Abstract
A constitutional guarantee of access to essential medicines has been identified as an important indicator of government commitment to the progressive realization of the right to the highest attainable standard of health. The objective of this study was to evaluate provisions on access to essential medicines in national constitutions, to identify comprehensive examples of constitutional text on medicines that can be used as a model for other countries, and to evaluate the evolution of constitutional medicines-related rights since 2008. Relevant articles were selected from an inventory of constitutional texts from WHO member states. References to states’ legal obligations under international human rights law were evaluated. Twenty-two constitutions worldwide now oblige governments to protect and/or to fulfill accessibility of, availability of, and/or quality of medicines. Since 2008, state responsibilities to fulfill access to essential medicines have expanded in five constitutions, been maintained in four constitutions, and have regressed in one constitution. Government commitments to essential medicines are an important foundation of health system equity and are included increasingly in state constitutions.
Introduction
Essential medicines are an indispensable component of health systems and are recognized as part of the right to health under international law.1 One indicator of a government’s commitment to access to medicines and health system equity is the inclusion of medicines-related rights in national constitutions.2 Constitutional rights to health and medicines set priorities for national health policies and programs, while also creating the mechanisms necessary to enforce these rights before domestic courts.3 An initial scoping study conducted in 2008 of 186 constitutions identified only three constitutions worldwide that included provisions on medicines, and a fourth that provided for essential goods and services.4 Since then, many constitutions have been amended or redrafted, coinciding with recent economic, political, and social tensions linked to the 2008 financial crisis, the 2009 European debt crisis, and the 2011 Arab Spring.5
Every constitutional review is an opportunity for the state to include health and medicines rights in the constitution, in line with human rights standards. The time is ripe to evaluate whether state constitutions recognize that essential medicines are part of the right to health. The starting point is a survey of the current provisions on essential medicines in national constitutions against the backdrop of states’ legal obligations under international human rights law and the right to health framework. We identify model text for policy makers and advocates seeking to enshrine these rights in their national constitutions. This study also develops an inventory of constitutional provisions on essential medicines in order to track their evolution since 2008.
Essential medicines in international law
The right to the progressive realization of the highest attainable standard of health first emerged as a social right in the World Health Organization (WHO) Constitution.6 It was also included in Article 25.1 of the Universal Declaration of Human Rights (UDHR) and confirmed in numerous international and regional treaties since then.7 The most important treaty in this respect, the binding International Covenant on Economic, Social and Cultural Rights (ICESCR), details the realization of the right to health through four concrete and targeted steps, including access to health facilities, goods, and services.8 The non-binding but highly respected General Comment No. 14, drafted by the Committee on Economic, Social and Cultural Rights (CESCR), further specifies in paragraph 43(d) that access to essential medicines is part of the right to health. Paragraphs 34-37 of General Comment No. 14 clearly describe governments’ legal obligations to respect, protect, and fulfill the right to health. The duty to respect is a negative obligation to refrain from interfering with the enjoyment of health rights. The duties to protect and to fulfill impose positive obligations on states to take measures to safeguard (to protect) and to take measures to ensure that health rights can be enjoyed (to fulfill).9 This tripartite typology helps to identify the specific legal obligations of states to realize the right to health. Moreover, the right to health framework in General Comment No. 14 (known as the AAAQ framework) enumerates the elements of the right to health as accessibility, availability, acceptability, and (assured) quality of goods and services, including essential medicines “as defined by the WHO Action Programme on Essential Drugs.”10
WHO defines essential medicines as “those that satisfy the priority healthcare needs of the population. Essential medicines are chosen with due regard to disease prevalence, efficacy, safety and comparative cost-effectiveness.”11 According to WHO, essential medicines are used for disease prevention, treatment, and control, and are applicable to most chronic and acute diseases.12 It is, therefore, of great concern that an estimated one-third of the population in developing countries is unable to access essential medicines on a regular basis.13 One way to address this unmet need is through national legislation that promotes a human rights approach to essential medicines.
The constitutional right to health and essential medicines
Most countries have ratified at least one of the international or regional treaties that include the right to health, including 163 state parties to the International Covenant on Economic, Social and Cultural Rights (ICESCR).14 Access to essential medicines as part of the progressive realization of the right to health is well founded in international law and binding on many states. But do these laws mean anything in practice? Hogerzeil et al. identified 59 court cases from 12 low- and middle-income countries in which access to essential medicines was claimed under the right to health. Half of these cases related to life-saving treatment of HIV/AIDS. The study showed that international treaties, if enforced through constitutional provisions, could indeed promote the realization of individual rights at the national level. The examples identified by Hogerzeil et al., most of which are from Latin America, show that individual court cases can generate entitlements across a population group, that the right to health is not restricted by limitations in social security coverage, that government policies have successfully been challenged in court, and that states have special obligations towards the poor and disadvantaged. In the countries studied, other factors in successfully generating entitlements are a link between the right to health and the right to life (in case of life-threatening disease) and support by public interest NGOs.15
There is much variation in de jure constitutional recognition of human rights and de facto application of these rights in practice.16 One example can be found in high-income countries, over half of which lack a constitutional right to health or medical care.17 At the same time, many high-income economies do have functioning health systems providing services and medicines on a regular basis. Despite this phenomenon, constitutional health rights are valuable tools to promote access to medicines. Hogerzeil et al. showed that one of the most important success factors for the practical implementation of access to essential medicines was the fact that the right to health principles of the international human right treaties had been incorporated into national constitutions.18 For this reason, the constitutional recognition of access to essential medicines is an indicator of government commitment to the right to health and health system equity.19
Given that national constitutions have proven so important in realizing the right to health, the next question is: which countries have incorporated access to essential medicines in their national constitution? Our baseline study in 2008 identified provisions on essential medicines, goods, and services in only four national constitutions worldwide: Mexico, Panama, the Philippines, and the Syrian Arab Republic.20 Since 2008, many governments have revised and amended existing constitutions or created new constitutions, sometimes in reaction to the changing political, social, and economic circumstances caused in part by the global financial crisis, the European debt crisis, and the Arab Spring.21
The purpose of this study was to track the evolution of national constitutions and identify those that now include a specific reference to the legal obligations to respect, protect, and fulfill access to essential medicines as part of the right to health. We identified examples of constitutional texts that include the AAAQ elements of the right to health. These constitutional texts may serve as a model for other countries interested in updating or strengthening their own constitutions. Model constitutional provisions could assist domestic legislators in formulating an inclusive constitutional health clause suited to national needs and resources. Patient and advocacy groups can also use the model texts in their efforts to advance universal access to essential medicines.
Methods
A comprehensive inventory of constitutional texts from WHO member states was created. We considered essential medicines to be encompassed by the terms “medicines” and “vaccines,” as well as by “essential goods”, “services”, “supplies” and “aids”.
