Abstract
The Ubiquitin proteasome system (UPS) plays roles in protein degradation, cell cycle control, and growth and inflammatory cell signaling. Dysfunction of UPS in cardiac diseases has been seen in many studies. Cholesterol acts as an inducer of cardiac hypertrophy. In this study, the effect of proteasome inhibitors on the cholesterol-induced hypertrophic growth in H9c2 cells is examined in order to observe whether UPS is involved in cardiac hypertrophy. The treatment of proteasome inhibitors MG132 and Bortezomib markedly reduced cellular surface area and mRNA expression of β-MHC in cholesterol-induced cardiac hypertrophy. In addition, activated AKT and ERK were significantly attenuated by MG132 and Bortezomib in cholesterol-induced cardiac hypertrophy. We demonstrated that cholesterol-induced cardiac hypertrophy was suppressed by proteasome inhibitors. Thus, regulatory mechanism of cholesterol-induced cardiac hypertrophy by proteasome inhibitors may provide a new therapeutic strategy to prevent the progression of heart failure. [BMB Reports 2016; 49(5): 270-275]
Keywords: Brotezomib, Cholesterol-induced cardiac hypertrophy, H9c2 cells, MG132, Proteasome inhibitors
INTRODUCTION
Cardiac hypertrophy, the first phase of cardiovascular disease, induces heart failure. Indeed, it is an important compensatory mechanism in response to physiological or pathological stimuli that involve regulation of cellular signaling mediators and transcript factors (1-3). Hypertrophic signals result in increased protein synthesis and regulated cell cycles (4). The ubiquitin-proteasome system (UPS) is a key pathway of the cellular processes, regulating many proteins involved in cell cycle progression, signal transduction, and apoptosis (5, 6). Alterations in UPS have been implicated in a variety of disease such as cancer and neurodegenerative diseases.
The relationship between UPS and cardiac diseases has been reported in recent years (7). For example, it has been reported that activation of the cardiac proteasome accompanies the pathogenesis of heart conditions such as ischemia-reperfusion, heart failure, hypertrophic myopathy, hypertension, and hypertrophy (8-10). According to one study, proteasome inhibition suppressed disease progression and dysfunction in stimuli-induced cardiac hypertrophy and early cardiac remodeling (11). Indeed, proteasome inhibitors altered the protein expression of cardiomyocytes and regulated the progression of cardiovascular diseases (12-14). MG132 significantly reduced cardiac fibrosis through downregulation of matrix metalloproteinases and collagens (15) and suppressed isoproterenol-induced cardiac hypertrophy in cultured cardiomyocyte (16). Another proteasome inhibitor, epoxomicin, has been reported to prevent pressure-overload-induced cardiac hypertrophic development (9) and improve cardiac function in hypertrophic cardiomyopathy mice (17). Likewise, PS-519, known as the irreversible proteasome inhibitor, has been reported to reduce isoprenaline-mediated hypertrophy in mice (18).
Cholesterol is a major structural component of eukaryotic cellular membranes and a key lipid that regulates the permeability, fluidity, curvature, and stiffness of membranes (19, 20). Previously, cholesterol was shown to contribute to induction of cardiac hypertrophy through activated AKT signaling pathway in H9c2 cells (21). Although some studies have demonstrated that proteasome inhibitors suppress or reverse cardiac hypertrophy, it remains unclear whether cholesterol-induced cardiac hypertrophy is affected by proteasome inhibitors.
Our study was designed to investigate the effects of proteasome inhibitors on cholesterol-induced cardiac hypertrophy. Our data reveal that proteasome inhibitors MG132 and Bortezomib have a suppressive effect on cholesterol-induced cardiac hypertrophy, decreasing cell surface area and expression of hypertrophic marker genes induced by cholesterol. These results showed that proteasome inhibitors MG132 and Bortezomib inhibit cholesterol activation of extracellular signal-regulated kinase (ERK) and AKT. Taken together, these data suggest that proteasome inhibitors play an important role in the suppression of cholesterol-induced cardiac hypertrophy.
