24.1, 22.6, 22.5, 18.5, 16.2, 14.1, −4.3; MS (EI) m/z 408.3/410.3 (M+); HRMS (ESI/APCI) m/z calcd for C23H38O2Si35Cl [M+H]+ 409.2324, found 409.2317.
4.6. (R)-[(3,7-Dimethyloct-6-en-1-yl)sulfonyl]benzene (14)
To a solution of 8-bromo-2,6-dimethyloct-2-ene (300 mg, 1.29 mmol) in DMF (5 mL) was added sodium benzenesulfinate (253 mg, 1.54 mmol) at room temperature. The resulting mixture was stirred overnight. Ethyl acetate (40 mL) was added and the resulting mixture was washed with water (20 mL) and brine (20 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 5:1) to afford compound 14 (310 mg, 81%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 7.55–7.92 (m, 5H), 5.02 (t, J = 7.2 Hz, 1H), 3.01–3.14 (m, 2H), 1.87–1.96 (m, 2H), 1.66–1.78 (m, 1H), 1.66 (s, 3H), 1.46–1.61 (m, 5H), 1.22–1.31 (m, 1H), 1.11–1.18 (m, 1H), 0.85 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 139.3, 133.7, 131.8, 129.4, 128.2, 124.2, 54.6, 36.5, 31.6, 29.2, 25.8, 25.3, 19.2, 17.8; MS (EI) m/z 280.1 (M+); HRMS (ESI/APCI) m/z calcd for C16H25O2S [M+H]+ 281.1570, found 281.1566.
4.7. tert-Butyl{[2,8-dimethyl-2(R)-(4,8S,12-trimethyltrideca-3E,11-dien-yl)chroman-6-yl]oxy}dimethylsilane (10)
To a solution of compound 14 (56 mg, 0.20 mmol) in THF/HMPA (3 mL/0.5 mL) at −78 °C was added n-BuLi (1.6 M in hexane, 125 uL, 0.24 mmol) dropwise. The resulting bright orange-red colored mixture was allowed to stir at −78 °C for 1 h. Compound 12 (82 mg, 0.20 mmol) in THF (0.5 mL) was added dropwise and the resulting mixture and the reaction was allowed to warm up to room temperature. After turning into a colorless solution (about 1h at room temperature), the reaction was quenched with saturated aqueous NH4Cl (5 mL) and extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 20:1) to afford compound 15 (108 mg, 83%) as a mixture of two diastereomers which were used directly to next step. Compound 15 (50 mg, 0.077 mmol) was then dissolved in THF/MeOH (2:5, 4 mL) and NaHPO4 (54 mg, 0.38 mmol) was added at 0 °C followed by sodium amalgam (5%, 354 mg, 0.77 mmol). After stirring at the same temperature for 2 h, water (10 mL) was added and the reaction was quenched by adding 1.0 M HCl dropwise to pH 7. The resulting mixture was extracted with ethyl acetate (3 × 10 mL) and the combined organic phases was washed with saturated aqueous NaHCO3 (15 mL), water (15 mL), and brine (15 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 30:1) to afford compound 10 (26 mg, 66%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 6.46 (d, J = 2 Hz, 1H), 6.37 (d, J = 2 Hz, 1H), 5.13–5.10 (m, 2H), 2.69–2.60 (m, 2H), 2.12–2.09 (m, 5H), 1.95–1.90 (m, 3H), 1.78–1.74 (m, 2H),1.69 (s, 3H), 1.68–1.61 (m, 1H), 1.60 (s, 3H), 1.57 (s, 3H), 1.55–1.45 (m, 1H), 1.32–1.26 (m, 4H), 1.26 (s, 4H), 1.26–1.22 (m, 1H), 1.20–1.00 (m, 2H), 0.97 (s, 9H), 0.86 (d, J = 6.4 Hz, 3H), 0.16 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 147.7, 146.5, 135.7, 131.1, 127.0, 125.2, 124.2, 121.0, 120.2, 117.4, 75.4, 40.1, 39.9, 37.3, 36.7, 32.5, 31.6, 29.9, 25.9, 25.7, 25.5, 24.2, 22.6, 22.3, 19.8, 18.3, 17.8, 16.2, 15.9, −4.3; MS (EI) m/z: 512.4 (M+); HRMS (ESI/APCI) m/z calcd for C33H57O2Si [M+H]+ 513.4122, found 513.4134.