We assembled an inventory of constitutional provisions from two points in time: those currently in force and the version in force in 2007. The Chronology of the Comparative Constitutions Project was used to determine these two time points for each country.22 Constitutions were retrieved in English (preferred), Spanish, or French from six online databases: the Comparative Constitutions Project, ConstitutionNet, Political Database of the Americas, International Constitutional Law, Constitution Finder, and the African Human Rights Law Document Database.23 When necessary, a search term ([country], constitution, [year]) was also used in Google and the first 20 results were consulted.
Once located, each constitution was searched for the keywords health, medical, medicines, pharmaceutical, drug, vaccination, and vaccine (located using the stem vaccin if permitted by the search tool). The original language texts were consulted in cases of uncertainty. All search results were included in the database.
Analytical framework
This study analyzed national constitutions through international human rights standards elaborated in General Comment No. 14 on the right to health. The legal obligations and AAAQ framework described in General Comment No. 14 use concise terminology to assert well-defined commitments to which governments can be held accountable. Moreover, clear references to human rights standards in national constitutions may facilitate their legal interpretation. Melton et al. suggest that the scope of the constitutional text, such as whether it is focused by topic rather than using complex cross-referencing, and the use of once-only words for clarity and brevity, are of greater importance for clear interpretation.24 Therefore, the legal obligations and the AAAQ framework were selected because they have brief, clear, and objective communication about governments’ commitments in realizing the right to health as related to essential medicines.
Legal obligations to essential medicines
Constitutional text was examined for each category of legal obligation: to respect, to protect, and to fulfill medicines-related rights. The first category, the obligation to respect the right to health as it relates to medicines, is described in paragraph 34 of General Comment No. 14, as a duty of non-interference.25 States must refrain from “denying or limiting equal access for all persons” and abstain from marketing unsafe medicines or otherwise interfering with the acceptability, availability, or accessibility of medicines.26 An example of denying equal access to medicines for all persons would be limiting access to available contraceptives due to the nature of the medicine or the familial status of the patient.
The second category, the obligation to protect the right to health as it relates to medicines, is explained in paragraph 35 of General Comment No. 14 as the duties:
to ensure that privatization of the health sector does not constitute a threat to the availability, accessibility, acceptability and quality of health facilities, goods and services;
[and] to control the marketing of medical equipment and medicines by third parties.27
This duty includes, for example, regulating the production and sale of medicines on the domestic market. In essence, states must take measures to protect against interventions by third parties that could damage the provision of acceptable, accessible, and available medicines of good quality. Relevant third parties include pharmaceutical manufacturers, and they should be required by law to adhere to good manufacturing practices in order to produce medicines of assured quality. Pharmacists and medical prescribers should be adequately trained to help patients use medicine appropriately and by prescribing the right medicine, for the right patient, in the right dose, and for the right duration.
The third category, the obligation to fulfill the right to health, was drawn from paragraph 37 of General Comment No. 14. In this provision, states are duty-bound to facilitate health rights by taking “positive measures that enable and assist individuals and communities to enjoy the right”.28 States must also provide for health rights when an individual or group is unable to provide medicines with the means at their own disposal.29 Finally states are obliged to promote the right to health by undertaking “actions that create, maintain and restore the health of the population”.30
The obligation to fulfill requires states to take positive actions to enable rights holders to enjoy their right to health. For example, states are responsible to develop and maintain a health care system through which medicines are available, accessible (affordable, within physical reach, and free of discrimination), acceptable, and of assured quality. States also have the duty to provide medicines to the impoverished and to individuals who cannot otherwise access them, such as prisoners or ethnic minorities.
We applied this typology to determine the prevalence of each category of obligation by WHO region and by national income.31 In addition, we noted the terminology used to describe duties to fulfill access to essential medicines.
AAAQ framework applied to essential medicines
In order to identify model text, we selected constitutional provisions in each category of legal obligations that satisfied the greatest number of right to health elements described in General Comment No. 14 as accessibility, availability, acceptability, and (assured) quality. We view these elements as being complimentary to the legal obligations in the sense that the AAAQ framework elaborates on the multiple elements needed to fully enjoy the respect, protection, and fulfillment of medicines-related rights.
The AAAQ elements, defined in paragraph 12 of General Comment No. 14, require that health goods and services be available in sufficient quantity, accessible to all without discrimination, within safe physical reach (called physical accessibility), and at an affordable price (called economic accessibility), as well as ensuring the accessibility of information about health issues (called information accessibility). Health goods and services must also be acceptable from a cultural and generational standpoint, and they must respect medical ethics. Medicines must be available in a form that is usable by unconscious patients and people with difficulty swallowing, as well as in very small dosages for neonates. Their quality must be assured and health goods and services must be scientifically and medically appropriate. Provisions that addressed the greatest number of AAAQ elements are presented as model text.
Finally, to understand how duties to fulfill medicines rights have changed since the 2008 global financial crisis, we compared texts in our database from constitutions in force in 2007 to those currently in force. The similarities and differences were noted.
Results
Overview of constitutional medicines rights worldwide
Current constitutions of 185 WHO member states were retrieved. Of the 185 constitutions, no constitutions include the obligation to respect medicines-related rights, while 14 constitutions (7.6%) include provisions to protect and 13 (7%) to fulfill medicines-related rights. (See Table 1 below.) The constitutional duties to protect and fulfill access to essential medicines were not mutually exclusive and a total of 22 constitutions enshrined at least one of these legal obligations. The constitutional duty to fulfill utilized the terminology access to medicines (n=3), vaccination (n=2), medicines (n=4), essential goods and services including medical aids (n=3), and mechanisms to control the costs of medicines (n=1).
Table 1.
Global view of medicines-related rights in national constitutions.