RESULTS
Cholesterol-induced cardiac hypertrophy is suppressed by proteasome inhibitor
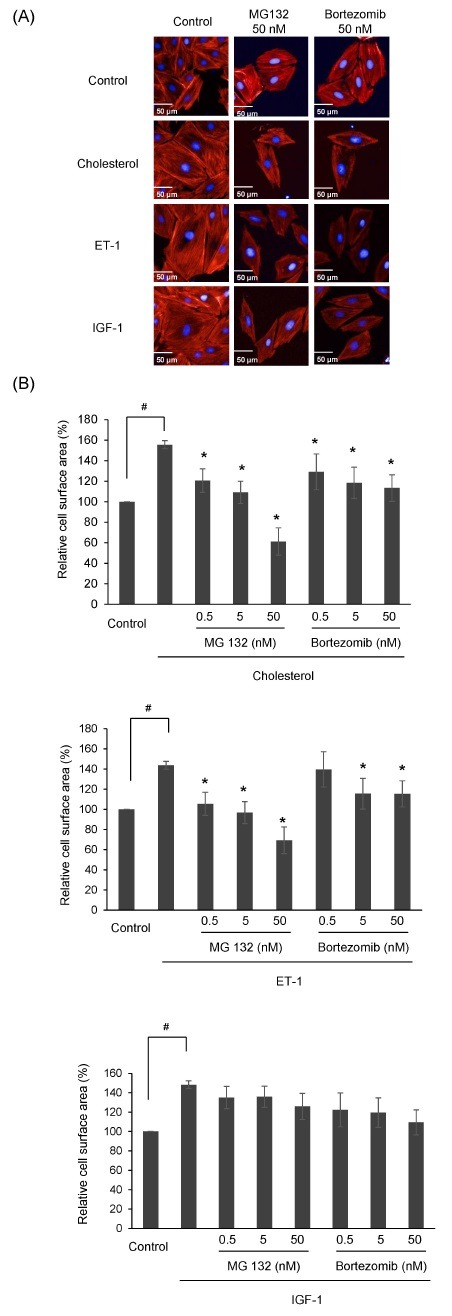
The effect of proteasome inhibitors on cholesterol-induced cardiac hypertrophy was examined by measuring cardiac cell surface area. H9c2 cells were treated with 0.5-50 nM MG132 or 0.5-50 nM Bortezomib, in the presence of 5 μg/ml cholesterol, IGF-1, or 100 nM ET-1 for 24 h. Cell surface was then measured after staining with rhodamine phalloidin and DAPI, as described in Materials and Methods (Fig. 1A). Consistent with a previous study, cholesterol itself significantly increased the cell surface area by 155%, as with ET-1 and IGF-1. Proteasome inhibitors MG132 and Bortezomib suppressed hypertrophic growth induced by cholesterol, ET-1, and IGF-1. When H9c2 cells were treated with more than 5 nM MG132 or 50 nM Bortezomib, cholesterol-induced hypertrophic growth was almost completely reversed (Fig. 1B). The effective concentration of MG132 on suppression of hypertrophic growth was a little lower than that of Bortezomib. In the case of ET-1-induced cardiac hypertrophy, the effect of proteasome inhibitors was almost similar to cholesterol. However, proteasome inhibitors did not show significant effect on the IGF-1 induced cardiac hypertrophy. Overall, proteasome inhibition by MG132 and Bortezomib suppressed cholesterol-induced cardiac hypertrophic growth.
Fig. 1. Effects of proteasome inhibitors on cell surface area of cholesterol-induced cardiac hypertrophic H9c2 cells. (A) H9c2 cells were cultured in serum-free medium for 4 h and treated with MG132 (0.5-50 nM) or Bortezomib (0.5-50 nM) in the presence of ET-1 (100 nM), IGF-1 (50 ng/ml), and cholesterol (5 μg/ml) for 24 h. Cells were fixed, and then stained with rhodamine phalloidin (red) and DAPI (blue) for 30 min to visualize F-actin and nuclei, respectively. (B) Images were acquired on an Operetta system (PerkinElmer, USA) and the cell surface area was analyzed using HarmonyⓇ High Content Imaging and Analysis Software. The values shown are the mean ± S.D. from three independent experiments. #P < 0.05 vs control; *P < 0.05 vs cholesterol or ET-1.
To confirm whether proteasome inhibitors have toxicity on cholesterol-induced cardiac hypertrophic cells, H9c2 cells were treated with MG132 or Bortezomib for 24 h in the presence of 5 μg/ml cholesterol after incubation with serum-free medium for 4 h before cells were analyzed by a WST-1 colorimetric assay. As shown in Fig. 2A, proteasome inhibition by MG132 had no effect on cell viability in the presence of cholesterol. Altogether, 0.5 to 50 nM MG132 and 0.5 to 500 nM Bortezomib had no cytotoxic effect on cholesterol-induced cardiac hypertrophy.