4.8. (2R,8′S,3′E,11′E)-δ-Tocodienol (1)
To a solution of compound 10 (13 mg, 0.025 mmol) in THF (3 mL) was added TBAF (10 mg, 0.038 mmol) at 0 °C. After stirring at the same temperature for 15 min, the reaction mixture was partitioned between ethyl acetate (10 mL) and water (5 mL). The aqueous layer was extracted with ethyl acetate (2 × 5 mL), and the combined organic layers were washed with water (3 mL) and brine (3 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was chromatographed on silica (hexanes/ethyl acetate 15:1–10:1) to afford 1 (10 mg, 100%) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 6.48 (d, J = 2.8 Hz, 1H), 6.38 (d, J = 2.8 Hz, 1H), 5.11 (m, 2H), 4.18 (br s, 1H), 2.65–2.74 (m, 2H), 2.12 (s, 3H), 2.08 – 2.13 (m, 2H), 1.85–1.98 (m, 3H), 1.60–1.80 (m, 2H), 1.68 (s, 3H), 1.62–1.66 (m, 1H), 1.60 (s, 3H), 1.58 (s, 3H), 1.52–1.56 (m, 1H), 1.18–1.38 (m, 9H), 1.04–1.17 (m, 2H), 0.85 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 147.8, 146.2, 135.7, 131.1, 127.5, 125.2, 124.2, 121.4, 115.7, 112.7, 75.5, 40.1, 39.9, 37.3, 36.7, 32.5, 31.5, 25.9, 25.7, 25.5, 24.2, 22.6, 22.3, 19.8, 17.8, 16.2, 15.9; MS (EI) m/z 398.3 (M+); HRMS (ESI/APCI) m/z calcd for C27H43O2 [M+H]+ 399.3258, found 399.3262.
Supplementary Material
Acknowledgments
This work was supported financially by the National Institute of General Medical Sciences of the NIH under grant number P20 GM109005. We thank Dr. Barrie Tan and American River Nutrition, Inc. for providing DeltaGold®.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data (Reaction procedures and GC-MS spectra for 10 from 6 and 16; 1H and 13C NMR spectra of compounds 1, 2, 3, 5, 6, 10, 13, and 16; antioxidant data) associated with this article can be found in the online version, at http://dx.doi.org/xxxxxx
References and notes
- 1.Tan B, Watson RR, Preedy VR. Tocotrienols: Vitamin E Beyond Tocopherols. 2nd. Boca Raton, FL: Taylor & Francis/CRC Press; 2012. [Google Scholar]
- 2.a Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao T, Hauer-Jensen M, Kumar KS. Int. J. Radiat. Biol. 2009;85:598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]; b Li XH, Fu D, Latif NH, Mullaney CP, Ney PH, Mog SR, Whitnall MH, Srinivasan V, Xiao M. Haematologica. 2010;95:1996–2004. doi: 10.3324/haematol.2010.026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yap SP, Yuen KH, Wong JW. J. Pharm. Pharmacol. 2001;53:67–71. doi: 10.1211/0022357011775208. [DOI] [PubMed] [Google Scholar]
- 4.Morley S, Cecchini M, Zhang W, Virgulti A, Noy N, Atkinson J, Manor D. J. Biol. Chem. 2008;283:17797–177804. doi: 10.1074/jbc.M800121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, Arai H, Inoue K. FEBS Lett. 1998;409:105–108. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 6.a Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A. J. Mol. Biol. 2003;331:725–734. doi: 10.1016/s0022-2836(03)00724-1. [DOI] [PubMed] [Google Scholar]; b Min KC, Kovall RA, Hendrickson WA. Proc. Natl. Acad. Sci. USA. 2003;100:14713–14718. doi: 10.1073/pnas.2136684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compadre CM, Singh A, Thakkar S, Zheng G, Breen PJ, Ghosh S, Kiaei M, Boerma M, Varughese KI, Hauer-Jensen M. Drug Dev. Res. 2014;75:10–22. doi: 10.1002/ddr.21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Uria U, Vila C, Lin M–Y, Rueping M. Chem. Eur. J. 2014;20:13913–13917. doi: 10.1002/chem.201403768. [DOI] [PubMed] [Google Scholar]; b Lecea M, Hernández-Torres G, Urbano A, Carreño MC, Colobert F. Org. Lett. 2010;12:580–583. doi: 10.1021/ol9027804. [DOI] [PubMed] [Google Scholar]; c Rein C, Demel P, Outten RA, Netscher T, Breit B. Angew. Chem. Int. Ed. 2007;46:8670–8673. doi: 10.1002/anie.200703268. [DOI] [PubMed] [Google Scholar]; d Jung ME, MacDougall JM. Tetrahedron Lett. 1999;40:6339–6342. [Google Scholar]