| Country | WHO Region | Income level | Year of adoption | Respect | Protect | Fulfill |
|---|---|---|---|---|---|---|
| Angola | AFRO | UMI | 2010 | ✓ | ||
| Bolivia | PAHO | LMI | 2009 | ✓ | ✓ | |
| Brazil | PAHO | UMI | 2014 | ✓ | ||
| Bulgaria | EURO | UMI | 2007 | ✓ | ||
| Cape Verde | AFRO | LMI | 2010 | ✓ | ||
| Cuba | PAHO | UMI | 2002 | ✓ | ||
| Czech Republic | EURO | HI | 2013 | ✓ | ||
| Dominican Republic | PAHO | UMI | 2010 | ✓ | ||
| Ecuador | PAHO | UMI | 2011 | ✓ | ✓ | |
| Egypt | EMRO | LMI | 2014 | ✓ | ||
| El Salvador | PAHO | LMI | 2009 | ✓ | ||
| Guatemala | PAHO | LMI | 1993 | ✓ | ||
| Honduras | PAHO | LMI | 2013 | ✓ | ||
| Mexico | PAHO | UMI | 2014 | ✓ | ||
| Mozambique | AFRO | LI | 2007 | ✓ | ||
| Niger | AFRO | LI | 2010 | ✓ | ||
| Panama | PAHO | UMI | 2004 | ✓ | ||
| Paraguay | PAHO | LMI | 2011 | ✓ | ✓ | |
| Philippines | WPRO | LMI | 1987 | ✓ | ✓ | |
| Portugal | EURO | HI | 2005 | ✓ | ✓ | |
| Suriname | PAHO | UMI | 1992 | ✓ | ||
| Syrian Arab Republic | EMRO | LMI | 2012 | ✓ |
Abbreviations used in this table: AFRO: WHO Africa Region; EMRO: WHO Eastern Mediterranean Region; EURO: WHO European Region; PAHO: WHO Pan-American Region; SEARO: WHO South East Asia Region; WPRO: WHO Western Pacific Region; LI: Low-income economy; LMI: Lower-middle-income economy; UMI: Upper-middle-income economy; HI: High-income economy
Worldwide, protecting and fulfilling medicines-related rights were most often enshrined in constitutions in the Pan-American (PAHO) region, followed by the European (EURO) and African (AFRO) regions. Two constitutions in the Eastern Mediterranean region (EMRO) enshrined duties to fulfill (9%) while duties to protect were absent in this region. Medicines-related rights are notably absent from the South-East Asia (SEARO) region and scarce in the Western Pacific region (WPRO)(4% of WPRO constitutions), with the exception of the Philippines.
The majority of provisions on medicines were identified in middle-income economies enshrining the obligation to protect (n=12) and to fulfill (n=10) . Only two high-income economies, Portugal and the Czech Republic, included duties to protect and/or to fulfill in their constitutions. Only one low-income economy, Mozambique, imposed the state responsibility to protect medicines-related rights. A second low-income economy, Niger, cited the duty to fulfill to essential needs and services in its constitution.
Model constitutional text to protect and fulfill the right to essential medicines
Model constitutional text was sought from our database and reproduced below in order to make it accessible for future constitutional framers.
We did not identify any constitutional text requiring states to respect medicines rights as part of the right to health.
We identified two types of provisions concerning the duty to protect access to essential medicines. The first is a state obligation to monitor pharmaceutical supplies to protect citizens against the provision of poor quality medicines by third parties. El Salvador’s Constitution provides:
The State shall be equipped with the necessary and indispensable resources for permanent control of the quality of chemical, pharmaceutical and veterinary products through surveillance organisms.32
This provision creates a duty to protect because the state is obliged to monitor the quality of medicinal products marketed by third parties. Interestingly, this text also requires the state to provide “indispensable resources” for it to carry out these activities without interruption. However, the text does have some deficiencies. While it refers to quality control, it does not address regulation of the safety, efficacy, and quality of medicines entering the market, which are equally important. In Portugal, the Constitution provides that the state shall be under a primary duty to “regulate and control the production, distribution, marketing, sale and use of chemical, biological and pharmaceutical products and other means of treatment and diagnosis.”33 This text, addressing the duty to protect, goes beyond the regulation of medicines produced by third parties. Here, the state is obliged to control medicines throughout their lifecycle by regulating the acts of third parties in their “production, distribution, marketing, sale and use.”34
The second type of protection of medicines rights is a novel addition to constitutional law: the declaration that international trade agreements shall not interfere with access to medicines. This new phrasing was introduced in two Latin American constitutions. In Bolivia, the Constitution provides that “the right to access medicine shall not be restricted by intellectual property rights and commercial rights, and it contemplates quality standards and first generation medicine.” 35 Moreover, the Bolivian Constitution specifies that negotiation, signing and ratification of international relations shall preserve “the right of the population to have access to all medications, primarily generic medications”.36
In Ecuador, the Constitution provides:
The application of international trade instruments shall not undermine, either directly or indirectly, the right to health, access to medicine, inputs, services or scientific and technological breakthroughs.37
These provisions protect the right to access medicines from being limited by the actions of third parties, including the application of international trade instruments and/or intellectual property rights.
The most comprehensive obligations to fulfill medicines entitlements embodied the AAAQ framework and were identified in the constitutions of Ecuador and Panama. In Ecuador, the Constitution provides that the state shall be responsible for:
“Guaranteeing the availability and access to quality, safe and effective medicines, regulating their marketing, and promoting the national production and use of generic drugs that meet the epidemiological needs of the population. With respect to access to medicine, public health interests shall prevail over economic and commercial interests.38
This text cites the state’s duty to ensure the availability, quality, and accessibility of medicines, although the type of accessibility is unclear. It is positive that the text also supports the rational use of medicines by regulating their marketing and promoting generic use; these aspects are often missing from government commitments despite states’ crucial role in creating conditions for appropriate use of medicines.
Panama’s Constitution identifies the State as having a primary obligation to develop certain activities and facilities with the aim of prevention, cure and rehabilitation, including:
“[The] Establishment, in accordance with the requirements of each region, of centers which provide comprehensive health care services, and supply medicines to all the people. These services and medicines shall be given free to those who lack economic means to purchase them.”39
In this text, the government is obliged to provide medicines universally and free of charge to those who cannot afford them; this is otherwise known as economic accessibility.
Evolution of the duty to fulfill access to essential medicines as part of the right to health
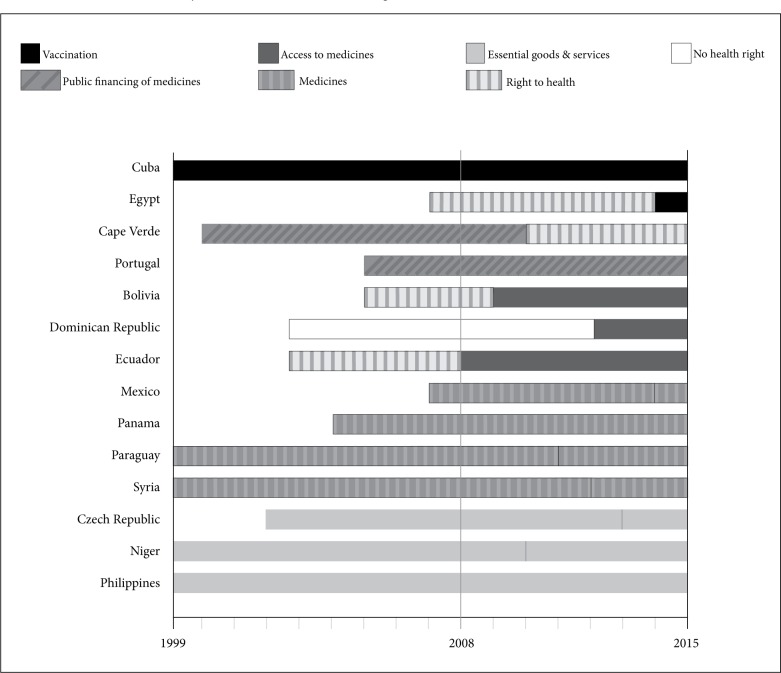
We retrieved 108 constitutions that have undergone review since 2008. Of these countries, the obligation to fulfill medicines-related rights was introduced in five constitutions, maintained in four constitutions, and has regressed in one constitution. Figure 1 compares the terminology used before and after the constitutional revision (marked by vertical black lines) in these 10 countries.