Fig. 2. The effect of proteasome inhibitors on cholesterol-induced cardiac hypertrophy. (A) H9c2 cells were plated on a 96-well plate and treated with MG132 (0.5-50 nM) or Bortezomib (0.5-50 nM) in the presence of ET-1 (100 nM), IGF-1 (50 ng/ml), and cholesterol (5 μg/ml) for 24 h after starvation for 4 h in a serum-free medium. After incubation with WST-1 for 1 h, the absorbance at 450 nm was measured using a Spectra MaxⓇ M3 Microplate Reader (Molecular Devices, USA). The relative cell viability was calculated by comparison to the control cells. The values shown are the mean ± S.D. from three independent experiments. (B) The proteasome activity in cell lysates was quantified using the Proteasome Activity Fluorometric Assay Kit (BioVision). Cell lysates were incubated with fluorogenic substrate and Succ-LLVY-AMC. Fluorescence intensity (350 nm excitation, 440 nm emission) was then measured using a Spectra MaxⓇ M3 Microplate Reader (Molecular Devices, USA). The relative proteasome activity was calculated by comparison to the control cells. The values shown are the mean ± S.D. from three independent experiments. *P <0.05 vs control. (C) Total RNA was isolated from cells with MG132 (0.5-50 nM) or Bortezomib (0.5-50 nM), with or without cholesterol (5 μg/ml) for 24h using TrizolⓇ reagent. qRT-PCR was performed for primers specific to β-MHC. The expression of the target genes was expressed as the relative expression level after normalization to the levels of actin, the internal control. The values shown are the mean ± S.D. from three independent experiments. **P < 0.01 vs control.
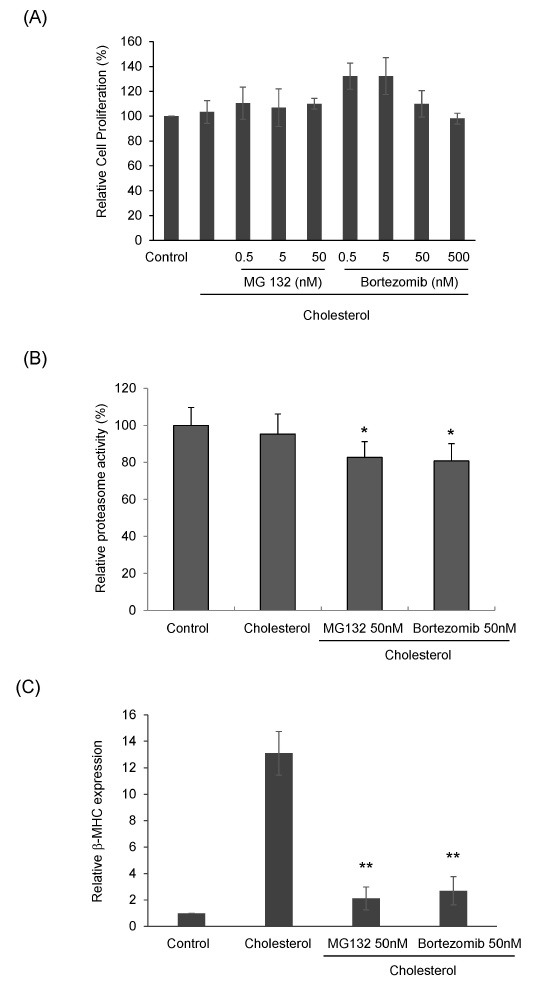
Next, we examined whether proteasome activity is suppressed by treatment of proteasome inhibitor. Proteasome activities in cell lysates were measured by fluorescence from the cleavage of fluorogenic substrate, Succ-LLVY-AMC. The treatment of cholesterol didn’t show any effect on the proteasome activity. The treatment of 50 nM MG132 or 50 nM Bortezomib suppressed proteasome activity by 82% and 80% compared with control (Fig. 2B). These results indicate that low doses of proteasome inhibitors partially suppress proteasome activity.
