Abstract
The level and activity of critical regulatory proteins in cells are tightly controlled by several tiers of post-translational modifications. HIF-1α is maintained at low levels under normoxia conditions by the collaboration between PHD proteins and the VHL-containing E3 ubiquitin ligase complex. We recently identified a new physiologically relevant mechanism that regulates HIF-1α stability in the nucleus in response to cellular oxygen levels. This mechanism is based on the collaboration between the SET7/9 methyltransferase and the LSD1 demethylase. SET7/9 adds a methyl group to HIF-1α, which triggers degradation of the protein by the ubiquitin-proteasome system, whereas LSD1 removes the methyl group, leading to stabilization of HIF-1α under hypoxia conditions. In cells from knock-in mice with a mutation preventing HIF-1α methylation (Hif1αKA/KA), HIF-1α levels were increased in both normoxic and hypoxic conditions. Hif1αKA/KA knock-in mice displayed increased hematological parameters, such as red blood cell count and hemoglobin concentration. They also displayed pathological phenotypes; retinal and tumor-associated angiogenesis as well as tumor growth were increased in Hif1αKA/KA knock-in mice. Certain human cancer cells exhibit mutations that cause defects in HIF-1α methylation. In summary, this newly identified methylation-based regulation of HIF-1α stability constitutes another layer of regulation that is independent of previously identified mechanisms. [BMB Reports 2016; 49(5): 245-246]
Keywords: HIF-1α, LSD1, Lysine methylation, SET7/9, Ubiquitin
Hypoxia-inducible factor-1α (HIF-1α) is a key transcriptional regulator responsible for the adaptation of cells and tissues to a state of low oxygen (hypoxia). Since uncontrolled expression of hypoxia-inducible genes is harmful to normal physiology, the cellular level of HIF-1α is tightly regulated, primarily by ubiquitin-mediated degradation. In the presence of physiological concentrations of oxygen (normoxia), HIF-1α is hydroxylated on proline residues by proline hydroxylase domain (PHD) proteins (PHD1/PHD2/PHD3). Hydroxylated proline residues serve as a marker for recognition by the VHL-containing CUL2 E3 ubiquitin ligase complex. On the other hand, the lack of oxygen in hypoxia triggers reduced hydroxylation and increased stability of HIF-1α, which leads to its translocation to the nucleus, where it heterodimerizes with HIF-1β and induces the expression of target genes. In addition to controlling HIF-1α transcriptional activity by regulating HIF-1α stability, other means of regulation include SUMOylaton, acetylation, and phosphorylation.
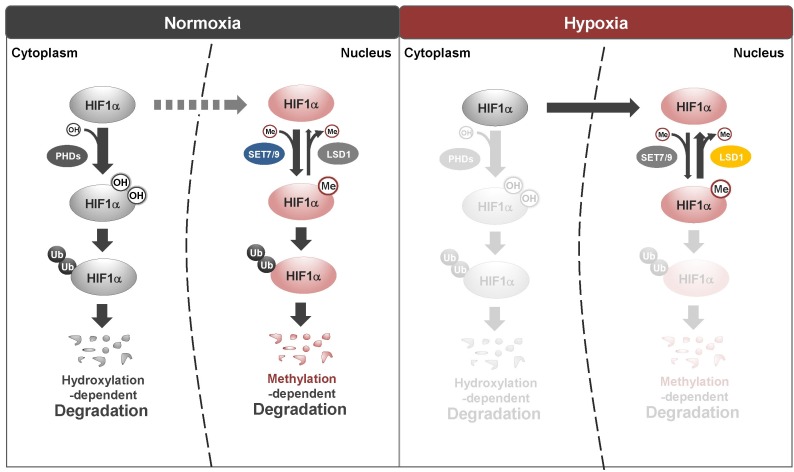
Recently, we identified another control mechanism of HIF-1α stability (Fig. 1). We will discuss the following three aspects of this new discovery: molecular mechanisms, physiological significance as revealed by a Hif1αKA/KA knock-in mouse model, and clinical relevance to human cancers. In general, the cellular level of HIF-1α is very low in normoxic conditions. In the presence of MG132, a proteasome inhibitor, we were able to identify methylation of HIF-1α on the 32nd lysine residue, and found that this methylation is mediated by SET7/9 methyltransferase in the nucleus. Interestingly, the level of HIF-1α methylation in the presence of MG132, which was measured by a HIF-1α methylation-specific antibody, is high in normoxia, decreases upon initiation of hypoxia, and increases again after longer exposure to hypoxia. This inversely correlates with the protein level of HIF-1α without proteasome inhibitor treatment. HIF-1α methylation could be a signal for poly-ubiquitination by an unidentified E3 ligase that results in the degradation of HIF-1α, which is independent of the cytosolic destabilization mechanism of HIF-1α. We sought to identify the underlying mechanism controlling the stabilization of HIF-1α from early to later periods of hypoxia. On the basis of decreased methylation of HIF-1α, we found that a demethylating enzyme, LSD1, removes a methyl group from HIF-1α to stabilize it. The HIF-1α protein level under hypoxia was higher in Lsd1-deficient mouse embryonic fibroblasts (MEFs) compared to control MEFs. Therefore, SET7/9 and LSD1 are newly identified regulators of hypoxia that control HIF-1α stability.