Figure 1.
Evolution of the duty to fulfill medicines-related rights in constitutions since 2008.
Of the five countries that have expanded health rights, Ecuador, Bolivia, and the Dominican Republic introduced medicines-related rights in their newly drafted constitutions for the first time in 2008, 2009, and 2010 respectively (see Figure 1 and Table 2). These three upper-middle-income economies introduced the term “access to medicines” into their constitutions. A fourth country, Niger, introduced the right to “essential needs and supplies” into its constitution in 2010. Finally, Egypt introduced the right of children to “free compulsory vaccinations” in its new constitution in 2014.
Table 2.
Government duties to fulfill medicines-related rights in national constitutions.
| Country | WHO Region | World Bank lending group | Current Constitution | Change in duty to fulfill since 2008 | ||
|---|---|---|---|---|---|---|
| Terminology describing duty to fulfill | Year of adoption | New or amended constitution | ||||
| Egypt | EMRO | LMI | Vaccination | 2014 | New | Added |
| Mexico | PAHO | UMI | Medicines | 2014 | Amended | Maintained |
| Czech Republic | EURO | HI | Medical aids | 2013 | Amended | Maintained |
| Syrian Arab Republic | EMRO | LMI | Medicines | 2012 | New | Unclear^ |
| Ecuador | PAHO | UMI | Access to medicines | 2011 | Amended* | Added |
| Paraguay | PAHO | LMI | Medicines | 2011 | Amended | Maintained |
| Dominican Republic | PAHO | UMI | Access to medicines | 2010 | New | Added |
| Niger | AFRO | LI | Essential needs and services | 2010 | New | Added |
| Bolivia | PAHO | LMI | Access to medicines | 2009 | New | Added |
| Portugal | EURO | HI | Public financing of medicines costs | 2005 | ||
| Panama | PAHO | UMI | Medicines | 2004 | ||
| Cuba | PAHO | UMI | Vaccination | 2002 | ||
| Philippines | WPRO | LMI | Essential goods and services | 1987 | ||
Abbreviations used in this table: AFRO : WHO Africa Region; EMRO : WHO Eastern Mediterranean Region; EURO : WHO European Region; PAHO : WHO Pan-American Region; SEARO : WHO South East Asia Region; WPRO : WHO Western Pacific Region; LI : Low-income economy; LMI : Lower-middle-income economy; UMI: Upper-middle-income economy; HI : High-income economy
Amended – A new constitution was adopted in Ecuador in 2008 that introduced the medicines-related rights described here for the first time. This constitution was last amended in 2011.
Please see the discussion section of this article for our comments on the limitations of determining whether this provision has changed since 2008.
Of the four constitutions in which health rights have remained static through recent constitutional revisions, three provide for “medicines” (Mexico, the Syrian Arab Republic, and Paraguay) and one provides for “medical aids” (Czech Republic) (see Table 2).
Provisions on medicines in Cape Verde’s Constitution regressed following amendments made in 2010 (See Figure 1 above). The state’s duty to promote the “socialization of medicines costs” was removed from the right to health.40
Discussion
Twenty-two constitutions now oblige governments to protect or to fulfill accessibility, acceptability, availability, and/or quality of medicines as part of the right to health. Thirteen of these constitutions create a state duty to fulfill medicines-related rights: four adopted before 2008 in which these rights were included; four amended after 2008 in which these rights were maintained; and five amended after 2008 in which these rights were expanded. Notably, terminology used to describe these rights has evolved over time to be more inclusive of human rights standards. Recently adopted constitutions enshrine the obligation to fulfill access to medicines and to protect medicines access from barriers in international trade agreements and/or intellectual property rights. Model text to protect and to fulfill essential medicines-related rights was identified and includes the elements of access, availability, and quality.
The most prevalent and comprehensive constitutional rights to medicines were identified in middle-income economies, many from the Pan-American region. Flood et al. describe several justifications of the so-called ‘resurgence’ of the right to health in international and domestic law that may explain why medicines-related rights are appearing more frequently in constitutions.41 Several applicable justifications are that health rights are a reaction to neoliberal economic policies or healthcare reform that may reduce government services and privatize health care, particularly in Latin America, possibly resulting in delayed or denied care.42 Another hypothesis behind the resurgence of the right to health is the rise of free trade agreements in which intellectual property (IP) protection and enforcement is at odds with the provision of affordable generic medicines.43 A final explanation may be governments’ own ambitions to “accelerate an equity and equality agenda” in nations with sizable income disparities resulting from dictatorship and apartheid.44
This study identified a lack of constitutional medicines-related rights in WHO South East Asia Region. In contrast, Heymann et al. demonstrated that the constitutional protection of free health care was most common in the South Asia region.45 Despite a legal culture of enshrining health commitments in South Asian constitutions, our findings suggest that essential medicines have not yet been explicitly embraced as part of these commitments.
No obligations to respect essential medicines were retrieved as part of the right to health, signaling that governments are perceived as having only positive duties towards medicines. As an example, positive duties include facilitating access through health services or providing health services and goods to the impoverished. Only one example of the duty to respect medicines-related rights was created in the context of a peace agreement between the government and the communist party, and subsequently included within the Constitution of Nepal (2010). Nepal’s Constitution states, “Both parties shall not hinder drug supplies and aid and health related campaigns.”46 Other governments confronted with conflict may wish to adopt similar language in order to establish the clear duty to respect the availability of medicines, and may consider further expanding the provision to include the duty to affordability, acceptability, and quality of medicines.
New strategies for articulating state commitments to medicines
This study has identified how constitutions frame state commitments to medicines and, in this process, has revealed new strategies to articulate these duties. The first strategy reveals a shift of medicines-related provisions nested within the right to health, to now also being a part of consumer rights. We observed this strategy in the Bolivian Constitution (2009), which states that users and consumers enjoy the right to the supply of pharmaceuticals “in harmless and quality condition, in sufficient and adequate quantity, and with efficient service and timely supply”.47 Users and consumers also have the right “to reliable information about the characteristics and contents of the products they consume and of the services they use.”48 Here, the Constitution references access to medicines of good quality; suitable availability of medicines, that is, “in sufficient and adequate quantity”; and access to information about medicinal products.49 This strategy aims to include access to information about medicines, as well as access to quality of medicines, as fundamental basic consumer rights.