We further examined the effect of proteasome inhibitors on the mRNA expression level of β-myosin heavy chain (β-MHC), known as a hypertrophic marker gene, by qRT-PCR. H9c2 cells were incubated with serum-free medium for 4 h and then treated with 50 nM MG132 or 50 nM Bortezomib in the presence of 5 μg/ml cholesterol for 24 h. As shown in Fig. 2C, β-MHC expression was 12-14 times higher in the cholesterol-treated sample than in the control. Moreover, qRT-PCR revealed that cholesterol-induced β-MHC expression was attenuated six times by proteasome inhibition with 50 nM MG132 and 50 nM Bortezomib (P < 0.01). There was no difference in the effective concentrations of MG132 and Bortezomib on β-MHC expression. Jointly, 50 nM MG132 and 50 nM Bortezomib significantly suppressed cholesterol-induced β-MHC expression in H9c2 cells.
Proteasome inhibitors suppress activation of AKT and ERK by cholesterol
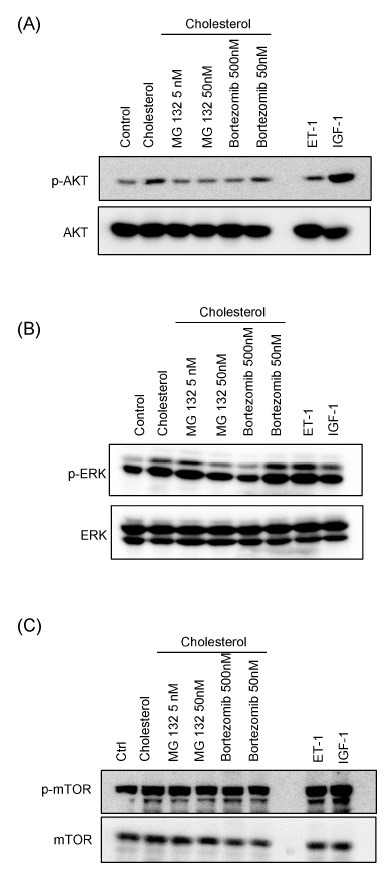
We further observed that proteasome inhibitors have an effect on cellular signal mediators in cholesterol-induced cardiac hypertrophy. In a previous study, we established that cholesterol-induced cardiac hypertrophy occurred through activation of AKT and MAPK signal pathway (21). Therefore, we investigated whether proteasome inhibitors have an effect on the signaling pathway activated by cholesterol using western blotting. H9c2 cells were treated with 5 or 50 nM MG132 or 50 or 500 nM Bortezomib for 4 h before treatment with 5 μg/ml cholesterol for 1 h. Cell lysates were then subjected to western blotting using antibodies against AKT, ERK1/2, and mTOR. As shown in Fig. 3A, AKT phosphorylation, the main pathway of cholesterol-induced cardiac hypertrophy, was significantly diminished in H9c2 cells by proteasome inhibitors. Indeed, treatment with low-dose MG132 and Bortezomib attenuated AKT activation effectively in cholesterol-induced cardiac hypertrophy. ERK1/2 phosphorylation was also suppressed by the indicated dose of MG132 and Bortezomib (Fig. 3B). Even though proteasome inhibitors reduced AKT and ERK1/2 activation, their expression was not changed. Following that, to assess the suppressive effect of proteasome inhibitors on other signal pathways of hypertrophy, we examined mTOR activation. Proteasome inhibitors were less effective in suppressing mTOR activation (Fig. 3C). These results suggest that proteasome inhibitors suppressed cholesterol-induced cardiac hypertrophy through reduction of AKT and ERK signaling effectively.
Fig. 3. Effects of proteasome inhibitor on hypertrophic signaling pathways activated by cholesterol. H9c2 cells were incubated with MG132 (5, 50 nM) or Bortezomib (50, 500 nM) for 4 h before treatment with ET-1 (100 nM), IGF-1 (50 ng/ml), and cholesterol (5 μg/ml). The phosphorylation of AKT (A), ERK (B), and mTOR (C) were analyzed using antibodies against phospho-AKTSer473 and AKT, phospho-ERK1/2Thr 202/Tyr 204, ERK, phosphomTORSer2448, mTOR, phospho-AKTSer473, and AKT.