Fig. 1. Schematic model of regulation of HIF-1α protein stability. Under normoxic conditions, HIF-1α protein stability is regulated by PHD-dependent hydroxylation in the cytoplasm. Hydroxylated HIF-1α is degraded by 26S proteasomes to maintain low HIF-1α protein levels. In contrast, SET7/9-dependent methylation and LSD1-dependent demethylation of HIF-1α regulate protein stability primarily in the nucleus in a hydroxylation-independent manner during normoxia. Upon hypoxia, HIF-1α is stabilized by LSD1-dependent demethylation in the nucleus.
The physiological relevance of this new mechanism was evaluated with a Hif1αKA/KA knock-in mouse model, in which HIF-1α resists methylation. These mice are largely indistinguishable from their wild-type counterparts in growth, fertility, and life-span. HIF-1α levels are slightly higher in several tissues from Hif1αKA/KA mice compared to those from wild-type mice; the difference becomes more prominent after treating the mice with a prolyl hydroxylase inhibitor, dimethyloxalylglycine (DMOG), which protects HIF-1α from cytosolic degradation. This phenomenon can be explained as follows: HIF-1α that resists degradation in the cytosol is normally degraded in the nucleus by SET7/9-mediated methylation followed by ubiquitination and proteasomal degradation. However, methylation-defective HIF-1α accumulates in the nucleus after resisting degradation in the cytosol. Along with higher levels of HIF-1α protein, Hif1αKA/KA mice display increased hematocrit, red blood cell count, and hemoglobin levels. One well-known function of HIF-1α is to promote angiogenesis by activating the transcription of angiogenic factors. Two types of angiogenesis, retinal and tumor-associated, were both significantly elevated in Hif1αKA/KA mice along with increased expression of VEGF in both cases. Although the phenotype was not prominent under normal physiological conditions, Hif1αKA/KA mice showed clear alterations in the methylation-derived clearance system in pathological situations.
Another intriguing characteristic of Hif1αKA/KA cells and mice is an increased tendency for tumorigenesis. MEFs derived from Hif1αKA/KA mice showed enhanced cell migration as well as colony formation. MDA-MB231 breast cancer cells that stably express methylation-resistant HIF-1α K32A protein form more and larger tumors in athymic nude mice compared to cells expressing wild-type HIF-1α. In order to determine if this newly found mechanism has any relevance to human cancer, we searched databases for HIF-1α mutations in human cancers and identified two frequently occurring mutations, S28Y and R30Q. Both are situated near the methylation site at K32; however, a mutation in K32 itself was not detected. Both S28Y and R30Q mutant HIF-1α proteins are resistant to methylationdependent degradation and cells expressing the mutant HIF-1α exhibit increased migration. Although this hypothesis requires systemic validation, it is highly possible that these HIF-1α mutations contribute to the development and/or progression of human cancers.
Acknowledgments
We thank Young Suk Yu for illustration. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) to S.H.B. (Research Center for Chromatin Dynamics, 2009-0081563) and to K.I.K. (NRF-2013R1A2A2A01067617).