The second strategy shows the introduction of constitutional provisions to protect access to medicines from potentially harmful IP standards in international trade agreements. The Bolivian and Ecuadorian governments have explicitly stated in their constitutions that IP rights or trade instruments shall not undermine access to medicines.50 This strategy likely evolved after a decade of academic debate about and civil society advocacy for a human rights approach to IP standards in international trade agreements, particularly in Latin America.51 Moreover, the constitutions of Ecuador and Bolivia include provisions on “generic medicines”, a phrase that could serve to shield legitimate generics from confusion with substandard and falsified medicines.52 Confusing legitimate generic medicines with substandard and falsified medicines is a misunderstanding with drastic consequences for affordable and accessible medicines in developing countries.53 The term “generic medicines” could also be used to proactively guide national policy and to create a supportive environment for local generic industry, generic procurement, and generic prescribing.
Promoting access to medicines through constitutional commitments
All in all, governments committed to improving access to medicines can include these rights in their national constitutions, which are counted among the most vital expressions of state responsibilities and individual rights. Admittedly much variation is noted worldwide between de jure constitutional law (the rights and duties in the constitution) and de facto law (the rights and duties actually enforced).54 However, enshrining medicines-related rights does facilitate a number of mechanisms to promote access to medicines. Constitutional medicines-related rights can guide the implementation and revision of national laws and policies. Two examples of this practice are the national health policies in South Africa and Bangladesh that give effect to the constitutional commitments to healthcare provision.55 Moreover, in a climate of austerity or political or social instability, a constitutional framework in which access to medicines is a clear priority can aid government decision making in order to prevent a regression of health rights.
Another advantage of clear constitutional commitments to access to medicines is that they can be monitored and targets can be set. Governments, researchers, and civil society organizations already conduct proactive monitoring. Since 2003, country surveys are undertaken using a method developed by Health Action International and WHO, and the results are publicly available online.56 In addition, some governments support household surveys to monitor financial and geographic accessibility, as well as institutional surveys to monitor the availability of medicines at health care facilities.57 Through these monitoring exercises, stakeholders can evaluate progress towards the progressive realization of health and medicines-related rights provided by their constitution. Some civil society organizations, such as the Treatment Action Campaign in South Africa, not only monitor access to medicines, but in cases of insufficient provision, they have also claimed their rights through the courts.58
Monitoring access from the perspective of public health and human rights
Public health and law propose distinct analytical frameworks through which pharmaceutical legislation can be analyzed. From a public health perspective, WHO’s Framework for Equitable Access to Essential Medicines proposes steps necessary to achieve access within a health system. First, essential medicines should be selected and used rationally, then they should be available at affordable prices, and sustainable financing mechanisms—such as public funding—should be in place. Second, reliable health and supply systems should regulate, procure, and distribute essential medicines.59 Legal frameworks, on the other hand, take a human rights approach derived from General Comment No. 14. This approach first considers a government’s negative duty to refrain from interfering with health rights, followed by its positive duty to protect individuals from deleterious interference by others (such as the sale of low-quality medicines). Last, states have a positive obligation to facilitate individuals’ enjoyment of medicines-related rights and to provide medicines to individuals unable to do so for themselves. This includes an immediate duty to satisfy minimum core obligations towards health, including the provision of essential medicines.60
When taken together, a combined public health and human rights framework holds the greatest potential to achieve universal access to medicines. The Global Health Law Groningen Research Centre applies this combined framework in its Essential Laws for Medicines Access project to identify sound domestic laws and policies that promote access.61
WHO supports countries to establish and implement their own comprehensive national policies on medicines. WHO’s Mid-Term Strategic Plan 2008-2013 offered several indicators to monitor and measure progress in countries. Indicators included measures of the following: a constitutional provision and/or domestic legislation recognizing the right to essential medicines (structural indicator); regulatory capacity (process indicator); vaccine quality, prescribing appropriateness; and essential medicines availability and price (outcome indicator).62
In 2008, Backman et al. expanded on these indicators to include measures on: a national policy on medicines and an essential medicines list (structural indicators); the public per capita spending on pharmaceuticals (process indicator); and the rate of immunization coverage (outcome indicator).63 The initial figures reported by Backman et al. for 194 countries serve as a baseline measurement of global access to medicines, rather than an analysis of the relationship between domestic laws, programs and access outcomes. At the same time, other single-country case studies have explored domestic pharmaceutical policy frameworks and their implications for access.64
Despite much interest in these indicators and case studies, the authors are not aware of any systematic or comparative research that studies the relationship between constitutional rights, domestic legislation and programs, and access to medicines on the ground. There is a demonstrated need for more research on domestic laws and policies that support universal access.
Potential barriers to access to medicines
Medicines are an essential part of every health system and, as such, access to medicines is highly interconnected to other factors that transcend domestic legislation and programs. Bigdeli et al. argue that the complexity of health systems can create potential barriers to access to medicines at multiple levels.65 First, barriers at the individual/community level include the cost and perceived quality of medicines, health-seeking behavior, and socio-cultural elements, such as poverty.66 Second, at the level of health service delivery, low availability, high price, and substandard quality of medicines are again barriers.67 Other barriers include the overall quality of health services--including irrational prescribing and dispensing—and competition between public and private health care services.68 Third, barriers can arise at the level of the health sector primarily concerning pharmaceutical sector governance (that is, at all stages in the lifecycle of medicines, from their registration on the market to their promotion to prescribers and consumers), medicines price control particularly in procurement, and overall health sector governance and pluralism.69 Fourth, overarching public policies can create barriers such as low public accountability and transparency, low priority of social sectors such as health, and corruption or the high burden of government bureaucracy.70
Conflict between trade and economic and public health goals for the pharmaceutical sector, especially in countries with strong local medicines production, can also stifle access to medicines.71 Finally, international and regional policies can also challenge access to medicines in situations where patents and IP rights are used unethically, international donors’ agendas are poorly aligned with disease burden, and/or research and development priorities are set by profitability rather than medical need.72 Access to medicines is best supported through a wide health systems approach where medicines are embedded and addressed at each level.73
The pharmaceutical industry has a significant role to play at all levels of the health system. Ethical medicines promotion can help limit irrational medicines-seeking behavior on the part of patients and curb inappropriate prescribing on the part of physicians. Transparent pricing policies, including differential pricing and market segmentation, can empower poorer members of the populations to obtain essential medicines at affordable prices. Drug companies can further embrace voluntary licenses to make a range of lifesaving medicines available in many markets.74 Concerning international trade agreements, companies can bring their market interests in line with global public health protections, such as the flexibilities in the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS), and refrain from seeking extensive IP protection for their products. Finally, companies can foster in-house or support external research and development into medicines for neglected diseases.75
It is important to note that, unlike our present research, our survey in 2008 identified only four constitutions that cited access to or the provision of essential goods and medicines. In contrast to this limited scope, our present study addresses duties to respect, protect, and fulfill; that is, not only to provide, but also to facilitate and promote essential medicines-related rights. Moreover, high-quality, reliable databases enabled us to locate constitutions in our present survey that we previously could not retrieve. These factors contribute to the higher numbers of relevant constitutional texts reported in this article than in our previous research.