- 9.a Chênevert R, Courchesne G. Tetrahedron Lett. 2002;43:7971–7973. [Google Scholar]; b Fuchs M, Simeo Y, Ueberbacher BT, Mautner B, Netscher T, Faber K. Eur. J. Org. Chem. 2009:833–840. [Google Scholar]; c Couladouros EA, Moutsos VI, Lampropoulou M, Little JL, Hyatt JA. J. Org. Chem. 2007;72:6735–6741. doi: 10.1021/jo0705418. [DOI] [PubMed] [Google Scholar]; d Chênevert R, Courchesne G, Pelchat N. Bioorg. Med. Chem. 2006;14:5389–5396. doi: 10.1016/j.bmc.2006.03.035. [DOI] [PubMed] [Google Scholar]; e Spivak AY, Shafikov RV, Odinokov VN. Arkivoc. 2011:67–75. [Google Scholar]; f Sugai T, Watanabe N, Ohta H. Tetrahedron: Asymmetry. 1991;2:371–376. [Google Scholar]; g Hyatt JA, Skelton C. Tetrahedron: Asymmetry. 1997;8:523–526. [Google Scholar]; h Takabe K, Okisaka K, Uchiyama Y, Katagiri T, Yoda H. Chem. Lett. 1985:561–562. [Google Scholar]; i Harada T, Hayashiya T, Wada I, Iwa-ake N, Oku A. J. Am. Chem. Soc. 1987;109:527–532. [Google Scholar]
- 10.Alternatively, DeltaGold® was treated directly with TBSCl in CH2Cl2 to form TBS-protected tocotrienols, which were then subjected to column separation. However, the separation between TBS-δ- and TBS-γ-tocotrienol proved to be very difficult.
- 11.Aldehyde 3 was inseparable from other components in the reaction mixture. To determine the reaction yields, the crude product was treated with NaBH4/EtOH to afford the corresponding primary alcohol, which was readily isolated by column chromatography in 10–17% yield (two steps).
- 12.Yu W, Mei Y, Kang Y, Hua Z, Jin Z. Org. Lett. 2004;19:3217–3219. doi: 10.1021/ol0400342. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaou KC, Adsool VA, Hale RH. Org. Lett. 2010;12:1552–1555. doi: 10.1021/ol100290a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The ee was determined on the corresponding primary alcohol of 3 by HPLC using a Chiralcel OD-H column.
- 15.Qian X, Auffrant A, Felouat A, Gosmini C. Angew. Chem. Int. Ed. 2011;123:10586–10589. doi: 10.1002/anie.201104390. [DOI] [PubMed] [Google Scholar]
- 16.a Grieco PA, Masaki Y. J. Org. Chem. 1974;39:2135–2136. [Google Scholar]; b Terao S, Kato K, Shiraishi M, Morimoto H. J. Chem. Soc. Perkin Trans. I. 1978:1101–1110. [Google Scholar]; c Sato K, Miyamoto O, Inoue S, Yamamoto T, Hirasawa Y. J. Chem. Soc. Chem. Commun. 1982:153–154. [Google Scholar]; d Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ. J. Med. Chem. 1992;35:3595–3606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]; e Li X, Lantrip D, Fuchs PL. J. Am. Chem. Soc. 2003;125:14262–14263. doi: 10.1021/ja0377596. [DOI] [PubMed] [Google Scholar]
- 17.Corey EJ, Kim CV, Takeda MA. Tetrahedron Lett. 1972;42:4339–4342. [Google Scholar]
- 18.a Normant H. Angew. Chem. Int. Ed. Engl. 1967;6:1046–1067. [Google Scholar]; b Normant H. Russ. Chem. Rev. (Engl. Transl.) 1970;39:457–484. [Google Scholar]
- 19.a Kotake H, Yamamoto T, Kinoshita H. Chem. Lett. 1982;82:1331–1334. [Google Scholar]; b Mohri M, Kinoshita H, Inomata K, Kotake H, Takagaki H, Yamazaki K. Chem. Lett. 1986:1177–1180. [Google Scholar]
- 20.The ratio between 10 and the combined amount of three isomers is about 60:40 based on GC-MS analysis.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.