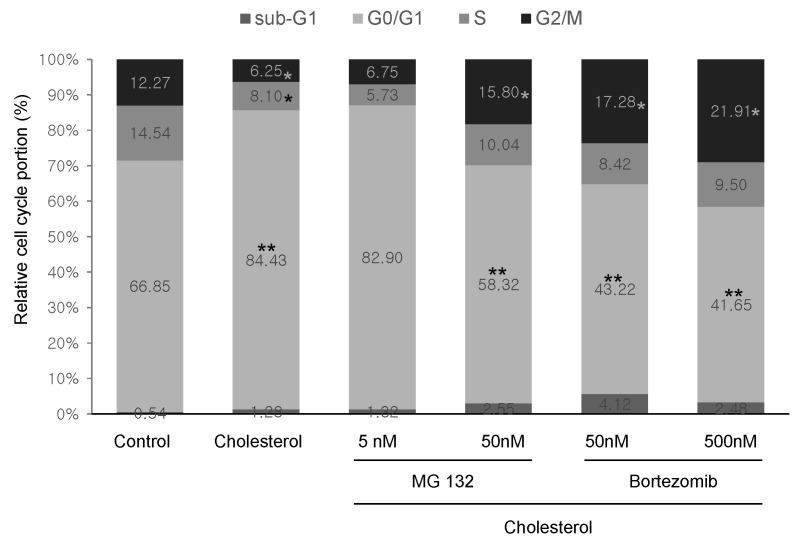
Suppressive effect of proteasome inhibitors on cell cycle of cholesterol-induced cardiac hypertrophy
It is reported that hypertrophic growth is related to upregulation of G1 cyclin or cyclin-dependent kinases, and that postmitotic cardiac myocytes reenter the cell cycle for proliferative growth (15, 22, 23). To assess how the cell cycle is regulated by cholesterol-induced hypertrophy and reversed by proteasome inhibitors, H9c2 cells were analyzed by flow cytometry assay after treatment with 5 μg/ml cholesterol in the presence or absence of 5 or 50 nM Bortezomib or 5 or 50 nM MG132 for 24 h, followed by staining with propidium iodide. Fig. 4 showed that the proportion of G0/G1 phase in H9c2 cells was increased by cholesterol (84.43%), compared with the control (66.85%). Conversely, the proportion of G0/G1 phase was significantly reduced by 50 nM MG132 (58.32%), 50 nM Bortezomib (43.22%), and 500 nM Bortezomib (41.65%). The G2 proportion of cholesterol-induced cardiac hypertrophy was increased by 50 nM MG132 and 50 nM Bortezomib from 6.25% to 15.8% and 17.28%, respectively. These results showed that proteasome inhibitors induce reentry of the cell cycle by G1/S phase transition in cholesterol-induced cardiac hypertrophy.
Fig. 4. The effect of proteasome inhibitors on cell cycle in cholesterol-induced hypertrophy. H9c2 cells were cultured in serum-free medium for 4 h and treated with MG132 (5, 50 nM), Bortezomib (50, 500 nM), ET-1 (100 nM), and cholesterol (5 μg/ml) for 24 h. Cells were fixed and then stained for 10 min with propidium iodide to visualize the nuclei. Data were acquired on FACSCaliber and the cell cycle was analyzed using CellQuestTM of FACSCalibur Software. *P < 0.05 vs control; **P < 0.01 vs control.
DISCUSSION
The importance of proteasome inhibitors has become evident for clinical treatment potential for human diseases such as cancer, neurological diseases, and cardiac diseases. Many studies have reported that proteasome inhibitors suppressed various cardiac diseases such as orthostatic hypotension, reperfusion injury, hypertension, and hypertrophy (8-10). Cardiac hypertrophy has also been found to be regulated by proteasome inhibitors. MG132, a proteasome inhibitor, has been reported to prevent artery restenosis and hypertrophic myopathy (24, 25). Recently, a study reported that MG132 suppresses isoproterenol-induced hypertrophy in cardiomyocytes in vitro (16). Another study shown that MG132 diminished pressure-overload-induced left ventricular (LV) hypertrophy in vivo (17). Bortezomib, the first proteasome inhibitor approved for use as clinical drug, has been reported to reduce cardiomyocyte surface area and inhibit angiotensin-induced cardiac hypertrophy (26). However, the effect of proteasome inhibitors on cholesterol-induced cardiac hypertrophy has not been examined. Here, we determined that proteasome inhibitors MG132 and Bortezomib suppress cholesterol-induced hypertrophic growth in H9c2 cells. We showed that low doses of proteasome inhibitors reduce surface area of H9c2 cells without a change in cell viability. Additionally, upregulation of β-MHC, known as a marker of cardiac hypertrophy, was attenuated by MG132 and Bortezomib. The effect of proteasome inhibitors on cell surface area was almost similar in both cholesterol or ET-1 induced cardiac hypertrophy. However, IGF-1-induced cardiac hypertrophy was not clearly reversed by proteasome inhibitors. ET-1 is known as a stimulator of pathological hypertrophy, but IGF-1 affects to physiological hypertrophy (27). The signaling pathway between ET-1 and IGF-1 is somewhat different. Most studies related to proteasome inhibition are performed in cardiac hypertrophy caused by pathological stimuli. Therefore, proteasome inhibition may be a new approach in the suppression of pathological cardiac hypertrophy.