Strengths and limitations of this research
The present study has several limitations. First, we were unable to retrieve the text of 10 constitutions. Any medicines-related rights in these laws are therefore not described in our study. Second, of the constitutions amended after 2008 (n=109), we were unable to retrieve 34 constitutions in force at the first point in time (2007). However, we did retrieve the constitutions currently in force from all 34 countries, and no provisions on medicines were identified in these documents. This result, together with the findings from our initial study in 2008, suggests that no provisions on medicines have gone unidentified in the 34 irretrievable constitutions from 2007, but we cannot be certain.
Similarly, we were unable to retrieve the Syrian Constitution in force at the first point in time (this was an amendment of the constitution adopted in 1973). Both of the constitutions adopted in 1973 and 2012 (currently in force), however, include medicines as part of the right to health, which strongly suggests that the provision of medicines is not a recent development; this is shown in Figure 1.
Third, constitutional law scholars Elkins, Ginsburg, and Melton articulated the difficulties of analyzing and comparing constitutional language based on their experience with the Comparative Constitutions Project. Two main difficulties of constitutional comparison are a lack of conceptual clarity of the topic studied and atypical or “fuzzy” constitutional text.76 Our present study addressed the former challenge by using the well-developed essential medicines concept from authoritative sources such as WHO and applying the tripartite typology from global human rights instruments. We minimized the latter challenge by consulting a second source or the original language text when the constitutional language was obscure.
Conclusion
This study demonstrates the value of a human rights approach to governments seeking to make clear commitments to essential medicines as part of the constitutional right to health. Our study illustrates that these commitments have multiplied since 2008 and are now found in 22 constitutions. In 13 of these constitutions, states are duty-bound to fulfill medicines-related rights, some of which now use access to medicines terminology. In general, medicines-related rights have been maintained or enhanced in constitutions revised since 2008. As an empirical measurement of the development of medicines-related rights, these results can be used to monitor the evolution of constitutional aspirations. The examples of constitutional text identified in this study can perhaps serve as a model to states motivated to achieve the universal right to health.
Future research can address the domestic interpretation and application of constitutional medicines provisions in order to further elucidate the role of constitutional commitments in promoting universal access to medicines. In any case, constitutional commitments to medicines as a part of the right to health are an important foundation of health system equity and are, encouragingly, being established in ever more countries.
Acknowledgements
The authors are grateful to Ferdinand Quist, as part of the Global Health Law Groningen Research Centre, for his research assistance in locating online versions of the constitutions, and they are indebted to Alicia Ely Yamin for her useful comments on an earlier draft of this article.
References
- 1.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health.
- 2.World Health Organization. Medium-term strategic plan 2008–2013. Geneva: WHO; 2008. pp. 96–98.http://apps.who.int/gb/ebwha/pdf_files/MTSP-08-13-PPB-10-11/mtsp-3en.pdf (Amended draft) Available at. [Google Scholar]; Backman G., Hunt P., Khosla R., et al. “Health systems and the right to health: An assessment of 194 countries,”. The Lancet. 2008;9655:2047–2085. doi: 10.1016/S0140-6736(08)61781-X. [DOI] [PubMed] [Google Scholar]
- 3.Hogerzeil H. V., Samson M., Casanovas J. V., Rahmani-Ocora L. “Is access to essential medicines as part of the fulfilment of the right to health enforceable through the courts?”. The Lancet. 2006;9532:305–311. doi: 10.1016/S0140-6736(06)69076-4. [DOI] [PubMed] [Google Scholar]
- 4.Perehudoff S., Laing R., Hogerzeil H. “Access to essential medicines in national constitutions,”. Bulletin of the World Health Organization. 2010;11:800. doi: 10.2471/BLT.10.078733. http://www.who.int/bulletin/volumes/88/11/10-078733/en/ Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]; Perehudoff S. Health, human rights & national constitutions. Amsterdam: Vrije Universiteit Amsterdam; 2008. http://www.who.int/medicines/areas/human_rights/Perehudoff_report_constitutions_2008.pdf Available at. [Google Scholar]
- 5.Gallala-Arndt I. Constitutional reforms in Tunisia, Egypt, Morocco and Jordan: A comparative assessment. Barcelona: European Institute of the Mediterranean; 2012. http://www.gallala_en.pdf Available at. [Google Scholar]; Contiades X. “Introduction: The global financial crisis and the constitution,”. In: Contiades X., editor. Constitutions in the global financial crisis. Surrey: Ashgate; 2013. pp. 1–5.http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2298601 Available at. [Google Scholar]
- 6.Constitution of the World Health Organization. 1946. 1948. preamble.
- 7.Universal Declaration of Human Rights (UDHR) G.A. Res. 217A (III); Art. 25; 1948. [Google Scholar]
- 8.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health.
- 9.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000:34–37. Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health. para.
- 10.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000:34–37. Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health. para. para. 12 and 43.
- 11.WHO Expert Committee. The selection and use of essential medicines, Geneva: WHO; 2003. http://apps.who.int/medicinedocs/en/d/Js4875e/ WHO Technical Report Series No. 914. Available at. [Google Scholar]
- 12.WHO Expert Committee. The selection and use of essential medicines, Geneva: WHO; 2003. http://apps.who.int/medicinedocs/en/d/Js4875e/ WHO Technical Report Series No. 914. Available at. [Google Scholar]
- 13.World Health Organization. Trade, foreign policy, diplomacy and health: Access to medicines. Geneva: WHO; 2016. http://www.who.int/trade/glossary/story002/en/ Available at. [Google Scholar]
- 14.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health.
- 15.Hogerzeil H. V., Samson M., Casanovas J. V., Rahmani-Ocora L. “Is access to essential medicines as part of the fulfilment of the right to health enforceable through the courts?”. The Lancet. 2006;9532:305–311. doi: 10.1016/S0140-6736(06)69076-4. [DOI] [PubMed] [Google Scholar]
- 16.Elkins Z., Ginsburg T., Melton J. “Lessons from the decoding and coding of national constitutions,”. Comparative Democratization. 2011;1:2, 11–14. http://www.ned.org/sites/default/files/February-2011-APSA-CD.pdf Available at. [Google Scholar]
- 17.Heymann J., Cassola A., Raub A., Mishra L. “Constitutional rights to health, public health and medical care: The status of health protections in 191 countries,”. Global Public Health. 2013;6:639–653. doi: 10.1080/17441692.2013.810765. http://dx.doi.org/10.1080/17441692.2013.810765 Available at. [DOI] [PubMed] [Google Scholar]
- 18.Hogerzeil H. V., Samson M., Casanovas J. V., Rahmani-Ocora L. “Is access to essential medicines as part of the fulfilment of the right to health enforceable through the courts?”. The Lancet. 2006;9532:305–311. doi: 10.1016/S0140-6736(06)69076-4. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Medium-term strategic plan 2008–2013. Geneva: WHO; 2008. pp. 96–98.http://apps.who.int/gb/ebwha/pdf_files/MTSP-08-13-PPB-10-11/mtsp-3en.pdf (Amended draft) Available at. [Google Scholar]; Backman G., Hunt P., Khosla R., et al. “Health systems and the right to health: An assessment of 194 countries,”. The Lancet. 2008;9655:2047–2085. doi: 10.1016/S0140-6736(08)61781-X. [DOI] [PubMed] [Google Scholar]
- 20.Perehudoff S., Laing R., Hogerzeil H. “Access to essential medicines in national constitutions,”. Bulletin of the World Health Organization. 2010;11:800. doi: 10.2471/BLT.10.078733. http://www.who.int/bulletin/volumes/88/11/10-078733/en/ Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallala-Arndt I. Constitutional reforms in Tunisia, Egypt, Morocco and Jordan: A comparative assessment. Barcelona: European Institute of the Mediterranean; 2012. http://www.gallala_en.pdf Available at. [Google Scholar]; “Introduction: The global financial crisis and the constitution,”. In: Contiades X., editor; Contiades X., editor. Constitutions in the global financial crisis. Surrey: Ashgate; 2013. pp. 1–5.http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2298601 Available at. [Google Scholar]
- 22.Comparative Constitutions Project. Chronology. 2009 http://www.constituteproject.org/ontology/chronology?lang=en Available at.
- 23.Comparative Constitutions Project. http://www.constituteproject.org Available at.; ConstitutionNet. http://www.constitutionnet.org Available at.; Political Database of the Americas. 1995-2008 http://pdba.georgetown.edu/Constitutions/constudies.html Available at.; International Constitutional Law. 1994-2010 http://www.servat.unibe.ch/icl/index.html Available at.; Constitution Finder. http://confinder.richmond.edu/contact.html Available at.; African Human Rights Law Document Database. http://www.chr.up.ac.za/index.php/documents-by-country-database.html Available at.
- 24.Melton J., Elkins Z., Ginsburg T., Leetaru K. “On the interpretability of law: Lessons from the decoding of national constitutions,”. British Journal of Political Science. 2013;02:399–423. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2191145 Available at. [Google Scholar]
- 25.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health.
- 26.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health.
- 27.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health. para. 35.
- 28.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health. para 37.
- 29.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health.
- 30.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health.
- 31.World Health Organization. WHO Regional offices. 2016 http://www.who.int/about/regions/en/ Available at. [Google Scholar]; World Bank. Country and lending groups. 2016 http://data.worldbank.org/about/country-and-lending-groups Available at.
- 32.Constitution of El Salvador. 2009 http://pdba.georgetown.edu/Constitutions/ElSal/constitucion.pdf art. 69. Available at.
- 33.Constitution of Portugal. 1976 http://www.constituteproject.org/constitution/Portugal_2005.pdf amended 2005), art. 64(3)(e). Available at.
- 34.Constitution of Portugal. 1976 http://www.constituteproject.org/constitution/Portugal_2005.pdf amended 2005), art. 64(3)(e). Available at.
- 35.Constitution of the Plurinational State of Bolivia. 2009 http://www.constituteproject.org/constitution/Bolivia_2009.pdf art. 41(III). Available at.
- 36.Constitution of the Plurinational State of Bolivia. 2009 http://www.constituteproject.org/constitution/Bolivia_2009.pdf art. 41(III). Available at. art. 255.
- 37.Constitution of Ecuador. 2008 http://www.constituteproject.org/constitution/Ecuador_2011.pdf amended 2011), art. 421. Accessed.
- 38.Constitution of Ecuador. 2008 http://www.constituteproject.org/constitution/Ecuador_2011.pdf amended 2011), art. 421. Accessed. art. 363(7)
- 39.Constitution of Panama. 1972 http://www.constituteproject.org/constitution/Panama_2004.pdf amended 2004), art. 110(5). Available at.
- 40.Constitution of Cape Verde. 1992 http://workspace.unpan.org/sites/internet/Documents/UNPAN042808.pdf amended 1999), art. 68, 70(3)(e). Available at.
- 41.Flood C. M., Gross A. “Litigating the right to health: What can we learn from a comparative law and health care systems approach,”. Health and Human Rights. 2014;16(2):62–72. http://www.hhrjournal.org/2014/09/25/litigating-the-right-to-health-what-can-we-learn-from-a-comparative-law-and-health-care-systems-approach-2/ Available at. [PubMed] [Google Scholar]
- 42.Flood C. M., Gross A. “Litigating the right to health: What can we learn from a comparative law and health care systems approach,”. Health and Human Rights. 2014;16(2):62–72. http://www.hhrjournal.org/2014/09/25/litigating-the-right-to-health-what-can-we-learn-from-a-comparative-law-and-health-care-systems-approach-2/ Available at. [PubMed] [Google Scholar]
- 43.Flood C. M., Gross A. “Litigating the right to health: What can we learn from a comparative law and health care systems approach,”. Health and Human Rights. 2014;16(2):62–72. http://www.hhrjournal.org/2014/09/25/litigating-the-right-to-health-what-can-we-learn-from-a-comparative-law-and-health-care-systems-approach-2/ Available at. [PubMed] [Google Scholar]
- 44.Flood C. M., Gross A. “Litigating the right to health: What can we learn from a comparative law and health care systems approach,”. Health and Human Rights. 2014;16(2):62–72. http://www.hhrjournal.org/2014/09/25/litigating-the-right-to-health-what-can-we-learn-from-a-comparative-law-and-health-care-systems-approach-2/ Available at. [PubMed] [Google Scholar]
- 45.Heymann J., Cassola A., Raub A., Mishra L. “Constitutional rights to health, public health and medical care: The status of health protections in 191 countries,”. Global Public Health. 2013;6:639–653. doi: 10.1080/17441692.2013.810765. http://dx.doi.org/10.1080/17441692.2013.810765 Available at. [DOI] [PubMed] [Google Scholar]
- 46.Constitution of Nepal. http://www.constituteproject.org/constitution/Nepal_2010.pdf Schedule 4: The Comprehensive Peace Accord concluded between the Government of Nepal and Communist Party of Nepal (Maoist) (2006, amended 2010), Schedule 4, art. 7(5)(3). Available at.