Even though the mechanism of the protective effect of proteasome inhibitors on cardiac hypertrophy is not thoroughly understood, inactivation of signaling molecules in the hypertrophic pathway is suggested as the main reason. Pressureoverload-induced (AAB) cardiac hypertrophy was markedly reduced through decreased ERK1/2 and JNK activation by MG132 (28). Angiotensin 2-induced hypertrophy was attenuated through activation of angiotensin type 1 receptor-mediated p38MAPK and STAT3 signal pathway as proteasome inhibition by Bortezomib (26). Our data also showed that phosphorylation of ERK1/2 and AKT in cholesterol-induced cardiac hypertrophy was decreased by MG132 and Bortezomib. In a previous study, we showed that cholesterol induces cardiac hypertrophy by activating the AKT pathway. The treatment of LY294002, a PI3K inhibitor, inhibited cholesterol-induced cardiac hypertrophy (21). Therefore cholesterol level is very important for activation of PI3K/AKT signaling pathway. It is reported that proteasome inhibitors reduce cholesterol accumulation through regulating the stability and transcription of cholesterol export proteins (29, 30). Thus, PI3K/AKT signaling pathways may be inhibited by alteration of cholesterol level with the treatment of proteasome inhibitors as an underlying mechanism.
To know whether cholesterol has an effect on the proteasome directly, we examined the changes of proteasome activity. Cholesterol treatment did not enhance proteasome activity. However, low doses of proteasome inhibitors, to suppress cholesterol induced cardiac hypertrophy, induced partial inhibition of proteasome activity. These results suggest that the change of proteasome activity caused by proteasome inhibitors may not be a major cause of attenuation of cholesterol-induced hypertrophy. Proteasome inhibitors are reported to show inverse effects according to dose (31). High doses of proteasome inhibitor induce apoptosis but low doses of proteasome inhibitor suppress apoptosis (31). Therefore, partial inhibition of proteasomes by low doses of proteasome inhibitor may have an effect on the growth and the hypertrophy signaling pathway by different mechanisms from high doses of proteasome inhibitor.
The suppressive effects of MG132 and Bortezomib on cardiac hypertrophy were accompanied by alteration of the cell cycle. The induction of cardiac hypertrophy results in G0 or non-G0 arrest (G1 and G2) of cardiomyocytes. Inhibition or reversal of cardiac hypertrophy was characterized by reentry into G1/S phase through increased cyclin D1 activation (32, 33). On the contrary, p16 and p21 inhibited reentry into G1/S phase and G1 CDK activation in vitro and in vivo. Our results showed that the population of G0/G1 phase cells in proteasome inhibitor-treated cells decreased compared to cholesterol-treated cells. In addition, the proportion of G0/G1 phase cells returned to control levels in response to 50 nM MG132 or 50 nM Bortezomib. Although we did not examine the expression level of cyclins or CDK, proteasome inhibitors may result in the changes of protein level of cyclins and CDK to inhibit degradation by proteasome as shown in previous reports (34, 35).
Our data revealed the response by proteasome inhibitors in detail in cholesterol-induced cardiac hypertrophy. They decrease cell surface area and expression of hypertrophic marker genes induced by cholesterol. These results showed that proteasome inhibitors MG132 and Bortezomib inhibit the activation of ERK and AKT by cholesterol. These results suggest that proteasome inhibition may offer a new approach in the suppression of cholesterol-induced cardiac hypertrophy.
MATERIALS AND METHODS
See supplementary information for this section excepting measurement of cell surface area.
Measurement of cell surface area
H9c2 cells were plated on a 96-well plate (Corning, CA, USA). Cells were starved for 4 h in a serum-free medium before treatment with cholesterol (5 μg/ml), ET-1 (100 nM), or IGF-1 (50 ng/ml) for 24 h. Briefly, after washing twice with cold PBS, the cells were fixed in 4% paraformaldehyde at room temperature for 20 min and washed with PBS containing 2% bovine serum albumin and 0.1% Triton X-100.
Cells were stained with rhodamine phalloidin (Invitrogen, Carlsbad, CA, USA) and DAPI (Invitrogen, Carlsbad, CA, USA) (36). Images were acquired on an Operetta System and cell surface area was analyzed using HarmonyⓇ High Content Imaging and Analysis Software (Perkin Elmer, Massachusetts, USA).
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (20110021713 and 2015R1A2A2A04005596) and the intramural grant by Korea Institute of Science and Technology (2E25360).
References
- 1.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. (2004);109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 2.Glenn DJ, Rahmutula D, Nishimoto M, et al. Atrial natriuretic peptide suppresses endothelin gene expression and proliferation in cardiac fibroblasts through a GATA4-dependent mechanism. Cardiovasc Res. (2009);84:209–217. doi: 10.1093/cvr/cvp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eom GH, Kook H. Role of histone deacetylase 2 and its posttranslational modifications in cardiac hypertrophy. BMB Rep. (2015);48:131–138. doi: 10.5483/BMBRep.2015.48.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glickman MH, Ciechanover A. The ubiquitinproteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. (2002);82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 5.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. (1998);67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 6.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. (1999);68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 7.Birks EJ, Latif N, Enesa K. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res. (2008);79:472–480. doi: 10.1093/cvr/cvn083. [DOI] [PubMed] [Google Scholar]
- 8.Powell SR, Wang P, Katzeff H, et al. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: essential role of the proteasome. Antioxid Redox Signal. (2005);7:538–546. doi: 10.1089/ars.2005.7.538. [DOI] [PubMed] [Google Scholar]
- 9.Depre C, Wang Q, Yan L, et al. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation. (2006);17:1821–1828. doi: 10.1161/CIRCULATIONAHA.106.637827. [DOI] [PubMed] [Google Scholar]
- 10.Bahrudin U, Morisaki H, Morisaki T, et al. Ubiquitin-proteasome system impairment caused by a missense cardiac myosin-binding protein C mutation and associated with cardiac dysfunction in hypertrophic cardiomyopathy. J Mol Biol. (2008);384:896–907. doi: 10.1016/j.jmb.2008.09.070. [DOI] [PubMed] [Google Scholar]
- 11.Oliver MF. Fatty acids and recovery during first hours of acute myocardial ischemia. Am J Cardiol. (2014);113:285–286. doi: 10.1016/j.amjcard.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Ding Q, Dimayuga E, Markesbery WR, Keller JN. Proteasome inhibition induces reversible impairments in protein synthesis. FASEB J. (2006);8:1055–1063. doi: 10.1096/fj.05-5495com. [DOI] [PubMed] [Google Scholar]
- 13.Doll D, Sarikas A, Krajcik R, Zolk O. Proteomic expression analysis of cardiomyocytes subjected to proteasome inhibition. Biochem Biophys Res Commun. (2007);2:436–442. doi: 10.1016/j.bbrc.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. (2001);8:739–758. doi: 10.1016/S1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 15.Meiners S, Hocher B, Weller A, et al. Downregulation of matrix metalloproteinases and collagens and suppression of cardiac fibrosis by inhibition of the proteasome. Hypertension. (2004);4:471–477. doi: 10.1161/01.HYP.0000142772.71367.65. [DOI] [PubMed] [Google Scholar]
- 16.Meiners S, Dreger H, Fechner M, et al. Suppression of cardiomyocyte hypertrophy by inhibition of the ubiquitin-proteasome system. Hypertension. (2008);2:302–308. doi: 10.1161/HYPERTENSIONAHA.107.097816. [DOI] [PubMed] [Google Scholar]