- 47.Constitution of the Plurinational State of Bolivia. 2009 http://www.constituteproject.org/constitution/Bolivia_2009.pdf art. 41(III). Available at. art. 75(1)
- 48.Constitution of the Plurinational State of Bolivia. 2009 http://www.constituteproject.org/constitution/Bolivia_2009.pdf art. 41(III). Available at. art. 75(1) art. 75(2)
- 49.Constitution of the Plurinational State of Bolivia. 2009 http://www.constituteproject.org/constitution/Bolivia_2009.pdf art. 41(III). Available at. art. 75(1) 75(2) art. 75.
- 50.Constitution of the Plurinational State of Bolivia. 2009 http://www.constituteproject.org/constitution/Bolivia_2009.pdf art. 41(III). Available at. art. 75(1) 75(2) art. 75 art. 255.; Constitution of Ecuador. 2008 http://www.constituteproject.org/constitution/Nepal_2010.pdf amended 2011), art. 421. Accessed. art. 421.
- 51.Malpani R., Bloemen S. Trading away access to medicines: How the European Union’s trade agenda has taken a wrong turn. Amsterdam: Oxfam International and Health Action International; 2009. http://www.haiweb.org/20102009/OxfamHAIReportTradingAwayAccesstoMedicines.pdf Available at. [Google Scholar]
- 52.Constitution of the Plurinational State of Bolivia. 2009 http://www.constituteproject.org/constitution/Bolivia_2009.pdf art. 41(III). Available at.; Constitution of Ecuador. 2008. amended 2011), art. 421. Accessed. art. 363(7)
- 53.Maleche A., Day E. “Right to health encompasses right to access essential generic medicines: Challenging the 2008 Anti-Counterfeit Act in Kenya,”. Health and Human Rights. 2014;16(2):96–104. http://www.hhrjournal.org/wp-content/uploads/sites/13/2014/12/Maleche-final1.pdf Available at. [PubMed] [Google Scholar]
- 54.Elkins Z., Ginsburg T., Melton J. “Lessons from the decoding and coding of national constitutions,”. Comparative Democratization. 2011;1:2, 11–14. http://www.ned.org/sites/default/files/February-2011-APSA-CD.pdf Available at. [Google Scholar]
- 55.Nevondwe L., Odeku K. O. “The right of access to health care services: Pitfalls and prospects,”. Mediterranean Journal of Social Sciences. 2013;13:837–845. http://www.mcser.org/journal/index.php/mjss/article/view/1811 Available at. [Google Scholar]; Uddin J., Momtaz S., Islam M. S. “State Obligation towards the fulfillment of the right to health: A study in Bangladesh perspective,”. Mediterranean Journal of Social Sciences. 2013;13:73–86. http://www.mcser.org/journal/index.php/mjss/article/view/1490/1507 Available at. [Google Scholar]
- 56.Health Action International and WHO. Database of medicine prices, availability, affordability and price components. 2015 http://www.haiweb.org/MedPriceDatabase/ Available at.
- 57.Republic of Uganda Ministry of Health. Access to and use of medicines by households in Uganda. Kampala: Republic of Uganda Ministry of Health; Dec, 2008. http://apps.who.int/medicinedocs/documents/s16374e/s16374e.pdf?ua=1 Available at. [Google Scholar]
- 58.Heywood M. “South Africa’s Treatment Action Campaign: Combining law and social mobilization to realize the right to health,”. Journal of Human Rights Practice. 2009;1:14–36. http://jhrp.oxfordjournals.org/content/1/1/14.abstract Available at. [Google Scholar]; Hogerzeil H. V., Samson M., Casanovas J. V., Rahmani-Ocora L. “Is access to essential medicines as part of the fulfilment of the right to health enforceable through the courts?”. The Lancet. 2006;9532:305–311. doi: 10.1016/S0140-6736(06)69076-4. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. Equitable access to essential medicines: a framework for collective action. Geneva: WHO; 2004. http://whqlibdoc.who.int/hq/2004/who_edm_2004.4.pdf Available at. [Google Scholar]
- 60.International Covenant on Economic. UN Doc. E/C.12/2000/4. 2000 Social and Cultural Rights (ICESCR), G.A. Res. 2200A (XXI), Art. 12 (1966). UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, The Right to the Highest Attainable Standard of Health. para. 43(d)
- 61.Global Health Law Groningen Research Centre, Essential Laws for Medicines Access Project. 2015 http://www.rug.nl/research/groningen-centre-for-law-and-governance/onderzoekscentra/ghlg/access-to-medicines?lang=en Available at.
- 62.World Health Organization. Medium-term strategic plan 2008–2013. Geneva: WHO; 2008. pp. 96–98.http://apps.who.int/gb/ebwha/pdf_files/MTSP-08-13-PPB-10-11/mtsp-3en.pdfpp. 96 (Amended draft) Available at. [Google Scholar]
- 63.Backman G., Hunt P., Khosla R., et al. “Health systems and the right to health: An assessment of 194 countries,”. The Lancet. 2008;9655:2047–2085. 2057. doi: 10.1016/S0140-6736(08)61781-X. [DOI] [PubMed] [Google Scholar]
- 64.Seoane‐Vazquez E., Rodriguez‐Monguio R. “Access to essential drugs in Guyana: a public health challenge,”. The International journal of health planning and management. 2010;25:2–16. doi: 10.1002/hpm.949. http://onlinelibrary.wiley.com/doi/10.1002/hpm.949/full Available at. [DOI] [PubMed] [Google Scholar]; Zaidi S., Bigdeli M., Aleem N., Rashidian A. “Access to essential medicines in Pakistan: policy and health systems research concerns,”. PloS one. 2013;8:1–10. doi: 10.1371/journal.pone.0063515. http://dx.plos.org/10.1371/journal.pone.0063515.g001 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bigdeli M., Jacobs B., Tomson G., et al. “Access to medicines from a health system perspective,”. Health Policy and Planning. 2013;28:692–704. doi: 10.1093/heapol/czs108. http://heapol.oxfordjournals.org/content/early/2012/11/21/heapol.czs108.long Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hunt P., Khosla R. “Are drug companies living up to their human rights responsibilities? The perspective of the former United Nations Special Rapporteur (2002-2008),”. PLoS medicine. 2013;7:1–3. doi: 10.1371/journal.pmed.1000330. http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000330 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunt P., Khosla R. “Are drug companies living up to their human rights responsibilities? The perspective of the former United Nations Special Rapporteur (2002-2008),”. PLoS medicine. 2013;7:1–3. doi: 10.1371/journal.pmed.1000330. http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000330 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elkins Z., Ginsburg T., Melton J. “Lessons from the decoding and coding of national constitutions,”. Comparative Democratization. 2011;1:2, 11–14. http://www.ned.org/sites/default/files/February-2011-APSA-CD.pdf Available at. [Google Scholar]