- 17.Schlossarek S, Singh SR, Geertz B, et al. Proteasome inhibition slightly improves cardiac function in mice with hypertrophic cardiomyopathy. Front Physiol. (2014);5:484. doi: 10.3389/fphys.2014.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stansfield WE, Tang RH, Moss NC, et al. Proteasome inhibition promotes regression of left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. (2008);2:645–650. doi: 10.1152/ajpheart.00196.2007. [DOI] [PubMed] [Google Scholar]
- 19.Kedi X, Ming Y, Yongping W, et al. Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis. (2009);207:123–130. doi: 10.1016/j.atherosclerosis.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Rong JX, Shapiro M, Trogan E, Fisher EA. Trans differentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A. (2003);100:13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Yoo YS, Lee D, Song EJ. Cholesterol induces cardiac hypertrophy by activating the AKT pathway. J Steroid Biochem Mol Biol. (2013);138:307–313. doi: 10.1016/j.jsbmb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Preisig PA. Compensatory renal hypertrophy is mediated by a cell cycle-dependent mechanism. Kidney Int. (2002);62:1650–1658. doi: 10.1046/j.1523-1755.2002.00620.x. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqi S, Sussman MA. The heart: mostly postmitotic or mostly premitotic? Myocyte cell cycle, senescence, and quiescence. Can J Cardiol. (2014);30:1270–1278. doi: 10.1016/j.cjca.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreger H, Westphal K, Weller A, et al. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc Res. (2009);83:354–361. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- 25.Lüss H, Schmitz W, Neumann J. A proteasome inhibitor confers cardioprotection. Cardiovasc Res. (2002);54:140–151. doi: 10.1016/S0008-6363(02)00232-8. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Wang HX, Han QY, et al. Activation of the cardiac proteasome promotes angiotension II-induced hypertrophy by down-regulation of ATRAP. J Mol Cell Cardiol. (2015);79:303–314. doi: 10.1016/j.yjmcc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Weeks KL, McMullen JR. The athlete's heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology. (2011);26:97–105. doi: 10.1152/physiol.00043.2010. [DOI] [PubMed] [Google Scholar]
- 28.Chen B, Ma Y, Meng R, et al. MG132, a proteasome inhibitor, attenuates pressure-overload-induced cardiac hypertrophy in rats by modulation of mitogen-activated protein kinase signals. Acta Biochim Biophys Sin. (2010);42:253–258. doi: 10.1093/abbs/gmq012. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh V, Kim MJ, Gelissen IC, et al. Cellular cholesterol regulates ubiquitination and degradation of the cholesterol export proteins ABCA1 and ABCG1. J Biol Chem. (2014);289:7524–7536. doi: 10.1074/jbc.M113.515890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macías-Vidal J, Girós M, Guerrero M, et al. The proteasome inhibitor bortezomib reduced cholesterol accumulation in fibroblasts from Niemann-Pick type C patients carrying missense mutations. FEBS J. (2014);281:4450–4466. doi: 10.1111/febs.12954. [DOI] [PubMed] [Google Scholar]
- 31.Meiners S, Ludwig A, Stangl V, Stangl K. Proteasome inhibitors: poisons and remedies. Med Res Rev. (2008);28:309–327. doi: 10.1002/med.20111. [DOI] [PubMed] [Google Scholar]
- 32.Blagosklonny MV. Cell senescence: hypertrophic arrest beyond the restriction point. J Cell Physiol. (2006);209:592–597. doi: 10.1002/jcp.20750. [DOI] [PubMed] [Google Scholar]
- 33.Busk PK, Bartkova J, Strøm CC, et al. Involvement of cyclin D activity in left ventricle hypertrophy in vivo and in vitro. Cardiovasc Res. (2002);1:64–75. doi: 10.1016/S0008-6363(02)00510-2. [DOI] [PubMed] [Google Scholar]
- 34.Gavilán E, Giráldez S, Sánchez-Aguayo I, et al. Breast cancer cell line MCF7 escapes from G1/S arrest induced by proteasome inhibition through a GSK-3β dependent mechanism. Sci Rep. (2015);5:10027. doi: 10.1038/srep10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masamha CP, Benbrook DM. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. (2009);69:6565–6572. doi: 10.1158/0008-5472.CAN-09-0913. [DOI] [PubMed] [Google Scholar]
- 36.Hu L, Su P, Li R, et al. Knockdown of microtubule actin crosslinking factor 1 inhibits cell proliferation in MC3T3-E1 osteoblastic cells. BMB Rep. (2015);48:583–588. doi: 10.5483/BMBRep.2015.48.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]